Key Points
Elevated levels of BCA-1, sTNFR2, and sVEGFR2 are associated with increased risk for NHL several years after blood collection.
Abstract
Although severe immune dysregulation is an established risk factor for non-Hodgkin lymphoma (NHL), the importance of subclinical immunologic effects is unclear. We compared baseline serum levels of 67 immune and inflammation markers between 301 patients with NHL diagnosed 5+ years after blood collection and 301 control patients within the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. We observed associations with NHL for elevated B-cell-attracting chemokine 1 (BCA-1; fourth quartile vs first: odds ratio [OR], 2.7; 95% confidence interval [CI], 1.7-4.2; Ptrend = 1.0 × 10−6), soluble tumor necrosis factor receptor 2 (sTNFR2; OR, 3.4; 95% CI, 2.0-5.8; Ptrend = 1.1 × 10−6), and soluble vascular endothelial growth factor receptor 2 (sVEGFR2; OR, 2.3; 95% CI, 1.4-3.9; Ptrend = .0005) that remained significant after Bonferroni correction, simultaneous model adjustment, and restriction to cases diagnosed 8 to 13 years after blood collection. Associations with other markers were observed, although none remained associated with NHL after adjustment for BCA-1, sTNFR2, and sVEGFR2. Our findings suggest that circulating BCA-1, sTNFR2, and sVEGFR2 are associated with NHL risk well in advance of diagnosis. Additional research is needed to replicate these findings and elucidate the underlying biologic mechanisms.
Introduction
Conditions characterized by severe immune dysregulation such as immunosuppression, AIDS, and Sjögren syndrome have been established as strong risk factors for non-Hodgkin lymphoma (NHL).1 However, it is unclear whether subclinical immunologic perturbations influence NHL risk. Recently, NHL studies within general population cohorts incorporating serologic measurements of cytokines, chemokines, and other immune markers have provided important evidence supporting a role for subtle immunologic effects in lymphomagenesis. In an investigation of 11 immune markers among 297 case patients and 297 control patients within the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO), we found elevated circulating levels of the immune activation markers soluble CD27 (sCD27) and sCD30 to be strongly associated with increased future risk for NHL, as well as limited evidence of association with the proinflammatory markers soluble tumor necrosis factor receptor 1 (sTNFR1) and sTNFR2.2,3 A subsequent study of 5 analytes within the Women’s Health Initiative (WHI; 491 case patients, 491 control patients) confirmed our findings for sCD27 and sCD30, and observed strong NHL associations with 2 additional immune markers: sCD23 and B-cell-attracting chemokine 1 (BCA-1, also known as CXCL13).4 Findings from 2 small studies in the European Prospective Investigation into Cancer and Nutrition (EPIC-Italy) cohort (86 case patients, 86 control patients; 16 analytes measured) and the New York University–Women's Health Study (NYU-WHS; 92 case patients, 184 control patients; 32 analytes) have also suggested NHL associations with sCD30, sTNFR2, and other immune markers.5-7
The studies of circulating immune markers and risk for NHL conducted to date have been limited in terms of sample size (EPIC-Italy, NYU-WHS) or the number of evaluated analytes (PLCO, WHI). To more broadly investigate the effects of different immunologic and other mechanisms on NHL risk, we conducted a new nested case-control study within PLCO investigating associations with prediagnostic serum levels of 67 immune and inflammation markers.
Materials and methods
Study design
Detailed descriptions of the PLCO Trial have been reported.8,9 In brief, between 1993 and 2001, approximately 155 000 subjects aged from 55 to 74 years were recruited in 10 cities from the general population and randomly assigned to either the screening or nonscreening group of the study. All participants in the screening group provided nonfasting blood samples at baseline and 5 subsequent annual medical examinations. Samples were processed and frozen within 2 hours of collection and stored at −70°C. Individuals were followed-up for all cancer diagnoses by annual mailed questionnaires. All cancer diagnoses were pathologically confirmed through medical record abstraction. Institutional review boards of the National Cancer Institute (NCI) and the 10 study centers approved the trial, and all participants provided written informed consent in accordance with the Declaration of Helsinki. This case-control study was approved by the institutional review board of the NCI.
We identified 301 first-primary cases of NHL diagnosed 5 or more years after baseline blood collection from among eligible participants in the screening group, followed-up through December 31, 2008. Eligibility criteria included the availability of an unthawed baseline serum sample, consent to biochemical studies, completion of the baseline questionnaire, and no history of cancer (other than nonmelanoma skin cancer) before NHL diagnosis. Cases were classified according to the International Classification of Diseases for Oncology, Second Edition, and categorized into histologic subtypes on the basis of the Interlymph proposed hierarchical classification of lymphoid neoplasms for epidemiologic research.10 Of the 301 cases, 125 had been included in our earlier PLCO investigation.2,3 Controls were individually matched to cases on a 1:1 ratio on the basis of age at baseline (55-59, 60-64, 65-69, or 70-74 years), sex, race (white, African American or other), study center, and time (am, pm) and date (3-month categories) of baseline blood draw from among eligible subjects who had not been diagnosed with any type of malignancy (excluding nonmelanoma skin cancer) at the time of the case diagnosis date.
Laboratory methods
Using baseline serum specimens, we measured circulating levels of 83 immune and inflammation markers, including cytokines, chemokines, extracellular matrix proteins, growth factors, and soluble products of immune activation (supplemental Table 1). These markers were selected on the basis of a recent methodologic study that our group conducted to evaluate the performance and reproducibility of multiplexed immune/inflammation assays.11 Markers were measured using 6 Luminex bead-based commercial assay panels (Millipore Inc.; supplemental Table 1). All assays were performed in the same laboratory using the same instrument. Concentrations of markers within the range of 3.2 to 10 000 pg/mL were calculated using a 5-parameter standard curve. Serum samples were assayed in duplicate and were averaged to calculate concentrations. Samples with values below the assay lower limit of detection (LLOD) were assigned a value of half the LLOD. Cases and matched controls were included in the same analytical batch, each of which included 37 unique samples. To evaluate assay performance, we included 76 replicate samples from 19 PLCO participants within and across batches and a single replicate sample from a quality control pool in each batch. Assay reproducibility (as measured by coefficients of variation and intraclass correlation coefficients of log-transformed concentrations) was high for a majority of markers (supplemental Table 1), and measurements from quality control pool replicates suggested no laboratory drift across batches. We excluded from further study 16 markers with less than 20% of sample measurements above the LLOD (supplemental Table 1). The remaining 67 markers were included in the statistical analysis.
