Key Points
The Jak/Stat3 pathway promotes the expression of IL-17F in malignant CTCL cells.
IL-17F is highly expressed in a subset of CTCL patients and associated with progressive disease.
Abstract
Inappropriately regulated expression of interleukin (IL)-17A is associated with the development of inflammatory diseases and cancer. However, little is known about the role of other IL-17 family members in carcinogenesis. Here, we show that a set of malignant T-cell lines established from patients with cutaneous T-cell lymphoma (CTCL) spontaneously secrete IL-17F and that inhibitors of Janus kinases and Signal transducer and activator of transcription 3 are able to block that secretion. Other malignant T-cell lines produce IL-17A but not IL-17F. Upon activation, however, some of the malignant T-cell lines are able to coexpress IL-17A and IL-17F, leading to formation of IL-17A/F heterodimers. Clinically, we demonstrate that IL-17F messenger RNA expression is significantly increased in CTCL skin lesions compared with healthy donors and patients with chronic dermatitis. IL-17A expression is also increased and a significant number of patients express high levels of both IL-17A and IL-17F. Concomitantly, we observed that the expression of the IL-17 receptor is significantly increased in CTCL skin lesions compared with control subjects. Importantly, analysis of a historic cohort of 60 CTCL patients indicates that IL-17F expression is associated with progressive disease. These findings implicate IL-17F in the pathogenesis of CTCL and suggest that IL-17 cytokines and their receptors may serve as therapeutic targets.
Introduction
Cutaneous T-cell lymphoma (CTCL) is characterized by the expansion of malignant T cells in a chronic inflammatory environment. In the predominant clinical variant, mycosis fungoides (MF), skin lesions initially present as erythematous patches or plaques resembling benign inflammatory skin disorders. The lesions may develop into overt tumors and the malignant T cells can sometimes spread to lymph nodes and internal organs.1-3 Patients diagnosed in early stages often experience an indolent disease course and have a favorable prognosis with a life expectancy similar to that of age-matched controls. However, in a subgroup of patients diagnosed with early MF, the disease follows a more aggressive and occasionally fatal clinical course.4,5 Sézary syndrome (SS) is a less frequent but very aggressive form of CTCL characterized by erythroderma, generalized lymphadenopathy, and the presence of neoplastic T cells (Sézary cells) in the peripheral blood.1
Interleukin (IL)-17A and IL-17F are 2 highly homologous proinflammatory cytokines that are produced by the Th17 subset of CD4+ T cells. Their inflammatory capacities mainly appear to be mediated by their ability to induce expression of proinflammatory cytokines (eg, tumor necrosis factor-α, granulocyte colony-stimulating factor, IL-1, and IL-6), chemokines (eg, IL-8, CCL2, CCL7, CCL20, and CXCL1), angiogenic factors (eg, vascular endothelial growth factor [VEGF]), and matrix metalloproteases (eg, MMP1, MMP3, MMP-9, and MMP-13) from nonlymphoid cell types, including keratinocytes, fibroblasts, endothelial cells, and epithelial cells. Both cytokines are crucial for the host’s defenses against a range of extracellular pathogens, but as a double-edged sword, they can also promote the development of inflammatory and autoimmune diseases. Several studies have further implicated IL-17A in carcinogenesis demonstrating both pro- and anticarcinogenic properties depending on the type and stage of cancer. In line with their high degree of homology, IL-17A and IL-17F bind the same receptor complex that is comprised of the 2 subunits IL-17 receptor (IL-17RA) and IL-17RC and consequently exhibit similar biological activities in many aspects.6,7 However, recent reports have provided evidence that these 2 cytokines can also mediate distinct and even opposing effects.8-10
It was previously documented that malignant T cells from some CTCL patients have the capacity to produce IL-17A and that such expression can be increased or induced by T-cell receptor-activating signals and by activation of the Janus kinase (Jak)/Signal transducer and activator of transcription 3 (Stat3) pathway.11,12 Accordingly, IL-17A is also expressed in skin lesions from a subset of CTCL patients, suggesting that it contributes to chronic inflammation in these patients.11,12 In contrast, little is known about the possible role of IL-17F in the pathogenesis of this cancer. Here, we show that IL-17F expression is increased in CTCL skin lesions and is driven by the Jak/Stat3 pathway in malignant T cells. Furthermore, we find that IL-17F is significantly associated with progressive disease as previously proposed in microarray and reverse transcription-polymerase chain reaction (PCR) profiling studies.13,14
Methods
Antibodies and reagents
The antibody against Erk1/2 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), the antibody against Stat3 from Cell Signaling Technology (Beverly, MA), and the phospho-Stat3 (Y705) antibody from nanoTools (Denzlingen, Germany). Jak inhibitor I (P6) and Jak3 inhibitor II (WHI-P154) were purchased from Merck Millipore (Darmstadt, Germany) and Sta-21 and Tyrphostin Ag1478 inhibitors were purchased from Enzo Life Sciences (Plymouth Meeting, PA). Dimethylsulfoxide, phorbol 12-myristate 13-acetate (PMA), and ionomycin chemical reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Cell lines
The malignant T-cell lines MyLa2000 (MF2000), PB2B, SeAx, and Sez-4 as well as the nonmalignant T-cell lines MySi and MF1850 were obtained from patients with CTCL.15-17 The Jurkat T-cell line has been described elsewhere.18,19 MF2000, PB2B, and Jurkat cells were grown in conditional media (RPMI 1640, 2 mm l-glutamine and 100 mg/mL penicillin/streptomycin; all from Sigma-Aldrich) supplemented with 10% fetal bovine serum (Life Technologies, Roskilde, Denmark). SeAx, Sez-4, MF1850, and MySi were cultured in conditional media supplemented with 10% pooled human serum (Blood Bank, State University Hospital, Copenhagen, Denmark) and 103 U mL–1 IL-2 (Proleukin) (Chiron, Emeryville, CA).
