Key Points
Homozygosity for the F5 c.1296+268A>G splicing mutation causes life-threatening factor V deficiency.
Mutation-specific antisense molecules can correct this splicing defect and restore factor V synthesis in the patient’s megakaryocytes.
Abstract
Antisense molecules are emerging as a powerful tool to correct splicing defects. Recently, we identified a homozygous deep-intronic mutation (F5 c.1296+268A>G) activating a cryptic donor splice site in a patient with severe coagulation factor V (FV) deficiency and life-threatening bleeding episodes. Here, we assessed the ability of 2 mutation-specific antisense molecules (a morpholino oligonucleotide [MO] and an engineered U7 small nuclear RNA [snRNA]) to correct this splicing defect. COS-1 and HepG2 cells transfected with a F5 minigene construct containing the patient’s mutation expressed aberrant messenger RNA (mRNA) in excess of normal mRNA. Treatment with mutation-specific antisense MO (1-5 µM) or a construct expressing antisense U7snRNA (0.25-2 µg) dose-dependently increased the relative amount of correctly spliced mRNA by 1 to 2 orders of magnitude, whereas control MO and U7snRNA were ineffective. Patient-derived megakaryocytes obtained by differentiation of circulating progenitor cells did not express FV, but became positive for FV at immunofluorescence staining after administration of antisense MO or U7snRNA. However, treatment adversely affected cell viability, mainly because of the transfection reagents used to deliver the antisense molecules. Our data provide in vitro and ex vivo proof of principle for the efficacy of RNA therapy in severe FV deficiency, but additional cytotoxicity studies are warranted.
Introduction
Coagulation factor V (FV) is a large glycoprotein produced in the liver and released in the bloodstream as an inactive precursor. Once activated, it acts as a nonenzymatic cofactor of factor Xa (FXa) in the conversion of prothrombin (PT) to thrombin, accelerating this reaction by several orders of magnitude.1 This essential role in thrombin formation makes FV indispensable to life, as demonstrated by the lethal phenotype of FV knock-out mice.2 Approximately 80% of FV circulates in plasma, the remaining 20% being stored in platelet α-granules.3 Although megakaryocytes can synthesize FV,4 platelet FV originates from secondary endocytosis of plasma FV.5-7
FV deficiency8-10 is a rare bleeding disorder inherited as an autosomal-recessive trait and is associated with mutations in the F5 gene. Homozygous and doubly heterozygous individuals present with a strikingly variable bleeding tendency,11 which is mainly determined by their residual platelet FV.12-14 Because no FV concentrate or recombinant FV preparation is available, replacement therapy still relies on the administration of fresh-frozen plasma or platelet concentrates, with all well-known associated risks (volume overload, transmission of infectious agents, immune reactions, and transfusion-related acute lung injury).
At least 10% of all F5 mutations are splicing defects,15 that is, they disrupt the process by which introns are removed from the primary transcript and exons are joined together to form the mature messenger RNA (mRNA). The resulting mature transcript often contains a premature stop codon and is rapidly degraded. Accordingly, F5 splicing mutations are usually associated with severe bleeding symptoms.14,16-21 Several studies on various genetic disorders have shown that splicing defects are amenable to “RNA therapy” with antisense molecules specifically designed to anneal to the mutant pre-mRNA (without triggering its degradation) and to direct its maturation into the correct transcript (reviewed in Cooper et al,22 Du and Gatti,23 Hammond and Wood,24 and Pinotti et al25 ). In particular, mutations that disrupt an existing donor splice site can be corrected with U1 small nuclear RNA (snRNA) specifically modified to recognize the mutated splice site,26 whereas mutations that create a new splice site can be targeted with short antisense oligonucleotides or snRNAs that mask the aberrant splice site and prevent its interaction with the spliceosome. A growing body of in vitro and ex vivo evidence supports the efficacy and safety of antisense-based RNA therapy in several genetic diseases,23,24,27 but only few studies have been conducted in bleeding disorders.28-30
The aim of the present study was to design and validate a personalized antisense-based RNA therapy for a previously described patient with severe FV deficiency (undetectable FV in both plasma and platelets),14 who recently suffered from a second spontaneous intracranial hemorrhage. The patient is homozygous for a deep-intronic splicing mutation (F5 c.1296+268A>G) activating a cryptic donor splice site in intron 8 and causing the inclusion of a pseudo-exon with an in-frame stop codon in the mature transcript.14 The efficacy and cytotoxicity of 2 different antisense molecules targeting this mutation were tested in an in vitro model and ex vivo on patient-derived megakaryocytes.