Statistical analysis
For each of the 67 markers included in the analysis, we used conditional logistic regression models to compute odds ratios (ORs) and 95% confidence intervals (CIs) relating serum marker concentration and NHL risk. Marker levels were categorized into groups on the basis of the proportion of samples with measurements above the LLOD as follows: Markers with more than 75% of measurements above the LLOD (n = 41) were categorized into quartiles on the basis of the distribution among controls, markers with 50% to 75% of measurements above the LLOD (n = 4) were categorized into 3 groups (lower than LLOD, lower than median detectable level among controls, median or higher), and markers with less than 50% of measurements above the LLOD (n = 22) were categorized into 2 groups (lower than LLOD and detectable level). Tests for trend were conducted for markers with 3 or more categories by modeling the intracategory medians as a continuous parameter.
In addition, we carried out analyses adjusted for baseline body mass index (BMI; <25.0, 25.0-29.9, and ≥30.0 kg/m2), use (yes, no), and frequency (none, <2 times per month, 2-3 times per month, 1 time per week, 2 times per week, 3-4 times per week, 1 times per day, and 2+ times per day) of aspirin and ibuprofen in the 12 months before enrollment, and smoking status (never, former, current), restricted to white subjects and stratified by sex and median length of follow-up from blood collection to case diagnosis (5-7 and 8-13 years). We evaluated evidence of multiplicative statistical interaction of markers with sex, age at baseline, BMI, aspirin use, ibuprofen use, and smoking through likelihood ratio tests constructed using deviance statistics from models with and without interaction terms. We also fit polytomous regression models to compute associations between dichotomized analyte levels (more than vs less than or equal to control median for markers with 50% or more of measurements above the LLOD; detectable vs undetectable for other markers) and common NHL histologic subtypes (chronic lymphocytic leukemia/small lymphocytic lymphoma [CLL/SLL], diffuse large B-cell lymphoma [DLBCL], and follicular lymphoma [FL]) and to test for OR homogeneity across the 3 NHL subtypes.
Statistical tests were 2-sided at an α = 0.05. However, we also applied a Bonferroni-corrected α of 0.00075 to P values from tests of association across all 67 markers (tests for trend for markers with ≥50% of measurements above the LLOD; tests of detectable vs nondetetectable for markers with <50% of measurements above the LLOD) to control the family-wise error rate.
Results
The distributions of cases and controls by selected characteristics are summarized in Table 1. Participants were an average of 63.6 years of age at enrollment and were predominantly men (64% of participants) and white (97%). The most common NHL histologic subtypes were CLL/SLL (N = 106; 35% of cases), DLBCL (N = 71; 24% of cases), and FL (N = 41; 14% of cases). The median length of follow-up from blood collection to case diagnosis was 8.0 years (range, 5.0-13.9 years). The distribution by histology varied slightly with longer follow-up. Compared with cases diagnosed 5 to 7 years from blood collection, cases diagnosed 8 to 13 years postcollection were less likely to be DLBCL (20% vs 27%, respectively), were equally likely to be FL (14% vs 14%), and were more likely to be CLL/SLL (36% vs 34%) or of other/not otherwise specified histology (30% vs 25%).
Selected characteristics of NHL cases and individually matched controls selected from the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (1993-2008)
| . | Cases (n = 301) . | Controls (n = 301) . | ||
|---|---|---|---|---|
| . | N . | % . | N . | % . |
| Age at enrollment (years) | ||||