Enzyme-linked immunosorbent assay
Concentrations of IL-17A, IL-17F, and IL-17A/F heterodimers in cell culture supernatants were measured using human DuoSet enzyme-linked immunosorbent assay development kits from R&D Systems (Minneapolis, MN) in accordance with the manufacturer’s instructions.
Protein extraction and western blotting
Protein extraction and western blotting were performed as described earlier.20 To ensure equal loading, the total protein concentration of each lysate was determined by Bio-Rad protein Assay (Bio-Rad, Hercules, CA).
Luciferase assay
Jurkat T cells were cotransfected with a pGL4 luciferase reporter plasmid (Promega, Madison, WI) containing the proximal human IL-17F promoter region (−250 to +1) and a Renilla luciferase plasmid (pRL-CMV; Promega) as well as either an empty pcDNA3.1 plasmid or a pcDNA3.1 plasmid encoding a constitutive active form of Stat3. At 24 hours after transfection, the cells were lysed and the luciferase activities were determined with the use of the Dual-Luciferase Reporter Assay from Promega. The coexpressed Renilla luciferase activity was used for normalization of transfection efficiency.
RNA purification, cDNA synthesis, and QPCR on cell lines
Total cellular messenger RNA (mRNA) was purified and reverse transcribed into complementary DNA (cDNA) as previously described.21 Quantitative PCR (QPCR) was subsequently performed using the Brilliant II SYBR Green QPCR kit from Stratagene (La Jolla, CA) in accordance with the manufacturer’s instructions and the samples were analyzed on a Mx3000P Real-Time PCR System (Stratagene). For amplification, the following primers were used: IL-17F-forward 5′-TTCCAAAAGCCTGAGAGTTG-3′, IL-17F-reverse 5′-GCCCAAGTTCCTACACTGG-3′, glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-forward 5′-AAGGTGAAGGTCGGAGTCAA-3′, and GAPDH-reverse 5′-AATGAAGGGGTCATTGATGG-3′.
Patients
To assess IL-17F/IL-17A expression in skin biopsies, subjects with mycosis fungoides (MF; n = 57) were recruited from the Skin Lymphoma Clinics of the British Columbia Cancer Agency (Vancouver, BC, Canada) and Peking University First Hospital (Beijing, China) with approval from the corresponding local Clinical Ethics Boards. The diagnosis and clinical staging were according to the diagnostic criteria of CTCL.22 In addition, volunteers with normal healthy skin (n = 12) and benign inflammatory dermatoses (n = 22) were recruited from the outpatient dermatology clinic of the University of British Columbia (Vancouver, BC, Canada) with informed consent in accordance with the Declaration of Helsinki. These included 9 cases of psoriasis and 13 patients with benign chronic dermatitis. With informed consent, full-thickness lesional skin were obtained by 4-mm punch biopsies under local anesthesia as described previously.23 Skin biopsies were immediately placed in RNAlater and used for RNA extraction by TRIZOL reagent (Invitrogen, Carlsbad, CA). The purification of Sézary cells from Sézary patients (n = 9) and benign control CD4 T cells from healthy donors (n = 8) was performed as previously described.24-27 Total cellular RNA was extracted using the RNeasy Mini Kit (Qiagen, Mississauga, ON, Canada) according to the manufacturer’s instructions. RNA was reverse transcribed using random primers and SuperScript III reverse transcriptase (Invitrogen). Primers for IL-17A, IL-17F, IL-17RA, IL-17RC, and GAPDH were designed and purchased from Integrated DNA Technologies. Real-time PCR was performed and analyzed with GAPDH serving as internal control. The results are expressed as copies of specific genes per 1000 copies of GAPDH. The formula for the calculation of transcript abundance was previously reported.24,27 The historic cohort of patients from Boston (n = 60) was previously described in multiple publications.13,14,28,29 For these patients, 6-year clinical follow-up data are available for disease progression and disease-specific mortality, and they were accordingly used to analyze if there was a correlation between IL-17F expression and disease progression or disease-specific survival. The expression of IL-17F mRNA was analyzed in a previous study.13 Samples in the upper 90 percentile of IL-17F expression was classified as IL-17F+ and the remaining samples as IL-17F−, while disease progression was defined as the advancement toward a higher clinical stage and/or incidence of a disease-related death.13 For the univariate analysis, the data were subjected to Kaplan-Meier analyses, and significance was determined using the Log-rank test. Finally, the multivariate analysis was performed using the Cox proportional hazards regression method taking into account multiple progression events for each patient.