Materials and methods
Antisense molecules
Morpholino oligonucleotide.
Morpholino oligonucleotides (MOs) are uncharged analogs of nucleic acids with a phosphorodiamidate backbone. Two 25-mer MOs were purchased from GeneTools, one (rescuer) with a sequence complementary to the mutant F5 pre-mRNA (5′-GGTCACATGGTATCTACTTACCTGT-3′, mutation site underlined) and the other (control) with an irrelevant sequence (5′-CCTCTTACCTCAGTTACAATTTATA-3′).
Engineered U7snRNA.
U7snRNAs are a class of snRNAs involved in the 3′-end processing of histone pre-mRNAs.31 The U7-SmOPT construct32 (kindly provided by Dr D. Schümperli, Bern, Switzerland) consists of a murine U7snRNA gene (including its natural promoter and 3′ sequences) cloned in the pSP64 vector. This construct expresses a 62-nt U7snRNA in which the U7-specific Sm-binding site has been replaced by the Sm-binding site (SmOPT) found in spliceosomal snRNAs. Using a mutagenic primer, we replaced the anti-histone pre-mRNA sequence with a sequence complementary to the aberrant splice site in the mutant F5 pre-mRNA (5′-TCTACTTACCTGTGGCTTTA-3′, mutation site underlined). The ability of this engineered anti-F5 U7snRNA (rescuer) to correct splicing of the mutant F5 pre-mRNA was compared with that of the original anti-histone U7snRNA (control).
In vitro model: COS-1 or HepG2 cells transfected with F5 minigene constructs
Cloning of the F5 minigene.
A 2572-bp genomic fragment spanning F5 intron 7 through intron 9 and containing the c.1296+268A>G mutation was amplified from the patient’s genomic DNA using the PfuUltra II Fusion HS DNA Polymerase (Agilent Technologies) and cloned in the pTB expression vector33 between the unique Sac II and Nde I restriction sites (Figure 1). This mutant minigene construct was used as a template to obtain the corresponding wild-type construct by site-directed mutagenesis (QuikChange II XL Site-Directed Mutagenesis kit; Agilent Technologies). The sequences of both F5 minigenes were checked by direct sequencing.
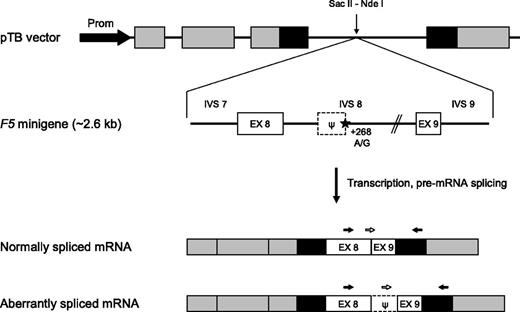
F5 minigene construct. (Top) Schematic representation of the F5 minigene construct. The pTB vector contains the promoter (black arrow) and exons 1 to 3 of the human α-globin gene (gray boxes) as well as exons 24 to 25 of the fibronectin-1 gene (black boxes). High transcription levels are ensured by an SV40 enhancer (not shown). The F5 minigene, encompassing the 3′ end of intron 7, exon 8, intron 8 (with or without the +268A>G mutation), exon 9 and the 5′ end of intron 9 of the F5 gene, was inserted between the vector’s unique Sac II and Nde I restriction sites. The position of the c.1296+268A>G mutation is marked by a star. The dotted box indicates the intronic pseudo-exon (ψ). (Bottom) Mature transcripts produced by the F5 minigene construct. The black arrows represent the primers used for qualitative analysis of the F5 mRNA; the white arrows represent the specific forward primers used to distinguish between the 2 differently spliced transcripts using real-time qPCR. EX, exon; IVS, intervening sequence (intron); Prom, promoter.
F5 minigene construct. (Top) Schematic representation of the F5 minigene construct. The pTB vector contains the promoter (black arrow) and exons 1 to 3 of the human α-globin gene (gray boxes) as well as exons 24 to 25 of the fibronectin-1 gene (black boxes). High transcription levels are ensured by an SV40 enhancer (not shown). The F5 minigene, encompassing the 3′ end of intron 7, exon 8, intron 8 (with or without the +268A>G mutation), exon 9 and the 5′ end of intron 9 of the F5 gene, was inserted between the vector’s unique Sac II and Nde I restriction sites. The position of the c.1296+268A>G mutation is marked by a star. The dotted box indicates the intronic pseudo-exon (ψ). (Bottom) Mature transcripts produced by the F5 minigene construct. The black arrows represent the primers used for qualitative analysis of the F5 mRNA; the white arrows represent the specific forward primers used to distinguish between the 2 differently spliced transcripts using real-time qPCR. EX, exon; IVS, intervening sequence (intron); Prom, promoter.