| 55-59 | 72 | 23.9 | 72 | 23.9 |
| 60-64 | 94 | 31.2 | 94 | 31.2 |
| 65-69 | 85 | 28.2 | 85 | 28.2 |
| 70-74 | 50 | 16.6 | 50 | 16.6 |
| Sex | ||||
| Female | 110 | 36.5 | 110 | 36.5 |
| Male | 191 | 63.5 | 191 | 63.5 |
| Race | ||||
| White | 293 | 97.3 | 293 | 97.3 |
| African American | 3 | 1.0 | 3 | 1.0 |
| Other | 5 | 1.7 | 5 | 1.7 |
| Trial center | ||||
| Birmingham, AL | 8 | 2.7 | 8 | 2.7 |
| Denver, CO | 27 | 9.0 | 27 | 9.0 |
| Detroit, MI | 28 | 9.3 | 28 | 9.3 |
| Honolulu, HI | 3 | 1.0 | 3 | 1.0 |
| Marshfield, WI | 46 | 15.3 | 46 | 15.3 |
| Minneapolis, MN | 65 | 21.6 | 65 | 21.6 |
| Pittsburgh, PA | 42 | 14.0 | 42 | 14.0 |
| Salt Lake City, UT | 28 | 9.3 | 28 | 9.3 |
| St. Louis, MO | 28 | 9.3 | 28 | 9.3 |
| Washington, DC | 26 | 8.6 | 26 | 8.6 |
| Year of enrollment | ||||
| 1993-1995 | 119 | 39.5 | 119 | 39.5 |
| 1996-1998 | 124 | 41.2 | 124 | 41.2 |
| 1999-2001 | 58 | 19.3 | 58 | 19.3 |
| NHL histologic subtype | ||||
| CLL/SLL | 106 | 35.2 | ||
| DLBCL | 71 | 23.6 | ||
| FL | 41 | 13.6 | ||
| Other/not otherwise specified | 83 | 27.6 | ||
| . | Cases (n = 301) . | Controls (n = 301) . | ||
|---|---|---|---|---|
| . | N . | % . | N . | % . |
| Age at enrollment (years) | ||||
| 55-59 | 72 | 23.9 | 72 | 23.9 |
| 60-64 | 94 | 31.2 | 94 | 31.2 |
| 65-69 | 85 | 28.2 | 85 | 28.2 |
| 70-74 | 50 | 16.6 | 50 | 16.6 |
| Sex | ||||
| Female | 110 | 36.5 | 110 | 36.5 |
| Male | 191 | 63.5 | 191 | 63.5 |
| Race | ||||
| White | 293 | 97.3 | 293 | 97.3 |
| African American | 3 | 1.0 | 3 | 1.0 |
| Other | 5 | 1.7 | 5 | 1.7 |
| Trial center | ||||
| Birmingham, AL | 8 | 2.7 | 8 | 2.7 |
| Denver, CO | 27 | 9.0 | 27 | 9.0 |
| Detroit, MI | 28 | 9.3 | 28 | 9.3 |
| Honolulu, HI | 3 | 1.0 | 3 | 1.0 |
| Marshfield, WI | 46 | 15.3 | 46 | 15.3 |
| Minneapolis, MN | 65 | 21.6 | 65 | 21.6 |
| Pittsburgh, PA | 42 | 14.0 | 42 | 14.0 |
| Salt Lake City, UT | 28 | 9.3 | 28 | 9.3 |
| St. Louis, MO | 28 | 9.3 | 28 | 9.3 |
| Washington, DC | 26 | 8.6 | 26 | 8.6 |
| Year of enrollment | ||||
| 1993-1995 | 119 | 39.5 | 119 | 39.5 |
| 1996-1998 | 124 | 41.2 | 124 | 41.2 |
| 1999-2001 | 58 | 19.3 | 58 | 19.3 |
| NHL histologic subtype | ||||
| CLL/SLL | 106 | 35.2 | ||
| DLBCL | 71 | 23.6 | ||
| FL | 41 | 13.6 | ||
| Other/not otherwise specified | 83 | 27.6 | ||
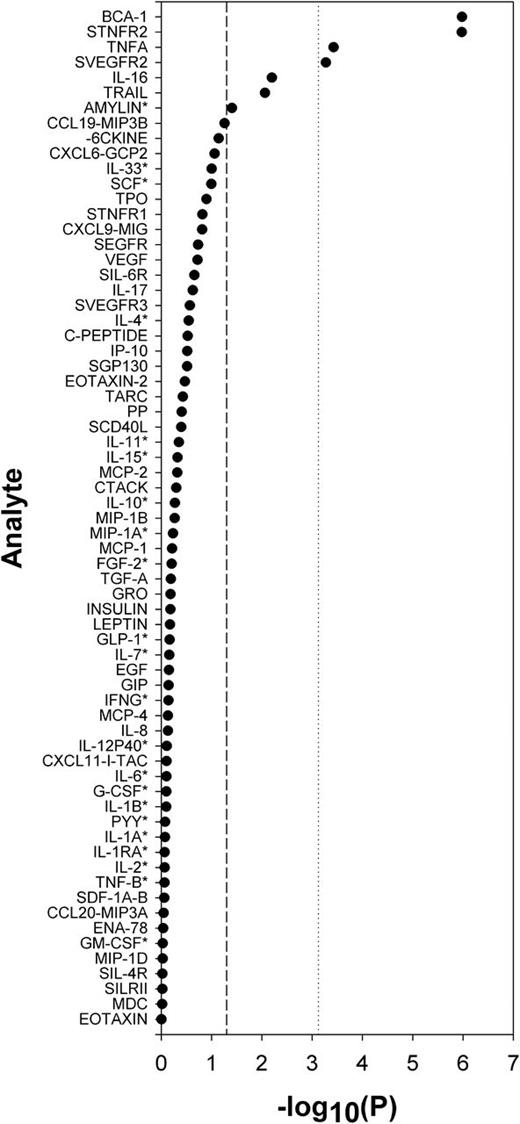
P values from tests of association across all 67 immune and inflammation markers are summarized in Figure 1. Serum levels of BCA-1, sTNFR2, TNF-α, and soluble vascular endothelial receptor 2 (sVEGFR2) were associated with NHL at a Bonferroni-corrected α of 0.00075, whereas interleukin 16 (IL-16), TNF-related apoptosis inducing ligand (TRAIL), and amylin were associated with risk at P < .05. More detailed results for these 7 markers are shown in Table 2 (see supplemental Table 2 for a summary of results for all markers). Cases had higher serum levels of BCA-1 (fourth quartile vs first: OR, 2.7; 95% CI, 1.7-4.2; Ptrend = 1.0 × 10−6), sTNFR2 (OR, 3.4; 95% CI, 2.0-5.8; Ptrend = 1.1 × 10−6), TNF-α (OR, 1.8; 95% CI, 1.1-2.9; Ptrend = .0004), sVEGFR2 (OR, 2.3; 95% CI, 1.4-3.9; Ptrend = .0005), IL-16 (OR, 2.1; 95% CI, 1.3-3.3; Ptrend = .006), TRAIL (1.6, 1.0-2.5; Ptrend = .009), and amylin (detectable vs < LLOD: OR 1.5, 95% CI 1.0-2.1; P = .04) compared with controls. These associations did not materially change with model adjustment for smoking status, aspirin frequency, ibuprofen frequency, or BMI (results not shown). For all 7 markers, the associations with NHL cases diagnosed 8 years or longer since blood collection were comparable to or stronger than those observed for cases diagnosed between 5 and 7 years postcollection (Table 2). We did not observe evidence of interaction with sex, age at baseline, smoking status, aspirin use, ibuprofen use, or BMI (results not shown).