Results
Malignant CTCL cell lines have the capacity to express IL-17F and/or IL-17A
To investigate if malignant CTCL cells produce IL-17F, we initially tested the expression of IL-17F in malignant and nonmalignant T-cell lines established from CTCL patients. Our data demonstrated that the malignant T-cell lines MF2000 and PB2B spontaneously produced high levels of IL-17F but little or no IL-17A (Figure 1). The reverse picture was observed in SeAx and SeZ-4 malignant T cells that spontaneously secreted IL-17A but essentially no IL-17F (Figure 1). Interestingly, activation of the malignant T cells by treatment with PMA and ionomycin was able to induce or up-regulate the expression of IL-17A and/or IL-17F (Figure 1). In some of the malignant T-cell lines, activation led to coexpression of IL-17A and IL-17F, resulting in the formation of IL-17A/F heterodimers (Figure 1). The transcription factor nuclear factor of activated T cells has previously been shown to be important for activation-induced expression of IL-17A in T cells.30 Indeed, the calcineurin inhibitor, FK506, strongly suppressed the PMA/ionomycin-mediated expression of IL-17A and IL17F, whereas an epidermal growth factor receptor inhibitor (Ag1478) had no effect, suggesting that the activation-induced IL-17 production was at least partly mediated through the nuclear factor of activated T cells pathway in the malignant T cells (supplemental Figure 1, available on the Blood Web site). In contrast to the malignant T-cell lines, the nonmalignant T-cell lines MySi and Myla1850 did not produce IL-17A or IL-17F even after activation with PMA and ionomycin (Figure 1; data not shown). Taken together, these findings show that the examined malignant CTCL cell lines spontaneously express IL-17F or IL-17A and demonstrate that the malignant T cells display a high degree of flexibility allowing for production of IL-17A, IL-17F, and IL-17A/F heterodimers, depending on their activation status.
Malignant CTCL cell lines express IL-17F and/or IL-17A. Nonmalignant (MySi) and malignant (MF2000, PB2B, SeZ-4, and SeAx) T-cell lines established from patients with CTCL were cultured for 24 hours in the absence (−) or presence (P + I) of PMA (50 ng/mL) and ionomycin (1 µg/mL). Subsequently, the concentrations of (A) IL-17F, (B) IL-17A, and (C) IL-17A/F heterodimers in the cell culture supernatants were determined by enzyme-linked immunosorbent assay. Bars represent mean + SEM.
Malignant CTCL cell lines express IL-17F and/or IL-17A. Nonmalignant (MySi) and malignant (MF2000, PB2B, SeZ-4, and SeAx) T-cell lines established from patients with CTCL were cultured for 24 hours in the absence (−) or presence (P + I) of PMA (50 ng/mL) and ionomycin (1 µg/mL). Subsequently, the concentrations of (A) IL-17F, (B) IL-17A, and (C) IL-17A/F heterodimers in the cell culture supernatants were determined by enzyme-linked immunosorbent assay. Bars represent mean + SEM.
