Key Points
The sensitivity and specificity of detecting the JAK2 p.V617F mutation in PB are both 100% compared with BM.
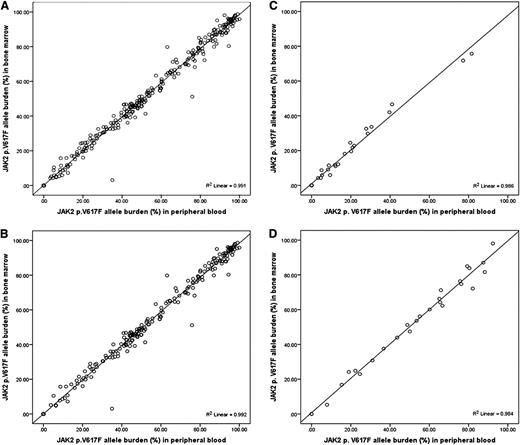
The JAK2 p.V617F allele burden measured in PB is equivalent to that in BM aspirate (R2 = 0.991; P < .0001).
Abstract
Detection of the JAK2 p.V617F mutation and measurement of its allele burden can be performed using both peripheral blood (PB) and bone marrow (BM) samples from patients with myeloproliferative neoplasms (MPNs). However, the diagnostic accuracy of detecting the JAK2 p.V617F mutation and quantifying its allele burden in PB and BM samples has not been systematically compared. We retrospectively analyzed 388 patients with MPN who had been tested for JAK2 p.V617F allele burden using both PB and BM samples within 3 months of each other. The sensitivity and specificity of detecting JAK2 p.V617F in PB when compared with BM were both 100%. Furthermore, the JAK2 p.V617F allele burden measured in PB and BM were equivalent by linear regression analysis (R2 = 0.991; P < .0001). We therefore conclude that PB is a reliable source for testing for the JAK2 p.V617F mutation and quantifying its allele burden in patients with MPN.
Introduction
Somatic mutation of the JAK2 gene (JAK2 p.V617F) can be detected in a variable proportion of patients with myeloproliferative neoplasms (MPNs).1 The JAK2 p.V617F mutation is observed in as much as 96% of patients with polycythemia vera (PV) and approximately 55% to 65% of those with essential thrombocythemia (ET) and primary myelofibrosis (PMF), and testing for its presence is an integral part of the diagnostic workup.2 In addition, quantitative measurement of the JAK2 p.V617F allele burden (ratio of mutant allele to total allele) has been associated with certain clinical phenotypes, such as a higher incidence of pruritus and splenomegaly and an increased risk of thrombosis in patients with PV and ET, although the exact association between allele burden and long-term outcome remains controversial, particularly in patients with myelofibrosis (MF).3-6 DNA extracted from peripheral blood (PB) granulocytes or bone marrow (BM) aspirates is most commonly used for testing for JAK2 p.V617F. In this study, we sought to answer two questions: (1) Are the sensitivity and specificity of detecting JAK2 p.V617F in PB and BM equivalent? and (2) Is the JAK2 p.V617F allele burden measured in PB and BM equivalent? Previous studies suggested the feasibility of detecting JAK2 p.V617F in both PB and BM samples.4,7-9 Furthermore, quantification of JAK2 p.V617F allele burden in PB and BM samples appeared to be equivalent in a small number of MPN cases.8,10 However, to date no systematic large-scale analysis comparing qualitative (sensitivity and specificity) and quantitative (allele burden) JAK2 p.V617F testing in PB and BM samples has been conducted for patients with MPN.
Patients and methods
By retrospective chart review, we identified 388 patients with a diagnosis of MPN (primary and secondary MF [N = 329] and ET and PV [N = 59]) who were referred to The University of Texas MD Anderson Cancer Center between January 2004 and July 2012 and were tested for the JAK2 p.V617F mutation using samples obtained from PB and BM during the same time period. For the purpose of this study, the sample analysis was considered to be conducted in the same time period if the analyses of PB and BM samples were conducted within 3 months of each other. Diagnosis of MF, ET, and PV strictly followed the criteria established by the World Health Organization in 2008.11 The study was based on a chart review protocol approved by the Institutional Review Board at MD Anderson Cancer Center and was conducted in accordance with the Declaration of Helsinki.
Genomic DNA was extracted from freshly obtained PB or BM aspirate samples using a semiautomated DNA extraction method following the manufacturer’s instructions (Gentra Autopure; Qiagen, Valencia, CA). The JAK2 p.V617F mutation was detected using polymerase chain reaction–based pyrosequencing, as previously described.12 The sensitivity of detecting the JAK2 p.V617F mutation by this assay was 5%. Correlation between the JAK2 p.V617F allele burden measured in PB and BM was evaluated by scatter plot. Linear regression was conducted to calculate the coefficient of determination (R2) and the statistical significance of the correlation. SPSS, version 21 (IBM Corp., Armonk, NY) was used for all statistical analyses.
Results and discussion
Among the 388 patients included in our analysis, 243 (63%) had a diagnosis of PMF, 38 (10%) had MF secondary to ET (post-ET MF), 48 (12%) had MF secondary to PV (post-PV MF), 32 (8%) had ET, and 27 (7%) had PV. The median time from diagnosis of MPN to JAK2 p.V617F testing was 5.9 months (range, 0-382) (Table 1).