Transfection of COS-1 and HepG2 cells.
COS-1 and HepG2 cells were cultured in a humidified incubator at 37°C and 5% CO2 in Dulbecco modified Eagle medium (DMEM; Lonza) supplemented with 2 mM l-glutamine, 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. For each experiment, 80% confluent cells were seeded in a 6-well plate. After 24 hours, cells were switched to serum-free OptiMEM (Invitrogen) and transiently transfected with the wild-type or mutant F5 minigene construct using Lipofectamine 2000 (Invitrogen).
Treatment with antisense molecules.
Cells were treated with a single dose of antisense molecule (MO or U7-SmOPT construct) administered at the time of transfection with the F5 minigene construct. MOs (1-10 μM) were delivered using EndoPorter Reagent (GeneTools, 6 µM) in aqueous formulation. The U7-SmOPT constructs (0.25-2 μg, corresponding to a 0.6-4.8× molar excess over the F5 minigene construct) were delivered by cotransfection with the F5 minigene using Lipofectamine 2000 (Invitrogen). After 48 hours, cells were harvested for mRNA analysis.
Qualitative mRNA analysis.
Total RNA was isolated using TRIzol reagent (Invitrogen) and quantified spectrophotometrically. Total RNA (2 μg) was reverse-transcribed for 2 hours at 37°C with MultiScribe reverse-transcriptase and random primers (Applied Biosystems). The mRNA (complementary DNA [cDNA]) transcribed from the F5 minigene construct was amplified using a forward primer located in F5 exon 8 and a reverse primer located in the distal fibronectin exon of the pTB vector (Figure 1, black arrows). This primer pair amplifies exclusively the F5 minigene mRNA (and not any endogenous F5 mRNA) and yields products of different sizes according to the absence (283 bp) or presence (394 bp) of the F5 pseudo-exon in the mature transcript. Polymerase chain reaction (PCR) products were analyzed by agarose gel electrophoresis and direct sequencing.
Quantification of the normal and aberrant F5 transcripts.
The normal and aberrant F5 transcripts were quantified by real-time quantitative PCR (qPCR) on a LightCycler 480 Real-Time PCR instrument (Roche Applied Science). Two different forward primers, located at the exon 8-9 junction (5′-TCAGAGACACACTCAAAATCG-3′) and in the pseudo-exon (5′-AGTAATACAAAGGATCTGAGAC-3′), respectively (Figure 1, white arrows), and a common reverse primer in the distal fibronectin exon of the pTB vector, were used in separate reactions to specifically amplify the normal (reference) and aberrant (target) F5 transcripts derived from the F5 minigene construct. Amplification reactions were carried out in a volume of 10 µL, including 100 ng of total cDNA, 5 µL of LightCycler 480 SYBR Green I Master mix (Roche), and 0.4 µM of each primer. An initial denaturation step (10 minutes at 95°C) was followed by 40 cycles of amplification (denaturation: 20 seconds at 95°C, annealing: 20 seconds at 57°C, extension: 30 seconds at 72°C). The specificity of amplification products was checked by melting curve analysis (42-95°C). Fluorescence curves were analyzed with LightCycler 480 Software (Version 1.5) and relative quantification was performed with the 2−ΔΔCt method. All samples were assayed in duplicate.
Cytotoxicity assays
Potential cytotoxic effects of the antisense molecules and/or delivery agents were evaluated with the xCELLigence System (Roche). Details are reported in the supplemental Methods (available on the Blood Web site).
Ex vivo model: patient’s ex vivo–differentiated megakaryocytes
Isolation and differentiation of circulating progenitor cells.
The study was approved by the Ethical Committee of Padua Academic Hospital and conducted according to the Declaration of Helsinki. After obtaining informed consent, venous blood was drawn from the FV-deficient patient and from a normal control in 0.129 M trisodium citrate. Megakaryocytes were differentiated from circulating hematopoietic progenitor cells as previously described.34 Briefly, mononuclear cells were isolated by centrifugation on Histopaque-1077 (Sigma-Aldrich) density gradient, resuspended in serum-free Iscove modified Dulbecco medium (Celbio) and seeded in 24-well plates containing a glass coverslip at the bottom of each well. Cells were cultured at 37°C and 5% CO2 in medium supplemented with 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (Invitrogen), and 1% insulin-transferrin-selenium (Invitrogen), as well as 50 ng/mL thrombopoietin (TPO; Peprotech) and 10 ng/mL interleukin-3 (IL-3; Peprotech) to induce differentiation toward the megakaryocytic lineage.