The Jak/Stat3 pathway promotes spontaneous expression of IL-17F in malignant T cells
Little is known about the transcriptional regulation of IL-17F in human T cells. To gain further insight into the regulation of IL-17F expression in malignant T cells, we investigated the molecular mechanisms responsible for its spontaneous production. A characteristic feature of CTCL is that the malignant T cells exhibit aberrant activation of the Jak/Stat3 pathway.31-33 We previously demonstrated that Jak/Stat3 activation up-regulates the expression of IL-17A in malignant CTCL cells.12 Hence, we tested the importance of this signaling pathway for the spontaneous secretion of IL-17F. Jak inhibitors blocked the activation of Stat3 and within 4 hours strongly inhibited the expression of IL-17F mRNA, whereas an epidermal growth factor receptor inhibitor had no effect (Figure 2A-B). Consistent with this finding, inhibition of Jak activity almost completely abrogated the expression of IL-17F at the protein level (Figure 2C; supplemental Figure 2). An inhibitor of Stat3 also repressed IL-17F expression at the mRNA (Figure 2B) and protein levels (Figure 2C; supplemental Figure 2), indicating that the Jak/Stat3 signaling pathway is involved in the spontaneous expression of IL-17F. Moreover, forced expression of a constitutive active form of Stat3 in Jurkat T cells increased the transcriptional activity of the proximal human IL-17F promoter in a luciferase reporter assay (Figure 2D). This finding further supports that under the right circumstances, Stat3 can promote the expression of IL-17F in malignant T cells.
The Jak/Stat3 pathway promotes the expression of IL-17F in malignant T cells. (A) Malignant T cells (PB2B) were incubated with Jak inhibitors (Jak3 inhibitor II/WHI-P154, 40 µM; Jak inhibitor I/P6, 1 µM), an inhibitor against Stat3 (Sta-21, 40 µM), an inhibitor against the EGF receptor (Ag1478, 200 ng mL–1) or vehicle (dimethylsulfoxide) and the expression of phosphorylated Stat3 (pYStat3), total Stat3, and Erk 1/2 analyzed by western blotting. (B) Malignant T cells (PB2B) were cultured with inhibitors as described above for 4 hours. Subsequently, the cells were harvested and the relative levels of IL-17F and GAPDH mRNA determined by QPCR. In each sample, the level of IL-17F mRNA was normalized to that of GAPDH mRNA and depicted as the fold change compared with cells cultured with vehicle. (C) Malignant T cells (PB2B) were cultured for 24 hours with inhibitors as described above and the concentration of IL-17F in the cell culture supernatants determined by enzyme-linked immunosorbent assay. The expression of IL-17F is shown as the percent expression relative to cells treated with vehicle. (D) Jurkat T cells were cotransfected with a luciferase reporter plasmid containing the proximal human IL-17F promoter and a Renilla luciferase plasmid as well as either an empty pcDNA3.1 plasmid or a pcDNA3.1 plasmid encoding a constitutive active form of Stat3 (Stat3c). At 24 hours after transfection, the cells were lysed and the luciferase activities determined. The coexpressed Renilla luciferase activity was used for normalization of transfection efficiency. Bars represent mean + SEM.
The Jak/Stat3 pathway promotes the expression of IL-17F in malignant T cells. (A) Malignant T cells (PB2B) were incubated with Jak inhibitors (Jak3 inhibitor II/WHI-P154, 40 µM; Jak inhibitor I/P6, 1 µM), an inhibitor against Stat3 (Sta-21, 40 µM), an inhibitor against the EGF receptor (Ag1478, 200 ng mL–1) or vehicle (dimethylsulfoxide) and the expression of phosphorylated Stat3 (pYStat3), total Stat3, and Erk 1/2 analyzed by western blotting. (B) Malignant T cells (PB2B) were cultured with inhibitors as described above for 4 hours. Subsequently, the cells were harvested and the relative levels of IL-17F and GAPDH mRNA determined by QPCR. In each sample, the level of IL-17F mRNA was normalized to that of GAPDH mRNA and depicted as the fold change compared with cells cultured with vehicle. (C) Malignant T cells (PB2B) were cultured for 24 hours with inhibitors as described above and the concentration of IL-17F in the cell culture supernatants determined by enzyme-linked immunosorbent assay. The expression of IL-17F is shown as the percent expression relative to cells treated with vehicle. (D) Jurkat T cells were cotransfected with a luciferase reporter plasmid containing the proximal human IL-17F promoter and a Renilla luciferase plasmid as well as either an empty pcDNA3.1 plasmid or a pcDNA3.1 plasmid encoding a constitutive active form of Stat3 (Stat3c). At 24 hours after transfection, the cells were lysed and the luciferase activities determined. The coexpressed Renilla luciferase activity was used for normalization of transfection efficiency. Bars represent mean + SEM.