Summary of JAK2 p.V617F testing in patients with MPN
| . | . | JAK2 p.V617F N (% each diagnosis) . | JAK2 p.V617F allele burden* . | ||
|---|---|---|---|---|---|
| N (% total) . | PB . | BM . | PB . | BM . | |
| All patients | 388 | 264 (68) | 264 (68) | 52.7 (3.3-100) | 51.4 (3.1-98.7) |
| PMF | 243 (63) | 156 (64) | 156 (64) | 48.8 (3.8-100) | 47.4 (3.1-98.7) |
| Post-ET MF | 38 (10) | 13 (34) | 13 (34) | 62.6 (24.4-94.5) | 63.8 (21.3-89.7) |
| Post-PV MF | 48 (12) | 48 (100) | 48 (100) | 92.6 (5.2-98.8) | 91.5 (10.5-98.1) |
| Primary ET | 32 (8) | 22 (69) | 22 (69) | 18.3 (3.3-81.6) | 18.9 (4.3-75.7) |
| Primary PV | 27 (7) | 25 (93) | 25 (93) | 60.2 (7.9-92.4) | 60.1 (5.3-98.1) |
| . | . | JAK2 p.V617F N (% each diagnosis) . | JAK2 p.V617F allele burden* . | ||
|---|---|---|---|---|---|
| N (% total) . | PB . | BM . | PB . | BM . | |
| All patients | 388 | 264 (68) | 264 (68) | 52.7 (3.3-100) | 51.4 (3.1-98.7) |
| PMF | 243 (63) | 156 (64) | 156 (64) | 48.8 (3.8-100) | 47.4 (3.1-98.7) |
| Post-ET MF | 38 (10) | 13 (34) | 13 (34) | 62.6 (24.4-94.5) | 63.8 (21.3-89.7) |
| Post-PV MF | 48 (12) | 48 (100) | 48 (100) | 92.6 (5.2-98.8) | 91.5 (10.5-98.1) |
| Primary ET | 32 (8) | 22 (69) | 22 (69) | 18.3 (3.3-81.6) | 18.9 (4.3-75.7) |
| Primary PV | 27 (7) | 25 (93) | 25 (93) | 60.2 (7.9-92.4) | 60.1 (5.3-98.1) |
Median and range are shown.
All patients who were negative for JAK2 p.V617F in PB samples were also negative in BM samples and vice versa, making the sensitivity and specificity of JAK2 p.V617F mutation detection in PB samples both 100% in the current MPN cohort (using BM testing as a reference).
Among the patients who tested positive for JAK2 p.V617F, the median JAK2 p.V617F allele burden in PB and BM was 52.7% (range, 3.3-100) and 51.4% (range, 3.1-98.7), respectively, in all MPN patients (Table 1). Patients with post-PV MF had the highest JAK2 p.V617F allele burden (median, 92.6% in PB and 91.5% in BM), whereas patients with primary ET had the lowest (median, 18.3% in PB and 18.9% in BM). The correlation between the JAK2 p.V617F allele burden measured in PB and BM samples in all MPN patients was very strong (R2 = 0.991, P < .0001, Figure 1A). The correlation remained strong for each MPN subtype (Figure 1B-D).
Correlation between JAK2 p.V617F allele burden measured in PB granulocytes and BM aspirates. Samples from (A) all MPN patients (N = 388), (B) MF patients (N = 329), (C) ET patients (N = 32), and (D) PV patients (N = 27). Coefficient of determination (R2) was calculated by linear regression.
Correlation between JAK2 p.V617F allele burden measured in PB granulocytes and BM aspirates. Samples from (A) all MPN patients (N = 388), (B) MF patients (N = 329), (C) ET patients (N = 32), and (D) PV patients (N = 27). Coefficient of determination (R2) was calculated by linear regression.
In summary, our data from a large cohort of patients with MPN (MF, ET, and PV) confirmed the consistency of detecting JAK2 p.V617F between PB and BM samples by the method used in our study. Moreover, our data confirmed that the JAK2 p.V617F allele burden measured in PB is equivalent to that measured in BM, which is in agreement with results from earlier published studies.8,10 These findings are valuable from a clinical perspective. Our results show that BM testing is not required to detect JAK2 p.V617F mutation or quantify JAK2 p.V617F allele burden, because PB testing is highly reliable. Hence, a negative result of the JAK2 p.V617F mutation in a PB sample can be considered to be reliable, and obtaining a BM sample for further testing does not seem to be necessary. In other words, our results dispel the notion that one needs to obtain a BM aspirate and biopsy specimen to verify the presence or absence of the JAK2 p.V617F mutation. Further, our results strongly suggest that heterogeneity of sampling (PB vs BM) can be essentially ignored when interpreting clinical correlation studies of JAK2 p.V617F allele burden.3 Comparisons could be made of levels done from the BM with those of the PB and the values can be used interchangeably for those in which initial values were obtained from the BM. This makes it much more feasible for long-term monitoring of JAK2 p.V617F allele burden in clinical trials with new agents, as well as in a setting of post–hematopoietic stem cell transplant, because JAK2 p.V617F allele burden seems to function as a minimal residual disease marker.8,13,14 It should be noted, however, that the sensitivity of the assay used in the current study is 5%. Because the presence of a very low level of residual JAK2 p.V617F allele burden can be clinically significant in a posttransplant setting, a future study to confirm the qualitative and quantitative accuracy between PB and BM samples using more sensitive assays is needed.15
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kate Newberry, a senior research scientist supported by Anderson Cancer Center, for her excellent scientific editing.
This study is funded by Kimberly Patterson Leukemia Research Fellowship (K.T.), Celgene Future Leaders in Hematology Award (K.T.), and the Anderson Cancer Center Support Grant CA016672.
Authorship
Contribution: K.T. designed the study, collected and analyzed the data, and wrote the manuscript; H.K. and J.C. treated the patients and reviewed the manuscript; K.P.P. and R.L. conducted molecular analysis and reviewed the manuscript; S.P. collected the data and reviewed the manuscript; and S.V. designed the study concept, guided the project, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Srdan Verstovsek, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: sverstov@mdanderson.org.