Treatment with antisense molecules.
At day 9 of culture, part of the cells were treated with rescuer MO (2.5 μM or 5.0 μM) or rescuer U7snRNA construct (200 ng or 400 ng), as described previously in “Treatment with antisense molecules.” After 24 hours, glass coverslips were retrieved and processed for FV immunostaining. Untreated and treated cells from other wells were collected in TRIzol reagent (Invitrogen, 200 μL/well) for F5 mRNA analysis.
F5 mRNA analysis.
Total RNA isolation, reverse transcription, and quantification of F5 transcripts by real-time qPCR were carried out as described previously in “Quantification of the normal and aberrant F5 transcripts,” except for the use of a different reverse primer (located in F5 exon 10).
FV immunostaining.
Cells adhering to the glass coverslips were fixed with 2% paraformaldehyde and permeabilized with 0.5% Triton X-100. FV expression was visualized with a mouse monoclonal antibody directed against the human FV heavy chain (Hematologic Technologies) and a fluorescein isothiocyanate (FITC)-labeled goat anti-mouse immunoglobulin G (IgG) secondary antibody (Chemicon International). Cell nuclei were stained with 1.5 μg/mL Hoechst 33258 (Sigma-Aldrich). Slides were examined with a Leica DMI6000CS fluorescence microscope (Leica Microsystems CMS) using a 63×/1.40 oil-immersion objective. Images were acquired by means of a DFC365FX camera and analyzed with Leica LAS-AF 3.1.0 software.
Results
Splicing correction strategy
The F5 c.1296+268A>G mutation introduces a strong donor splice site deep in intron 8, causing the inclusion of an intronic pseudo-exon with an in-frame stop codon in the mature F5 mRNA. To correct this aberrant splicing event, we designed antisense (“rescuer”) MO and engineered U7snRNA molecules targeting the mutation site, with the aim to induce pseudo-exon skipping. MO and U7snRNA molecules with irrelevant sequences were used as controls.
In vitro model
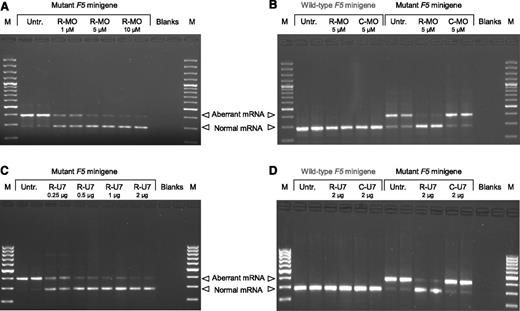
To develop an in vitro model to test the antisense molecules, we transiently transfected COS-1 cells with a F5 minigene construct containing the c.1296+268A>G mutation (Figure 1) and analyzed minigene mRNA by qualitative and real-time qPCR. The F5 minigene produced aberrantly spliced mRNA (containing the pseudo-exon) and correctly spliced mRNA (not containing the pseudo-exon) in a typical ratio of 5-10:1 (Figure 2A first two lanes). Treatment of transfected cells with 1 to 10 μM rescuer MO caused a dose-dependent decrease in the aberrant transcript and a parallel increase in the normal transcript, indicating progressive correction of F5 pre-mRNA splicing (Figure 2A). The ratio between aberrant and normal mRNA decreased from 9.50 in untreated cells to 0.09 in cells treated with 10 μM rescuer MO, corresponding to a relative enrichment in the normal mRNA of 103 times. To verify whether the effect of rescuer MO on F5 pre-mRNA splicing was specific, we performed additional transfection experiments to compare rescuer and control MO (Figure 2B). Treatment of transfected cells with 5 μM rescuer MO caused most of the F5 minigene pre-mRNA to be spliced correctly, whereas the same concentration of control MO was ineffective. The ratio between aberrant and normal mRNA decreased from 4.42 in untreated cells to 0.16 in cells treated with rescuer MO (correction factor 28-fold), whereas it hardly changed in cells treated with control MO (ratio 6.08, correction factor 0.7-fold). Finally, when COS-1 cells were transfected with the wild-type F5 minigene construct, not carrying the c.1296+268A>G mutation, only the correctly spliced mRNA was expressed and the administration of either rescuer or control MO did not affect its expression or splicing pattern (Figure 2B).