Summary of P values from tests of association with NHL for all analytes included in the analysis (N = 67).P values for analytes detectable in less than 50% of samples (marked by an asterisk), computed using Wald statistic of regression model parameters for dichotomized analyte level (< lower limit of detection vs detectable). P values for all other analytes were computed using Wald statistic of regression model parameters for analyte intracategory medians modeled as a continuous variable.
Summary of P values from tests of association with NHL for all analytes included in the analysis (N = 67).P values for analytes detectable in less than 50% of samples (marked by an asterisk), computed using Wald statistic of regression model parameters for dichotomized analyte level (< lower limit of detection vs detectable). P values for all other analytes were computed using Wald statistic of regression model parameters for analyte intracategory medians modeled as a continuous variable.
Associations with NHL for baseline serum concentrations of selected immune and inflammation markers, overall and stratified by median time from blood collection to case diagnosis
| Analyte concentration (pg/mL)* . | . | . | . | Years from blood collection to case diagnosis . | ||
|---|---|---|---|---|---|---|
| Overall . | 5-7 . | 8-13 . | ||||
| NCont/NCase . | OR† (95% CI) . | NCont/NCase . | OR† (95% CI) . | NCont/NCase . | OR† (95% CI) . | |
| BCA-1 | ||||||
| <12.44 | 76/52 | 1.0 | 29/28 | 1.0 | 47/24 | 1.0 |
| 12.44-15.56 | 75/52 | 1.0 (0.6-1.6) | 36/26 | 0.8 (0.4-1.7) | 39/26 | 1.1 (0.6-2.3) |
| 15.57-20.15 | 75/61 | 1.3 (0.8-2.1) | 46/29 | 0.6 (0.3-1.4) | 29/32 | 2.2 (1.1-4.6) |
| ≥20.16 | 75/136 | 2.7 (1.7-4.2) | 42/70 | 1.7 (0.9-3.5) | 33/66 | 3.7 (1.9-7.2) |
| Ptrend‡ | 1.0 × 10−6 | .007 | 3.8 × 10−5 | |||
| sTNFR2 | ||||||
| <4021.67 | 76/45 | 1.0 | 37/25 | 1.0 | 39/20 | 1.0 |
| 4021.67-4896.02 | 75/52 | 1.3 (0.8-2.3) | 36/22 | 1.0 (0.5-2.2) | 39/30 | 1.7 (0.8-3.6) |
| 4896.03-5792.83 | 75/82 | 2.1 (1.2-3.5) | 37/42 | 2.0 (1.0-4.3) | 38/40 | 2.2 (1.0-4.6) |
| ≥5792.84 | 75/122 | 3.4 (2.0-5.8) | 43/64 | 3.1 (1.4-6.7) | 32/58 | 3.8 (1.8-8.1) |
| Ptrend | 1.1 × 10−6 | .001 | .0003 | |||
| TNF-α | ||||||
| <4.07 | 76/68 | 1.0 | 39/32 | 1.0 | 37/36 | 1.0 |
| 4.07-5.03 | 75/41 | 0.6 (0.4-1.1) | 34/21 | 0.8 (0.4-1.7) | 41/20 | 0.5 (0.2-1.0) |
| 5.04-6.35 | 75/74 | 1.2 (0.7-1.9) | 35/40 | 1.4 (0.7-2.8) | 40/34 | 0.5 (0.2-1.0) |
| ≥6.36 | 75/118 | 1.8 (1.1-2.9) | 45/60 | 1.7 (0.9-3.3) | 30/58 | 2.0 (1.0-3.9) |
| Ptrend | .0004 | .03 | .004 | |||
| sVEGFR2 | ||||||
| <10 043.05 | 77/54 | 1.0 | 43/28 | 1.0 | 34/26 | 1.0 |
| 10 043.05-11 279.22 | 74/57 | 1.2 (0.7-1.9) | 35/29 | 1.2 (0.6-2.5) | 39/28 | 1.1 (0.5-2.3) |
| 11 279.23-13 357.80 | 75/87 | 1.9 (1.1-3.1) | 36/43 | 2.0 (1.0-3.9) | 39/44 | 1.7 (0.8-3.7) |
| ≥13 357.81 | 75/103 | 2.3 (1.4-3.9) | 39/53 | 2.3 (1.2-4.6) | 36/50 | 2.3 (1.0-5.1) |
| Ptrend | .0005 | .01 | .02 | |||
| IL-16 | ||||||
| <13.60 | 76/51 | 1.0 | 31/23 | 1.0 | 44/28 | 1.0 |
| 13.60-24.89 | 76/77 | 1.5 (0.9-2.5) | 39/40 | 1.4 (0.7-2.9) | 38/37 | 1.6 (0.8-3.2) |
| 24.90-35.26 | 74/74 | 1.6 (1.0-2.7) | 42/39 | 1.3 (0.6-2.9) | 33/35 | 1.9 (0.9-3.9) |
| ≥35.27 | 75/99 | 2.1 (1.3-3.3) | 41/51 | 1.7 (0.8-3.5) | 34/48 | 2.4 (1.2-4.7) |
| Ptrend | .006 | .15 | .02 | |||
| TRAIL | ||||||
| <24.09 | 76/68 | 1.0 | 35/42 | 1.0 | 41/26 | 1.0 |
| 24.09-31.40 | 75/48 | 0.7 (0.4-1.2) | 36/28 | 0.6 (0.3-1.3) | 39/20 | 0.8 (0.4-1.7) |
| 31.41-41.95 | 75/80 | 1.2 (0.8-1.9) | 39/35 | 0.8 (0.4-1.4) | 36/45 | 2.2 (1.1-4.4) |