The expression of IL-17F and IL-17A is increased in MF skin lesions
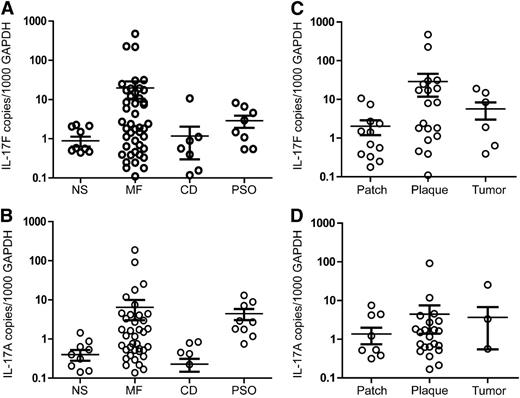
Although it is intriguing that malignant T cells are able to express IL-17A and IL-17F in culture, this raised the question whether these cytokines are also up-regulated in CTCL skin lesions. Consequently, we examined the expression of IL-17F and IL-17A in lesional skin from MF patients. As shown in Figure 3A-B, the expression of IL-17F and IL-17A mRNA was significantly increased in skin samples from MF patients compared with normal skin (IL-17A, P = .043; IL-17F, P = .023) or skin affected by chronic dermatitis (IL-17A, P = .046; IL-17F, P = .027). Expectedly, the expression of IL-17A and IL-17F was also significantly increased in lesional skin from psoriasis patients compared with healthy donors (IL-17A, P = .002; IL-17F, P = .0184). This finding is consistent with previous reports of enhanced expression of Th17 cytokines in psoriasis.34-38 MF patients displayed a considerable heterogeneity in IL-17A and IL-17F expression, with a significant subset of patients displaying high or very high expression levels compared with healthy donors and even psoriasis patients (Figure 3A-B). However, many patients also displayed expression levels similar to those observed in healthy donors (Figure 3A-B). Several patients (26 of 57) expressed high levels of both IL-17A and IL-17F, others (19 of 57) predominantly expressed one of the cytokines, while a minor fraction (12 of 57) expressed low levels of both cytokines (supplemental Figure 3). Although the difference did not reach significance, a higher average expression of IL-17A and IL-17F was observed in plaque (IL-17A, P = .165; IL-17F, P = .063) and tumor stages (IL-17A, P = .246; IL-17F, P = .116) compared with the patch stage (Figure 3C-D), suggesting that the inflammatory environment in MF lesions evolves during disease progression. Based on our findings, the expression of IL-17F did not correlate with the amount of CD4 mRNA in the biopsy samples (r = 0.014; supplemental Figure 4), suggesting that the observed increase in IL-17F was not merely a reflection of increased numbers of CD4 T cells. Likewise, it has previously been shown that the expression of IL-17A is not related to the number of CD4 T cells in CTCL lesions.11 Although MF is generally confined to the skin and lymph nodes, some inflammatory mediators have been shown to be increased in serum from patients with advanced disease.39 Accordingly, we addressed whether increased levels of IL-17F were present in serum from MF patients. However, we did not observe a significant difference (P = .394) in the average serum levels between 28 patients with various clinical stages (IB-IVB) of CTCL and 8 healthy donors, indicating that the increased expression of IL-17F is generally restricted to the skin lesions (supplemental Figure 5). In a similar fashion, IL-17A protein has previously been shown to be present in CTCL skin lesions but not in serum.12
Quantification of IL-17F and IL-17A mRNA in MF and benign skin biopsies. Skin biopsies were obtained from individuals with MF, chronic dermatitis (CD), psoriasis (PSO), and volunteers with normal healthy skin (NS) and used for RNA extraction as described in the methods section. QPCR was performed using primers specific to IL-17F, IL-17A, and GAPDH mRNA. The expression of IL-17F and IL-17A was normalized to GAPDH so that the levels shown represent copies of IL-17F or IL-17A mRNA per 1000 copies of GAPDH mRNA. Depicted is the normalized expression of (A) IL-17F and (B) IL-17A mRNA in NS, MF, CD, and PSO as well as the normalized expression of (C) IL-17F and (D) IL-17A in different types of MF skin lesions. Bars denote the average and standard deviation.
Quantification of IL-17F and IL-17A mRNA in MF and benign skin biopsies. Skin biopsies were obtained from individuals with MF, chronic dermatitis (CD), psoriasis (PSO), and volunteers with normal healthy skin (NS) and used for RNA extraction as described in the methods section. QPCR was performed using primers specific to IL-17F, IL-17A, and GAPDH mRNA. The expression of IL-17F and IL-17A was normalized to GAPDH so that the levels shown represent copies of IL-17F or IL-17A mRNA per 1000 copies of GAPDH mRNA. Depicted is the normalized expression of (A) IL-17F and (B) IL-17A mRNA in NS, MF, CD, and PSO as well as the normalized expression of (C) IL-17F and (D) IL-17A in different types of MF skin lesions. Bars denote the average and standard deviation.