Splicing correction in COS-1 cells. (A-B) COS-1 cells were transfected with the mutant or wild-type F5 minigene construct and either left untreated or treated with the indicated concentrations of rescuer MO or control MO. (C-D) COS-1 cells were transfected with the mutant or wild-type F5 minigene construct and either left untreated or cotransfected with the indicated amounts of the U7-SmOPT construct expressing anti-F5 (rescuer) U7snRNA or control U7snRNA. The 2-μg U7-SmOPT construct corresponds to a molar excess of 4.8× over the F5 minigene construct. After 48 hours, RNA was isolated, reverse-transcribed, and analyzed by PCR and gel electrophoresis. Representative gels are shown. M, 100-bp marker; Untr., untreated; R-MO, rescuer MO; C-MO, control MO; R-U7, rescuer U7snRNA; C-U7, control U7snRNA.
Splicing correction in COS-1 cells. (A-B) COS-1 cells were transfected with the mutant or wild-type F5 minigene construct and either left untreated or treated with the indicated concentrations of rescuer MO or control MO. (C-D) COS-1 cells were transfected with the mutant or wild-type F5 minigene construct and either left untreated or cotransfected with the indicated amounts of the U7-SmOPT construct expressing anti-F5 (rescuer) U7snRNA or control U7snRNA. The 2-μg U7-SmOPT construct corresponds to a molar excess of 4.8× over the F5 minigene construct. After 48 hours, RNA was isolated, reverse-transcribed, and analyzed by PCR and gel electrophoresis. Representative gels are shown. M, 100-bp marker; Untr., untreated; R-MO, rescuer MO; C-MO, control MO; R-U7, rescuer U7snRNA; C-U7, control U7snRNA.
Similar experiments were carried out with the U7-SmOPT constructs. When COS-1 cells were cotransfected with the mutant F5 minigene construct and increasing amounts of rescuer U7snRNA construct (0.25-2 μg, corresponding to a 0.6-4.8× molar excess over the F5 minigene construct), a dose-dependent decrease in the aberrant transcript and a parallel increase in the normal transcript were observed (Figure 2C). The ratio between the aberrant and normal F5 mRNA decreased from 12.07 in untreated cells to 0.14 in cells treated with 2 μg of rescuer U7snRNA construct, indicating progressive correction of pre-mRNA splicing up to a correction factor of 84-fold. The ability of rescuer U7snRNA to specifically restore normal splicing of the F5 minigene transcript was confirmed in independent transfections (Figure 2D), where the rescuer U7snRNA construct (2 μg) decreased the ratio between aberrant and normal F5 mRNA from 2.89 in untreated cells to 0.03 (correction factor 106-fold), whereas the control U7snRNA construct showed no corrective potential (3.44, correction factor 0.8-fold). No effect of rescuer or control U7snRNAs was observed in COS-1 cells transfected with the wild-type F5 minigene construct.
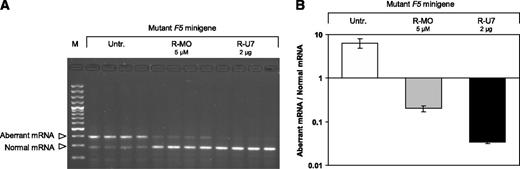
Because the liver is the physiological site of FV production in vivo, the ability of rescuer MO and U7snRNA to correct splicing of the mutant F5 pre-mRNA was also tested in HepG2 cells. These cells were transfected with the mutant F5 minigene construct and either left untreated or treated with 5 μM rescuer MO or 2 μg of the rescuer U7snRNA construct (Figure 3A). The ratio between aberrant and normal mRNA was 6.44 ± 1.64 (mean ± SD of 4 replicate transfections) in untreated cells and decreased to 0.20 ± 0.03 in cells treated with rescuer MO (correction factor 32-fold, P = .005) and to 0.034 ± 0.003 in cells treated with rescuer U7snRNA (correction factor 189-fold, P = .004) (Figure 3B), similar to what was observed in COS-1 cells. In a control experiment, control MO and U7snRNA with irrelevant sequences did not affect F5 pre-mRNA splicing (data not shown).