| ≥41.96 | 75/105 | 1.6 (1.0-2.5) | 43/48 | 0.9 (0.5-1.7) | 32/57 | 2.8 (1.4-5.6) |
| Ptrend | .009 | .95 | .0004 | |||
| Amylin | ||||||
| <LLOD | 171/148 | 1.0 | 82/76 | 1.0 | 89/72 | 1.0 |
| Detectable | 130/153 | 1.5 (1.0-2.1) | 71/77 | 1.2 (0.7-1.9) | 59/76 | 1.9 (1.1-3.2) |
| P§ | .04 | .46 | .03 | |||
| Analyte concentration (pg/mL)* . | . | . | . | Years from blood collection to case diagnosis . | ||
|---|---|---|---|---|---|---|
| Overall . | 5-7 . | 8-13 . | ||||
| NCont/NCase . | OR† (95% CI) . | NCont/NCase . | OR† (95% CI) . | NCont/NCase . | OR† (95% CI) . | |
| BCA-1 | ||||||
| <12.44 | 76/52 | 1.0 | 29/28 | 1.0 | 47/24 | 1.0 |
| 12.44-15.56 | 75/52 | 1.0 (0.6-1.6) | 36/26 | 0.8 (0.4-1.7) | 39/26 | 1.1 (0.6-2.3) |
| 15.57-20.15 | 75/61 | 1.3 (0.8-2.1) | 46/29 | 0.6 (0.3-1.4) | 29/32 | 2.2 (1.1-4.6) |
| ≥20.16 | 75/136 | 2.7 (1.7-4.2) | 42/70 | 1.7 (0.9-3.5) | 33/66 | 3.7 (1.9-7.2) |
| Ptrend‡ | 1.0 × 10−6 | .007 | 3.8 × 10−5 | |||
| sTNFR2 | ||||||
| <4021.67 | 76/45 | 1.0 | 37/25 | 1.0 | 39/20 | 1.0 |
| 4021.67-4896.02 | 75/52 | 1.3 (0.8-2.3) | 36/22 | 1.0 (0.5-2.2) | 39/30 | 1.7 (0.8-3.6) |
| 4896.03-5792.83 | 75/82 | 2.1 (1.2-3.5) | 37/42 | 2.0 (1.0-4.3) | 38/40 | 2.2 (1.0-4.6) |
| ≥5792.84 | 75/122 | 3.4 (2.0-5.8) | 43/64 | 3.1 (1.4-6.7) | 32/58 | 3.8 (1.8-8.1) |
| Ptrend | 1.1 × 10−6 | .001 | .0003 | |||
| TNF-α | ||||||
| <4.07 | 76/68 | 1.0 | 39/32 | 1.0 | 37/36 | 1.0 |
| 4.07-5.03 | 75/41 | 0.6 (0.4-1.1) | 34/21 | 0.8 (0.4-1.7) | 41/20 | 0.5 (0.2-1.0) |
| 5.04-6.35 | 75/74 | 1.2 (0.7-1.9) | 35/40 | 1.4 (0.7-2.8) | 40/34 | 0.5 (0.2-1.0) |
| ≥6.36 | 75/118 | 1.8 (1.1-2.9) | 45/60 | 1.7 (0.9-3.3) | 30/58 | 2.0 (1.0-3.9) |
| Ptrend | .0004 | .03 | .004 | |||
| sVEGFR2 | ||||||
| <10 043.05 | 77/54 | 1.0 | 43/28 | 1.0 | 34/26 | 1.0 |
| 10 043.05-11 279.22 | 74/57 | 1.2 (0.7-1.9) | 35/29 | 1.2 (0.6-2.5) | 39/28 | 1.1 (0.5-2.3) |
| 11 279.23-13 357.80 | 75/87 | 1.9 (1.1-3.1) | 36/43 | 2.0 (1.0-3.9) | 39/44 | 1.7 (0.8-3.7) |
| ≥13 357.81 | 75/103 | 2.3 (1.4-3.9) | 39/53 | 2.3 (1.2-4.6) | 36/50 | 2.3 (1.0-5.1) |
| Ptrend | .0005 | .01 | .02 | |||
| IL-16 | ||||||
| <13.60 | 76/51 | 1.0 | 31/23 | 1.0 | 44/28 | 1.0 |
| 13.60-24.89 | 76/77 | 1.5 (0.9-2.5) | 39/40 | 1.4 (0.7-2.9) | 38/37 | 1.6 (0.8-3.2) |
| 24.90-35.26 | 74/74 | 1.6 (1.0-2.7) | 42/39 | 1.3 (0.6-2.9) | 33/35 | 1.9 (0.9-3.9) |
| ≥35.27 | 75/99 | 2.1 (1.3-3.3) | 41/51 | 1.7 (0.8-3.5) | 34/48 | 2.4 (1.2-4.7) |
| Ptrend | .006 | .15 | .02 | |||
| TRAIL | ||||||
| <24.09 | 76/68 | 1.0 | 35/42 | 1.0 | 41/26 | 1.0 |
| 24.09-31.40 | 75/48 | 0.7 (0.4-1.2) | 36/28 | 0.6 (0.3-1.3) | 39/20 | 0.8 (0.4-1.7) |
| 31.41-41.95 | 75/80 | 1.2 (0.8-1.9) | 39/35 | 0.8 (0.4-1.4) | 36/45 | 2.2 (1.1-4.4) |
| ≥41.96 | 75/105 | 1.6 (1.0-2.5) | 43/48 | 0.9 (0.5-1.7) | 32/57 | 2.8 (1.4-5.6) |
| Ptrend | .009 | .95 | .0004 | |||
| Amylin | ||||||
| <LLOD | 171/148 | 1.0 | 82/76 | 1.0 | 89/72 | 1.0 |
| Detectable | 130/153 | 1.5 (1.0-2.1) | 71/77 | 1.2 (0.7-1.9) | 59/76 | 1.9 (1.1-3.2) |
| P§ | .04 | .46 | .03 | |||
CI, confidence interval; LLOD, lower limit of detection; NCont, number of controls; NCase, number of cases; OR, odds ratio.
Analyte concentrations categorized using quartiles among all controls as cutpoints.
Odds ratios computed using conditional logistic regression.
Wald test from regression analysis modeling analyte intra-category medians as a continuous variable.
Wald test P value from regression analysis modeling analyte as dichotomized variable (<LLOD, detectable).