The expression of IL-17RA is increased in MF skin lesions
IL-17A and IL-17F both signal through the IL-17 receptor complex composed of the 2 subunits IL-17RA and IL-17RC. Expression of IL-17RA and IL-17RC has been demonstrated in a large spectrum of tissues and cell types, including keratinocytes and endothelial cells.6,7,40 Not surprisingly, we detected IL-17RA and IL-17RC mRNA in normal and inflamed skin (Figure 4A-B). Most CTCL patients expressed normal or near-normal levels of IL-17RC compared with healthy individuals, whereas in some cases such expression was downregulated (Figure 4A). Strikingly, the expression of IL-17RA was significantly (P = .0012) increased in CTCL patients compared with normal skin (Figure 4B). The increased IL-17RA expression reflected an enhanced expression in the majority of CTCL patients. Yet, as for IL-17A and IL-17F, we observed a considerable heterogeneity in the IL-17RA expression levels among patients.
Quantification of IL-17RC and IL-17RA mRNA in MF and benign skin biopsies. Skin biopsies from individuals with MF, chronic dermatitis (CD), psoriasis (PSO), and normal healthy skin (NS) were analyzed by QPCR using primers specific to IL-17RC, IL-17RA, and GAPDH mRNA. The expression of IL-17RC and IL-17RA was normalized to GAPDH, so that the levels shown represent copies of (A) IL-17RC or (B) IL-17RA mRNA per 1000 copies of GAPDH mRNA. Bars denote the average and standard deviation for each skin type analyzed.
Quantification of IL-17RC and IL-17RA mRNA in MF and benign skin biopsies. Skin biopsies from individuals with MF, chronic dermatitis (CD), psoriasis (PSO), and normal healthy skin (NS) were analyzed by QPCR using primers specific to IL-17RC, IL-17RA, and GAPDH mRNA. The expression of IL-17RC and IL-17RA was normalized to GAPDH, so that the levels shown represent copies of (A) IL-17RC or (B) IL-17RA mRNA per 1000 copies of GAPDH mRNA. Bars denote the average and standard deviation for each skin type analyzed.
IL-17F expression correlates with increased risk of disease progression
Our findings of increased IL-17F expression in the lesional skin in a subset of MF patients raised the question of whether high expression of IL-17F has any prognostic significance. To answer this, we correlated IL-17F expression and clinical disease progression and/or survival in a historic cohort of 60 MF patients.13 Interestingly, by univariate analysis, we found that high expression of IL-17F (IL-17F+) was significantly (P = .008) associated with an increased risk of disease progression (Figure 5A). Moreover, there was a trend (P = .122) toward correlation between high IL-17F expression and poor survival, but statistical significance was not reached within the 6 years of clinical follow-up in the study (Figure 5B). The risk of disease progression has previously been reported to be influenced by a number of clinical parameters, including disease stage, sex, and age at the time of diagnosis.41-43 To address if IL-17F expression was an independent risk factor for disease progression, we performed a multivariate analysis including these covariates. In accordance with previous reports,42,43 we found that advanced clinical stage at the time of diagnosis was a strong independent risk factor (Table 1). Furthermore, a weak association was observed between male sex and disease progression (Table 1). In contrast, we did not find age to be an independent risk factor (Table 1). The studies that reported age and sex as independent risk factors for disease progression had very large sample populations, and it is likely that the lack of statistical significance is due to the relatively smaller sample population used in our study.42,43 Nevertheless, as in the univariate analysis, IL-17F+ patients had a significantly increased risk of disease progression (odds ratio = 2.75; P = .025) compared with IL-17- patients, indicating that high expression of IL-17F is an independent risk factor for progressive disease (Table 1).
High lesional expression of IL-17F is associated with progressive disease. Kaplan-Meier analyses of (A) disease progression and (B) disease-specific survival in the Boston historic cohort of CTCL patients (n = 60) stratified according to IL-17F mRNA expression in lesional skin. Seventeen patients were classified as IL-17F+ and 43 patients as IL-17F−. The Kaplan-Meier disease progression analysis was performed taking into account multiple progression events for each patient.
High lesional expression of IL-17F is associated with progressive disease. Kaplan-Meier analyses of (A) disease progression and (B) disease-specific survival in the Boston historic cohort of CTCL patients (n = 60) stratified according to IL-17F mRNA expression in lesional skin. Seventeen patients were classified as IL-17F+ and 43 patients as IL-17F−. The Kaplan-Meier disease progression analysis was performed taking into account multiple progression events for each patient.