Splicing correction in HepG2 cells. HepG2 cells were transfected with the mutant F5 minigene construct and either left untreated or treated with 5 μM rescuer MO or cotransfected with 2 μg of the U7-SmOPT construct expressing anti-F5 (rescuer) U7snRNA. The 2-μg U7-SmOPT construct corresponds to a molar excess of 4.8× over the F5 minigene construct. After 48 hours RNA was isolated, reverse-transcribed and analyzed by PCR and gel electrophoresis (A) and real-time qPCR (B). The gel shows the effects of 5 μM R-MO and 2 μg of rescuer U7snRNA construct on F5 pre-mRNA splicing in HepG2 cells transfected with the mutant F5 minigene (quadruplicate transfections). Results of the quantification experiments are expressed as the ratio between the aberrant and normal F5 mRNA, plotted on a logarithmic scale. The quantification data represent the mean ± SD of the 4 replicates. M, 100-bp marker; Untr., untreated; R-MO, rescuer MO; R-U7, rescuer U7snRNA.
Splicing correction in HepG2 cells. HepG2 cells were transfected with the mutant F5 minigene construct and either left untreated or treated with 5 μM rescuer MO or cotransfected with 2 μg of the U7-SmOPT construct expressing anti-F5 (rescuer) U7snRNA. The 2-μg U7-SmOPT construct corresponds to a molar excess of 4.8× over the F5 minigene construct. After 48 hours RNA was isolated, reverse-transcribed and analyzed by PCR and gel electrophoresis (A) and real-time qPCR (B). The gel shows the effects of 5 μM R-MO and 2 μg of rescuer U7snRNA construct on F5 pre-mRNA splicing in HepG2 cells transfected with the mutant F5 minigene (quadruplicate transfections). Results of the quantification experiments are expressed as the ratio between the aberrant and normal F5 mRNA, plotted on a logarithmic scale. The quantification data represent the mean ± SD of the 4 replicates. M, 100-bp marker; Untr., untreated; R-MO, rescuer MO; R-U7, rescuer U7snRNA.
Treatment with rescuer or control antisense molecules affected the viability and/or proliferation of COS-1 cells and (to a much lesser extent) HepG2 cells (supplemental Results and Figure).
Ex vivo model
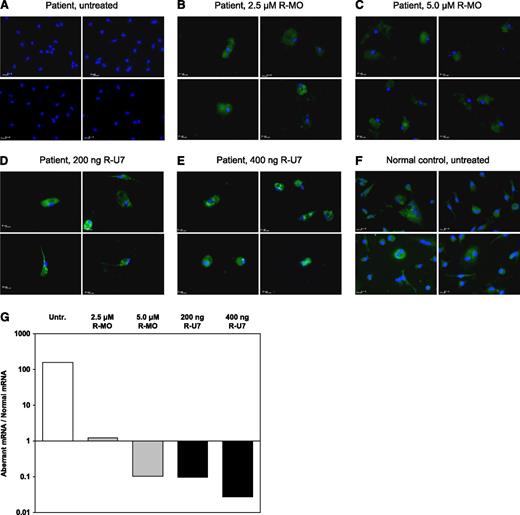
Hematopoietic progenitor cells from the FV-deficient patient and a normal control were cultured in serum-free medium in the presence of TPO and IL-3 to induce differentiation toward the megakaryocytic lineage. While control cells rapidly developed into FV-expressing megakaryocytes (Figure 4F), the patient’s cells did not show any sign of FV expression even after 9 days of culture (Figure 4A). However, treatment with rescuer MO (2.5-5.0 μM) or rescuer U7snRNA construct (200 ng and 400 ng) effectively restored FV expression in the patient’s cells (∼80% positivity, Figure 4B-E). Although this technique does not lend itself well to quantitative evaluation, green fluorescence was more intense (suggesting higher FV expression) in patient’s megakaryocytes treated with U7snRNA than in those treated with MO, whereas no clear-cut correlation with the intensity of treatment was noticed. Moreover, also in this model, administration of antisense molecules was associated with significant cytotoxicity, as demonstrated by the marked reduction in the number of viable cells following treatment.
Ex vivo splicing correction in patient’s megakaryocytes. Hematopoietic progenitor cells were isolated from peripheral blood and differentiated toward the megakaryocytic lineage as described in “Methods.” At day 9 of culture, the FV-deficient patient’s cells were either left untreated (A) or treated with rescuer MO or with the U7-SmOPT construct expressing rescuer U7snRNA at the indicated concentrations (B-E). Untreated cells from a normal control are shown for comparison (F). After immunofluorescence staining with Hoechst 33258 (cell nuclei, in blue) and with a FITC-labeled anti-mouse antibody recognizing the primary anti-FV antibody (FV, in green), cells were examined under a Leica DMI6000CS fluorescence microscope using a 63×/1.40 oil-immersion objective at room temperature. Images were acquired using a DFC365FX camera and analyzed with Leica LAS-AF 3.1.0 software. Each panel represents the overlay of 2 images of the same preparation stained with Hoechst 33258 and FITC-labeled antibody, respectively. Four representative microscope fields are shown for each condition. Following isolation and reverse transcription of total RNA, the F5 mRNA splicing pattern in untreated and treated patient’s megakaryocytes was analyzed by real-time qPCR (G). Results are expressed as the ratio between the aberrant and normal F5 mRNA, plotted on a logarithmic scale. Untr., untreated; R-MO, rescuer MO; R-U7, rescuer U7snRNA.