Serum levels of the 7 markers associated with NHL were weakly correlated with one another among controls, with Spearman rank correlation coefficients ranging from 0.03 (dichotomized amylin and sTNFR2) to 0.37 (sTNFR2 and TNF-α). When we simultaneously modeled the 4 markers that were significantly associated with NHL after Bonferroni correction, the associations for BCA-1, sTNFR2, and sVEGFR2 were attenuated (ORs for fourth quartile vs first 1.9, 2.3, and 1.9 respectively) but remained statistically significant (PTrend = .0007 for BCA-1 and sTNFR2, PTrend = .04 for sVEGFR2), whereas TNF- α was no longer associated with NHL (OR 1.0; PTrend = .63). Levels of IL-16, TRAIL, and amylin were also no longer associated with NHL after adjustment for BCA-1, sTNFR2, and sVEGFR2 (results not shown).
Table 3 summarizes associations between dichotomized levels of the 7 markers and risks for common NHL subtypes. The marker associations generally did not vary across subtypes, although associations with DLBCL and FL, but not CLL/SLL, were observed for above-median levels of BCA-1 and TNF-α (PHomogeneity = .03 and .008, respectively). Subtype-specific results for all 67 immune markers are summarized in supplemental Table 3; some additional markers were significantly associated with DLBCL (CXCL9-MIG, CCL19-MIP3B, IL-17, IL-33, and sCD40L) and CLL (SCF and TGF-α).
Associations with selected NHL subtypes for baseline serum concentrations of BCA-1, sTNFR2, sVEGFR2, IL-16, TRAIL, and amylin
| . | Controls . | CLL/SLL . | DLBCL . | FL . | Test of OR Homogeneity . | |||
|---|---|---|---|---|---|---|---|---|
| Analyte concentration (pg/mL)* . | N . | N . | OR† (95% CI) . | N . | OR† (95% CI) . | N . | OR† (95% CI) . | P . |
| BCA-1 | ||||||||
| <15.57 | 151 | 45 | 1.0 | 16 | 1.0 | 12 | 1.0 | |
| ≥15.57 | 150 | 61 | 1.4 (0.9-2.2) | 55 | 3.3 (1.8-6.2) | 29 | 2.8 (1.3-5.7) | .03 |
| BCA-1 | ||||||||
| <4896.03 | 151 | 31 | 1.0 | 18 | 1.0 | 14 | 1.0 | |
| ≥4896.03 | 150 | 75 | 2.6 (1.6-4.3) | 53 | 2.8 (1.5-5.2) | 27 | 2.2 (1.1-4.5) | .86 |
| TNF-α | ||||||||
| <5.04 | 151 | 46 | 1.0 | 23 | 1.0 | 6 | 1.0 | |
| ≥5.04 | 150 | 60 | 1.3 (0.8-2.1) | 48 | 2.1 (1.2-3.7) | 35 | 6.0 (2.4-15.0) | .008 |
| sVEGFR2 | ||||||||
| <11 279.23 | 151 | 38 | 1.0 | 25 | 1.0 | 17 | 1.0 | |
| ≥11 279.23 | 150 | 68 | 1.8 (1.1-2.9) | 46 | 2.1 (1.2-3.7) | 24 | 1.4 (0.7-2.8) | .67 |
| IL-16 | ||||||||
| <24.90 | 152 | 49 | 1.0 | 25 | 1.0 | 15 | 1.0 | |
| ≥24.90 | 149 | 57 | 1.2 (0.8-1.9) | 46 | 1.9 (1.1-3.3) | 26 | 1.9 (0.9-3.8) | .26 |
| TRAIL | ||||||||
| <31.41 | 151 | 33 | 1.0 | 28 | 1.0 | 15 | 1.0 | |
| ≥31.41 | 150 | 73 | 2.5 (1.5-4.1) | 43 | 1.5 (0.9-2.6) | 26 | 1.8 (0.9-3.7) | .29 |
| Amylin | ||||||||
| <LLOD | 171 | 48 | 1.0 | 34 | 1.0 | 19 | 1.0 | |
| Detectable | 130 | 58 | 1.7 (1.1-2.8) | 37 | 1.6 (0.9-2.8) | 22 | 1.5 (0.7-3.0) | .92 |
| . | Controls . | CLL/SLL . | DLBCL . | FL . | Test of OR Homogeneity . | |||
|---|---|---|---|---|---|---|---|---|
| Analyte concentration (pg/mL)* . | N . | N . | OR† (95% CI) . | N . | OR† (95% CI) . | N . | OR† (95% CI) . | P . |
| BCA-1 | ||||||||
| <15.57 | 151 | 45 | 1.0 | 16 | 1.0 | 12 | 1.0 | |
| ≥15.57 | 150 | 61 | 1.4 (0.9-2.2) | 55 | 3.3 (1.8-6.2) | 29 | 2.8 (1.3-5.7) | .03 |
| BCA-1 | ||||||||
| <4896.03 | 151 | 31 | 1.0 | 18 | 1.0 | 14 | 1.0 | |
| ≥4896.03 | 150 | 75 | 2.6 (1.6-4.3) | 53 | 2.8 (1.5-5.2) | 27 | 2.2 (1.1-4.5) | .86 |
| TNF-α | ||||||||
| <5.04 | 151 | 46 | 1.0 | 23 | 1.0 | 6 | 1.0 | |
| ≥5.04 | 150 | 60 | 1.3 (0.8-2.1) | 48 | 2.1 (1.2-3.7) | 35 | 6.0 (2.4-15.0) | .008 |
| sVEGFR2 | ||||||||
| <11 279.23 | 151 | 38 | 1.0 | 25 | 1.0 | 17 | 1.0 | |
| ≥11 279.23 | 150 | 68 | 1.8 (1.1-2.9) | 46 | 2.1 (1.2-3.7) | 24 | 1.4 (0.7-2.8) | .67 |
| IL-16 | ||||||||
| <24.90 | 152 | 49 | 1.0 | 25 | 1.0 | 15 | 1.0 | |
| ≥24.90 | 149 | 57 | 1.2 (0.8-1.9) | 46 | 1.9 (1.1-3.3) | 26 | 1.9 (0.9-3.8) | .26 |
| TRAIL | ||||||||
| <31.41 | 151 | 33 | 1.0 | 28 | 1.0 | 15 | 1.0 | |
| ≥31.41 | 150 | 73 | 2.5 (1.5-4.1) | 43 | 1.5 (0.9-2.6) | 26 | 1.8 (0.9-3.7) | .29 |
| Amylin | ||||||||
| <LLOD | 171 | 48 | 1.0 | 34 | 1.0 | 19 | 1.0 | |
| Detectable | 130 | 58 | 1.7 (1.1-2.8) | 37 | 1.6 (0.9-2.8) | 22 | 1.5 (0.7-3.0) | .92 |
CI, confidence interval; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; OR, odds ratio.
Analytes with 50% or more of samples above the LLOD were categorized using the median concentration among controls as cutpoint. Analytes with less than 50% of samples above the LLOD categorized as <LLOD vs detectable.
Odds ratios computed using polytomous regression with adjustment for age group at baseline, sex, race, study center, and blood collection date.
Discussion
In this prospective investigation of 67 circulating immune and inflammation markers, we observed strong evidence that elevated serum levels of BCA-1, sTNFR2, and sVEGFR2 are associated with future risk for NHL. Our findings for these markers were very robust; the associations remained significant after Bonferroni correction, were independent of one another in multivariable regression modeling, and were apparent for cases diagnosed as long as 8 to 13 years after blood collection. We also observed associations for levels of TNF-α, IL-16, TRAIL, and amylin, although these markers were no longer associated with NHL after adjustment for BCA-1, sTNFR2, and sVEGFR2. Our results generally did not vary by histologic subtype, although given the small subtype case numbers involved, we view these findings to be inconclusive.
Our findings for BCA-1, a chemokine that promotes the chemotaxis of B-cells to secondary lymphoid tissue,12 are in agreement with a recent prospective investigation of NHL conducted within the WHI.4 In both studies, elevated BCA-1 was strongly associated with NHL risk, with the association stronger for DLBCL and FL than for CLL/SLL. Unlike in the WHI study, where the BCA-1 association weakened with increasing time between blood collection and case diagnosis, we observed an association with serum BCA-1 for NHL cases diagnosed as far as 8 to 13 years postcollection. Elevated circulating BCA-1 has also been associated with increased risk for AIDS-related NHL in a cohort of HIV-positive men.13 It has been speculated that dysregulated BCA-1 expression may influence lymphomagenesis through inappropriate homing of B-cells to tissues and/or inappropriate B-cell activation.13 Elevated BCA-1 expression has been shown to be directly or indirectly induced by several types of B-cell lymphoma, including FL and CLL.14,15 However, our observed association with BCA-1 for cases diagnosed far from blood collection, and the absence of association with CLL, argue against reverse causation as an explanation for our findings.
sTNFR2 is a soluble fragment of TNF receptor 2 (TNFR2), 1 of 2 cell surface receptors mainly responsible for mediating the effects of TNF-α, a proinflammatory cytokine.16 Soluble TNF receptors have been measured in many studies as serologic markers of TNF-mediated inflammation, given the stability of their levels over time17 and the high sensitivities of assays for these markers.18 An association between circulating sTNFR2 and NHL was first suggested in a small study nested within the NYU-WHS cohort, although the finding did not reach statistical significance.5 In our earlier PLCO investigation, sTNFR2 was not clearly associated with NHL; an association was observed for cases diagnosed less than 3 years after blood collection, but not for those diagnosed thereafter.3 In our current study, we observed a strong, highly significant association with sTFNR2 that did not change with time from blood collection to case diagnosis and was highly consistent across major subtypes. Levels of TNF-α were also associated with NHL in our study, although not independently of sTNFR2, BCA-1, and sVEGFR2. Studies of TNF knockout mice19 and of genetic polymorphisms in humans20 suggest that TNF-α influences lymphomagenesis. Signals from TNFR2, expressed mainly by immune cells, activate the nuclear factor κ-B pathway (NF-ĸB), a positive mediator of T- and B-cell proliferation and survival.21 The NF-ĸB pathway plays an important role in NHL pathogenesis, with constitutive pathway activation shown to be a hallmark of several lymphoid malignancies.22 Our observed association between sTNFR2 and NHL risk 8 to 13 years postcollection suggests that upregulated NF-ĸB signaling may contribute to NHL development at a relatively early stage of lymphomagenesis.
Our study is, to our knowledge, the first to report an association between elevated circulating sVEGFR2 and future NHL risk. The biologic function of sVEGFR2 is unclear, although it appears to play a role in endothelial cell proliferation and vascular permeability.23 This soluble marker can bind VEGF and may act as a VEGF inhibitor, although it possesses a weaker binding affinity than sVEGFR1.23 Recombinant sVEGFR2 has been reported to exhibit antiangiogenic effects in animal models.24 Circulating levels of sVEGFR2 have been reported to be elevated among individuals with acute leukemia, obesity, and metabolic syndrome, and lower in patients with gastric cancer and systemic lupus erythematosus.25 Experimental findings suggest that levels of this marker may also be inversely associated with tumor progression.26-28 We note, however, that the elevated levels of prediagnostic sVEGFR2 among cases vs controls and the consistency of the NHL association across periods of follow-up from blood collection to case diagnosis argue against a disease-induced effect.
We also observed statistically significant associations with NHL for levels of the proinflammatory cytokines TNF-α, IL-16,29 and TRAIL,30 as well as for amylin, a polypeptide secreted by pancreatic B-cells involved in glycemic regulation.31 However, these analytes were no longer associated with NHL after adjustment for BCA-1, sTNFR2, and sVEGFR2. Several other markers demonstrated associations with NHL that approached statistical significance (Figure 1; supplemental Table 2). Findings for circulating TNF-α, a key member of the NF-kB pathway, have been inconsistent in previous prospective studies of NHL.5,6 To our knowledge, prediagnostic circulating levels of IL-16, TRAIL, and amylin have not been previously associated with NHL. The biologic bases for these associations, if real, are unclear, although low pretreatment IL-16 levels have been associated with good response to treatment in a study of NHL patients32 and genetic studies suggest that the TRAIL-1 and TRAIL-2 receptors act as tumor suppressors for lymphoma.33 We also note that circulating amylin levels are elevated in patients in the early stages of type 2 diabetes,31 a condition that may be associated with increased NHL risk.34 The attenuation in associations for these analytes on adjustment for BCA-1, sTNFR2, and sVEGFR2 may possibly reflect combinations of these inflammation/immune markers acting within a common biologic pathway; for example, TNF-α is the ligand for TNFR2, from which sTNFR2 is cleaved. Additional investigation of these markers will be needed to clarify their associations with NHL.
Circulating levels of other analytes have been associated with NHL in previous studies. Strong associations with increased risk have been reported for elevated levels of sCD23, sCD27, and sCD30,3,4,7 whereas weaker evidence of association has been observed for elevated sIL-2R, sTNFR1, and intercellular adhesion molecule.3,5,6 Of these, sTNFRI and sCD30 were measured on our multiplex panels, although we were unable to analyze sCD30 because of low assay sensitivity (detectable in 16% of samples). In our study, elevated sTNFRI was nonsignificantly associated with increased risk (supplemental Table 2), although this association did not remain after adjustment for BCA-1, sTNFR2, and sVEGFR2. Elevated levels of some analytes have been associated with a reduction in NHL risk in previous studies, including IL-2, interferon gamma (IFNG), IL-13, and IL-5.5,6 All of the analytes except for IL-13 were measured in our study. We did not observe an association with NHL for IL-2 or IFNG (supplemental Table 2), although our analysis of these markers was limited by low assay sensitivity (detectable levels of IL-2 in 30% of samples; IFNG, 41%). For IL-5, we had an even lower proportion of samples above the limit of detection (16%) that precluded analysis of this analyte. Our findings for these analytes are thus inconclusive.
Our study has several strengths. It is, to our knowledge, the broadest investigation of immune markers and NHL risk conducted to date, with 67 analytes evaluated. The study’s prospective design and restriction to cases diagnosed at least 5 years after blood collection minimized the potential for associations arising as a result of disease-induced effects. As well, the standardized blood collection and processing protocol employed in the PLCO Trial, which specified that blood specimens be centrifuged, processed, and frozen within 2 hours of blood collection, reduced the potential for such preanalytical factors to affect serum analyte levels.
The study also has limitations. Our statistical power, although adequate for the detection of analyte associations comparable in magnitude to those reported in previous studies of NHL (eg, 83% power at α = 0.05 to detect an OR of 1.9 for the fourth vs first quartile), was limited for detecting associations of weaker effect size or restricted to a specific NHL subtype. Moreover, the issue of small numbers limited our analysis of NHL subtypes to dichotomized analyte levels without stratification by follow-up period. We also were limited in our ability to investigate associations with NHL, or were unable to do so altogether, for some analytes with low levels of detectability (high-sensitivity panels were not used in this study). In particular, this issue limited our ability to investigate some markers that had been previously associated with NHL in earlier studies (sCD30, IL-2, IL-5, and IFNG). We did not have information on use at the time of blood collection of medications or other factors that may induce short-term changes in immune/inflammation levels. Such changes, if they occurred, would likely be nondifferential by case-control status and probably bias associations toward the null.
We also note that with 67 analytes analyzed (after excluding 16 analytes with less than 20% of sample measurements above the LLOD), there is the possibility of chance findings. We think it unlikely that our findings for BCA-1 and sTNFR2 arose by chance, given that they remained significant after a Bonferroni correction and are supported by results from previous studies. The sVEGFR2 finding is also robust to multiple testing; however, given its novelty, replication in other studies is needed before firm biologic inferences can be drawn. Similarly, the findings for TNF-α, IL-16, TRAIL, and amylin require further investigation in other studies. Last, we note that our serum measurements may not reflect analyte levels in focal sites of inflammation or immune modulation that may be relevant to NHL development (eg, Helicobacter pylori–mediated inflammation in the stomach and gastric mucosa–associated lymphoid tissue lymphoma). Studies investigating correlations with serum analyte levels for different types of tissue, using animal or human clinical specimens, could provide important insight into this question.
In conclusion, our prospective investigation of 67 immune markers provides additional evidence that elevated serum levels of BCA-1 and sTNFR2 are associated with increased future risk for NHL as far as 8 to 13 years after blood collection. Additional research, including investigations of inherited variants in genes encoding these analytes or influencing their expression, is needed to elucidate the biologic mechanisms underlying these associations; such findings may provide new insight into lymphomagenesis and suggest possible new therapeutic targets. Well-powered investigations into associations with these analytes for specific NHL subtypes are also needed. We also observed NHL associations for several novel markers, most notably sVEGFR2, that warrant further investigation in other studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute.
Authorship
Contribution: M.P.P. led the study design and statistical analysis and prepared the manuscript; Q.L. and N.R. contributed to the study design; T.J.K. conducted the assays in the laboratory of L.A.P.; L.A.P. supervised this laboratory work and contributed to the interpretation of the underlying physiology; A.K.C. and A.H. oversaw the data formatting and quality control checks of the assay data; J.N.H., R.M.P. and J.H.P. contributed to the statistical analysis; and all authors provided intellectual input into preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Corresponding author: Mark Purdue, Division of Cancer Epidemiology and Genetics, National Cancer Institute, 9609 Medical Center Dr, Room 6E604, Rockville, MD 20850-9701; e-mail: purduem@mail.nih.gov.