Patient characteristics associated with clinical disease progression
| Patient characteristics . | Odds ratio for progression (P value) . |
|---|---|
| Age | |
| <40 | 1.77 (P = .51) |
| 40-59 | 1.48 (P = .50) |
| ≥60 | 1.00 (reference) |
| Sex | |
| Male | 1.65 (P = .25) |
| Female | 1.00 (reference) |
| Clinical stage at the time of diagnosis | |
| Stage I | 1.00 (reference) |
| Stage II | 4.70 (P = .005) |
| Stage ≥III | 12.00 (P < .0001) |
| Expression of IL-17F | |
| IL-17F+ | 2.75 (P = .025) |
| IL-17F− | 1.00 (reference) |
| Patient characteristics . | Odds ratio for progression (P value) . |
|---|---|
| Age | |
| <40 | 1.77 (P = .51) |
| 40-59 | 1.48 (P = .50) |
| ≥60 | 1.00 (reference) |
| Sex | |
| Male | 1.65 (P = .25) |
| Female | 1.00 (reference) |
| Clinical stage at the time of diagnosis | |
| Stage I | 1.00 (reference) |
| Stage II | 4.70 (P = .005) |
| Stage ≥III | 12.00 (P < .0001) |
| Expression of IL-17F | |
| IL-17F+ | 2.75 (P = .025) |
| IL-17F− | 1.00 (reference) |
The expression of IL-17A but not IL-17F is increased in T cells isolated from the peripheral blood of SS patients
Recent data suggest that MF and SS are distinct disease entities.44,45 Our observations from the CTCL cell lines (Figure 1) indicated that malignant T cells derived from SS patients (SeAx and SeZ-4) preferentially produce IL-17A, whereas other CTCL cell lines preferentially produce IL-17F. In accordance with this observation, we found a heterogeneous but significantly increased expression of IL-17A mRNA in CD4 T cells isolated from peripheral blood of SS patients compared with CD4 T cells from healthy donors (Figure 6). In contrast, there was no difference in the average level of IL-17F mRNA in CD4 T cells from SS patients and healthy donors (Figure 6). Collectively, our findings thereby raise the possibility that IL-17F is differentially expressed in MF and SS.
Quantification of IL-17F and IL-17A mRNA in CD4 T cells isolated from the blood of SS patients and healthy donors. RNA purified from CD4 T cells freshly isolated from the blood of healthy donors (HD) and patients with SS was subjected to QPCR using primers specific for (A) IL-17A and (B) IL-17F. The expression of IL-17A and IL-17F was normalized to GAPDH, so that the level shown represents copies of IL-17A or IL-17F mRNA per 1000 copies of GAPDH mRNA. Bars denote the average and standard deviation for each skin type analyzed. *Indicates a statistically significant difference (P < .05), whereas NS indicates no significant difference (P > .05).
Quantification of IL-17F and IL-17A mRNA in CD4 T cells isolated from the blood of SS patients and healthy donors. RNA purified from CD4 T cells freshly isolated from the blood of healthy donors (HD) and patients with SS was subjected to QPCR using primers specific for (A) IL-17A and (B) IL-17F. The expression of IL-17A and IL-17F was normalized to GAPDH, so that the level shown represents copies of IL-17A or IL-17F mRNA per 1000 copies of GAPDH mRNA. Bars denote the average and standard deviation for each skin type analyzed. *Indicates a statistically significant difference (P < .05), whereas NS indicates no significant difference (P > .05).
Discussion
Little is known about the role of IL-17F in cancer. In the current work, we tested the expression of IL-17F in malignant and nonmalignant CTCL cell lines and documented that a number of malignant T-cell lines secrete IL-17F spontaneously or upon activation. Moreover, we provide evidence that similar to IL-17A, the expression of IL-17F is promoted by the Jak/Stat3 axis in malignant T cells. Consistent with the observed secretion of IL-17F and IL-17A from malignant CTCL cell lines, we found increased expression of both cytokines in lesional skin from a subset of MF patients compared with normal skin and skin from patients with chronic dermatitis. High lesional expression of IL-17F was associated with progressive disease, indicating that the cytokine may be playing a procarcinogenic role in MF.
In contrast to the present study, Miyagaki et al46 did not find an increased expression of IL-17F mRNA in a smaller cohort of CTCL patients. It is unknown whether the difference between the 2 studies is due to variations in patient populations, sample size, or tissue sampling or technical differences between the methods used in the 2 studies. Our findings of an increased expression of IL-17A and IL-17F in patients with psoriasis are in accordance with previous reports34-38 indicating a high level of robustness of the methods used in the present study.
Two of 3 previous studies reported that the expression of IL-17A is increased in CTCL,11,12 whereas a single study did not support a link between IL-17A and CTCL.46 The frequency of IL-17A positivity ranged from a fraction of patients in one study and up to more than two-thirds of all patients in another study.11,12 However, these studies used different techniques and enrolled only a few patients.11,12,46 In a much larger cohort of patients, we now report a significant upregulation of IL-17A in CTCL compared with healthy individuals and patients with chronic dermatitis. Importantly, IL-17A expression was not uniform among CTCL patients. Instead, some patients expressed clearly elevated IL-17A levels, whereas others expressed low levels comparable with those found in normal skin. The present findings are, therefore, not contradictory to the previous studies. On the contrary, all expression profiles (none, intermediate, and high) previously described11,12,46 were represented in our cohort of patients, suggesting that the apparent discrepancies between the aforementioned studies were possibly due to small sample sizes.