Ex vivo splicing correction in patient’s megakaryocytes. Hematopoietic progenitor cells were isolated from peripheral blood and differentiated toward the megakaryocytic lineage as described in “Methods.” At day 9 of culture, the FV-deficient patient’s cells were either left untreated (A) or treated with rescuer MO or with the U7-SmOPT construct expressing rescuer U7snRNA at the indicated concentrations (B-E). Untreated cells from a normal control are shown for comparison (F). After immunofluorescence staining with Hoechst 33258 (cell nuclei, in blue) and with a FITC-labeled anti-mouse antibody recognizing the primary anti-FV antibody (FV, in green), cells were examined under a Leica DMI6000CS fluorescence microscope using a 63×/1.40 oil-immersion objective at room temperature. Images were acquired using a DFC365FX camera and analyzed with Leica LAS-AF 3.1.0 software. Each panel represents the overlay of 2 images of the same preparation stained with Hoechst 33258 and FITC-labeled antibody, respectively. Four representative microscope fields are shown for each condition. Following isolation and reverse transcription of total RNA, the F5 mRNA splicing pattern in untreated and treated patient’s megakaryocytes was analyzed by real-time qPCR (G). Results are expressed as the ratio between the aberrant and normal F5 mRNA, plotted on a logarithmic scale. Untr., untreated; R-MO, rescuer MO; R-U7, rescuer U7snRNA.
For a more quantitative assessment of the efficacy of antisense molecules in patient’s megakaryocytes, total RNA was isolated from untreated and treated megakaryocytes and F5 mRNA was analyzed by real-time qPCR (Figure 4G). The ratio between aberrant and normal F5 transcripts in untreated megakaryocytes (158.3) was similar to that observed in the patient’s platelets (194.8, F.N. and E.C., unpublished observation. Platelet F5 mRNA was analysed as described previously in “F5 mRNA analysis.”) and indicated that <1% of F5 mRNA is spliced correctly in this patient. Treatment with rescuer MO and rescuer U7snRNA construct corrected the splicing pattern by 2 to 3 orders of magnitude in a roughly dose-dependent manner. At the concentrations used, rescuer U7snRNA was somewhat more effective than rescuer MO in restoring normal splicing, in line with the protein expression data and with the in vitro splicing correction experiments.
Discussion
Antisense-based RNA therapy is rapidly emerging as a promising tool to correct splicing defects.22-24,27 Unlike classical gene therapy, where a functional copy of the defective gene (cDNA) under control of a strong promoter is introduced in the patient’s cells, RNA therapy relies on short mutation-specific antisense oligonucleotides or engineered snRNAs that anneal to the mutant primary transcript and redirect its maturation into the correct mRNA. By acting at the RNA rather than DNA level, this form of molecular therapy does not affect the transcriptional regulation of the targeted gene (or any other gene).
Despite the encouraging results obtained in several genetic diseases, the potential utility of RNA therapy in coagulation disorders is only just beginning to be explored.25 Among all coagulation factor deficiencies, FV deficiency represents a particularly suitable target for RNA therapy, because (1) no FV concentrate or recombinant FV preparation is available for substitutive therapy; (2) the FV requirement for adequate hemostasis is extremely low, making a few percentages of FV sufficient to prevent life-threatening bleeding19,35-37 ; and (3) the large size of the F5 cDNA makes conventional gene therapy particularly challenging. Here, we present the first application of RNA therapy to severe FV deficiency and we provide in vitro and ex vivo evidence that mutation-specific antisense molecules can effectively rescue a F5 splicing mutation.
The mutation that we have targeted (F5 c.1296+268A>G) activates a cryptic donor splice site deep in intron 8, causing the retention of an intronic pseudo-exon in the mature mRNA.14 Although this is presently the only known F5 splicing defect that can be corrected through MO- or U7snRNA-mediated pseudo-exon skipping, deep-intronic splicing mutations may be more prevalent than previously suspected.38 In fact, deep-intronic splicing mutations potentially amenable to this corrective approach have been identified in the F8,39-42 FGB,30 and FGG43 genes.
COS-1 and HepG2 cells transfected with a F5 minigene construct containing the c.1296+268A>G mutation provided a suitable in vitro model to test the antisense molecules, even if the relative proportion of correctly spliced mRNA was much higher in this model (10%-20%) than in the patient’s platelets (∼0.5%, as estimated by real-time qPCR). This is not surprising considering that (1) the splicing pattern/efficiency is known to vary in different cell types; (2) the minigene transcript is different from the full-length F5 transcript, which may affect the susceptibility of the aberrantly spliced mRNA to nonsense-mediated decay; and (3) the transcript ratio in the minigene model also varied according to cell status and transfection conditions (data not shown). However, treatment of transfected cells with mutation-specific antisense molecules restored normal splicing in a specific and dose-dependent manner. In particular, the highest concentrations of antisense MO and U7snRNA increased the relative proportion of the correctly spliced mRNA by 1 to 2 orders of magnitude. Similar correction efficiencies, if achieved in vivo, would be more than sufficient to bring the patient’s FV level in the safe range.
The F5 c.1296+268A>G mutation was originally identified in the homozygous state in a patient with undetectable FV and a life-threatening bleeding diathesis.14 To prove that our antisense MO and U7snRNA could rescue the patient’s FV deficiency, we showed that they could effectively correct the F5 mRNA splicing pattern and restore FV expression in the patient’s ex vivo–differentiated megakaryocytes. Although megakaryocytes are not the physiological site of FV production in vivo,5-7 they have been shown to synthesize FV in culture,4 offering us the opportunity to test the antisense molecules on the patient’s own cells without a liver biopsy. Unfortunately, the antisense molecules could not be tested directly on the patient’s hepatocytes, but their efficacy in correcting the splicing defect in HepG2 cells transfected with the mutant F5 minigene construct suggests that they would be able to restore FV expression in the patient’s hepatocytes as well, especially considering the fact that the liver is a preferential site of accumulation of systemically injected MOs.44,45
Treatment with rescuer (and control) MO and U7snRNA molecules adversely affected the viability and/or proliferation of COS-1 and (to a much lesser extent) HepG2 cells in in vitro cytotoxicity assays. However, toxic effects were largely attributable to the transfection reagents used to deliver the antisense molecules rather than to the antisense molecules themselves. Because the delivery of antisense molecules to target cells in vivo does not rely on transfection reagents, but rather on free uptake by body cells (MOs)46 or on targeted delivery using engineered viral vectors (U7snRNAs),47 major adverse effects in treated patients appear unlikely. In fact, antisense molecules have shown excellent safety profiles in several in vivo studies of other genetic diseases, both in animal models and in human volunteers, being well tolerated even after systemic administration.44,47 Nevertheless, because each antisense molecule has a unique sequence, off-target effects can never be excluded.
In summary, our data show that specific antisense molecules targeting the c.1296+268A>G mutation can correct splicing of the mutant F5 pre-mRNA in vitro and restore FV synthesis in the patient’s megakaryocytes ex vivo. These findings provide proof of principle for the efficacy of RNA therapy for this particular F5 gene mutation and support RNA therapy as a possible alternative to substitutive therapy with blood derivatives in severe FV deficiency.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr D. Schümperli for sharing the U7-SmOPT construct, Prof C. P. Reutelingsperger and Mr N. Deckers for sharing the xCELLigence machine and for skillful assistance with the cytotoxicity assays, Mr M. Steenbergen (Roche Diagnostics) for donation of reagents and consumables, and Prof F. E. Baralle and Dr E. Buratti for critically reading the manuscript.
This work was supported by VIDI grant no. 917-76-312 from the Dutch Organisation for Scientific Research (E.C.) and by grant nos. CPDR077082/07 and 60A07-0203/09 from Ministero dell’Istruzione, dell’Università e della Ricerca (P.S.).
Authorship
Contribution: F.N. cloned the F5 minigene, performed the in vitro experiments, analyzed data, and drafted the manuscript; C.R. performed the ex vivo experiments; M.B. provided vital reagents and technical advice; L.S. collected the patient’s clinical history and blood samples for the ex vivo model; T.M.H. critically reviewed the manuscript; P.S. enrolled the patient and supervised the ex vivo experiments; E.C. designed and coordinated the whole study, supervised the in vitro experiments, analyzed data, and wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elisabetta Castoldi, Department of Biochemistry, Maastricht University, PO Box 616, 6200 MD Maastricht, The Netherlands; e-mail: e.castoldi@maastrichtuniversity.nl.
References
Author notes
F.N. and C.R. equally contributed to the study.