Even though the difference did not reach statistical significance, we found that the average expression of IL-17F and IL-17A was increased in the plaque and tumor stages compared with the patch stage. The lack of statistical significance is probably due to the heterogeneous expression of the cytokines in plaque and tumor stage lesions. Notably, our observations of increased expression of IL-17F in a subgroup of patients with plaque stage lesions and consequent association with progressive disease are in line with a recent large prospective study documenting that plaques in stage IA and IB patients are associated with a poor prognosis.42
Malignant CTCL cells display a considerable level of plasticity in their production of cytokines and expression of regulatory molecules.47,48 The fact that malignant T-cell lines that spontaneously produce IL-17A or IL-17F, under the right stimulating conditions, could also secrete IL-17A/IL-17F heterodimers supports the concept of malignant T-cell plasticity. In a similar fashion, IL-17A and IL-17F were not always expressed in tandem in lesional skin from MF patients. Some expressed high levels of neither or both, while others expressed high levels of IL-17A but not IL-17F or high levels of IL-17F but not IL-17A. We hypothesize that cytokines, antigens, and inflammatory factors in the local environment modulate the expression of IL-17 cytokines in CTCL.
IL-17 cytokines stimulate a multitude of biological responses in target cells expressing the appropriate IL-17 receptors. Multiple cell types residing in the skin, including keratinocytes and endothelial cells, express IL-17 receptors.6,7,40 Our data show that the expression of the receptor subunits for IL-17A and IL-17F is either increased (IL-17RA) or expressed to a similar extent (IL-17RC) as in healthy skin and therefore suggests that IL-17 cytokines influence the tumor microenvironment in MF. IL-17A and IL-17F are among cytokines known to promote: (1) angiogenesis and lymphangiogenesis via up-regulation of angiogenic and lymphangiogenic factors49,50 ; (2) production of prostaglandins such as PGE251 ; and (3) up-regulation of inflammatory chemokines and their receptors.6 Indeed, angiogenic factors such as VEGF and VEGF-C and their receptors are expressed in situ in lesional CTCL skin,33,52,53 and enhanced angiogenesis correlates with disease progression in CTCL patients.54,55 Prostaglandin E2 is a growth factor for malignant T cells56 and Celecoxib, an inhibitor of its production, is able to inhibit tumor growth in vivo in a CTCL xenograft model.57 Likewise, various chemokines and chemokine receptors have been implicated in CTCL development and progression.58,59
In conclusion, our study indicates that IL-17F is expressed and is likely to play a pathogenic role in MF. Combined with previous reports, our results suggest that IL-17 family cytokines as well as their corresponding receptors may serve as therapeutic targets in CTCL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Thomas Kupper from Harvard University for generously providing cDNA samples from CTCL patients for reverse transcription-PCR analysis. The authors also thank K. Kaltoft for providing the MyLa (MF) and SeAx cell lines.
This work was supported in part by research funding from the Carlsberg Foundation, the Danish Cancer Society, Dansk Kræftforsknings Fond, the Danish Research Councils, the Danish National Advanced Technology Foundation, the Copenhagen Cluster of Immunology, the Lundbeck Foundation, the Novo Nordic Foundation, the Beckett Foundation (Beckett-Fonden), the University of Copenhagen, and the National Cancer Institute (grant CA89194). This work was also supported by the Canadian Dermatology Foundation and the Fonds de recherche du Québec-Santé (research grants to D.S.).
Authorship
Contribution: T.K., I.V.L., Y.W., L.X., A.W-O., and A.W. performed experiments; T.K., I.V.L., Y.W., L.X., Y.Z., D.S., and N.O. analyzed and made the figures; and T.K., I.V.L., Y.W., L.X., A.W-O., S.K., K.L.K., C.M.B., M.A.W., C.G., A.W., Y.Z., D.S., and N.O. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Niels Odum, Department of International Health, Immunology and Microbiology, University of Copenhagen, Panum 22.5.34, Blegdamsvej 3c, DK-2200 Copenhagen N, Denmark; e-mail: ndum@sund.ku.dk; and Youwen Zhou, Department of Dermatology and Skin Science, University of British Columbia, 835 West 10th Ave, Vancouver, BC V5Z 4E8, Canada; e-mail: ywzhou@mail.ubc.ca.
References
Author notes
T.K., I.V.L., Y.W., and L.X. contributed equally to this study.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal