Key Points
Mice lacking mast cells have severely exacerbated GVHD compared with WT controls.
Engraftment of mast cells into C75BL/6-KitW-sh/W-sh mast cell–deficient animals improves survival but not if the mast cells are derived from IL-10 KO mice.
Abstract
To investigate the role of mast cells in hematopoietic cell transplantation, we assessed graft-versus-host disease (GVHD) in C57BL/6-KitW-sh/W-sh recipients, which virtually lack mast cells, compared with C57BL/6 WT recipients. GVHD was severely exacerbated in C57BL/6-KitW-sh/W-sh mice (median survival time = 13 vs 60 days in wild-type [WT] mice; P < .0001). The increased mortality risk in C57BL/6-KitW-sh/W-sh hosts correlated with increased T-cell numbers in lymph nodes, liver, and gastrointestinal tract sites, as indicated by bioluminescence imaging (P < .001). We did not detect any deficit in the number or function of CD4+CD25+ regulatory T cells (Tregs) in C57BL/6-KitW-sh/W-sh mice. Furthermore, Tregs were equally effective at reducing GVHD in C57BL/6-KitW-sh/W-sh recipients compared with WT recipients containing mast cells. Furthermore, we found that survival of C57BL/6-KitW-sh/W-sh mice during GVHD was significantly improved if the mice were engrafted with bone marrow–derived cultured mast cells from WT C57BL/6 mice but not from interleukin (IL)-10–deficient C57BL/6 mice. These data indicate that the presence of mast cells can significantly reduce GVHD independently of Tregs, by decreasing conventional T-cell proliferation in a mechanism involving IL-10. These experiments support the conclusion that mast cells can mediate a novel immunoregulatory role during hematopoietic cell transplantation.
Introduction
Traditionally, mast cells are best known as promoters of immunity and inflammation in response to pathogens, as well as in allergic disorders.1 However, there are other settings where mast cells appear to play an immunomodulatory role and suppress immune reactions. Mast cell precursors originate from hematopoietic stem cells and reside in vascularized tissue or serosal cavities, especially in surfaces that interact with the environment such as the gastrointestinal tract, airways, and skin.2-4 There they mature and can persist for long periods of time.2-4
There are several studies implicating mast cells in graft-versus-host disease (GVHD) pathogenesis. It has been noted that in connective tissue, particularly in the skin, mast cells disappear during chronic GVHD5,6 ; however, it was later demonstrated that the disappearance was merely a loss of toluidine blue staining of mast cell granules and that the mast cells remained.7 The conclusions of these studies were that mast cells were degranulating after activation, presumably by alloreactive donor T cells, resulting in the stimulation of fibroblasts, collagen secretion, and dermal fibrosis.7 Murphy et al8 demonstrated that in a CD8+ T cell–mediated model of GVHD, C57BL/6-KitW/Wv recipients, which lack mast cells, had prolonged survival over wild-type (WT) B6 recipients. Furthermore, they suggested that the degranulation of mast cells resulted in early epithelial injury. Another study used peptide antagonists for the immunoglobulin E/high-affinity Fc receptor for immunoglobulin E receptor interaction, which triggers activation of mast cells and other cell types after they are cross-linked by antigen.9 These peptides significantly inhibited GVHD development, again implicating mast cells as a driver of pathology in at least some models of GVHD.
In recent years, several studies have revealed that mast cells are much more functionally diverse than previously thought and can have potent immunoregulatory roles.1 In particular, Lu et al10 demonstrated that administration of anti-CD154 antibody together with an intravenous infusion of allogeneic cells (donor-specific transfusion) induced acceptance of skin allografts in WT but not in C57BL/6-KitW-sh/W-sh mice (which virtually lack mast cells). Furthermore, engraftment of mast cells in the skin extended graft survival in C57BL/6-KitW-sh/W-sh mice, which previously could not sustain allografts. Based on the increased presence of both mast cells and regulatory T cells (Tregs) in tolerated skin grafts, together with the production of interleukin (IL)-9 (a mast cell growth and activation factor) by Tregs, Lu et al proposed a functional role for mast cells as intermediaries in Treg-induced tolerance. In light of these findings in a model of allogeneic skin graft tolerance in C57BL/6-KitW-sh/W-sh mice, we reexamined the role of mast cells in GVHD in C57BL/6-KitW-sh/W-sh mice and investigated whether Treg suppression of GVHD may involve mast cells. Our results provide evidence for an anti-inflammatory and immunoregulatory role for mast cells in GVHD.
Methods
Mice
Experiments used gender-matched mice between 7 and 16 weeks old. FVB/N (H-2q), BALB/c(H-2d), C57BL/6(H-2b, CD45.2), and B6(Cg)-Tyrc-2J/J(H-2b, CD45.2) and IL-10−/− (B6.129P2-Il10tm1Cgn/J) mice were purchased from Jackson Laboratories (Sacramento, CA) and acclimated to the Stanford Animal Facility for a minimum of 3 days before every experiment. Genetically mast cell–deficient mice C57BL/6-KitW-sh/W-sh and C57BL/6-Cpa3-Cre; Mcl-1fl/fl mice (which had been back-crossed to C57BL/6J mice for >10 generations) are as described and maintained in the Stanford Animal Facility.11,12 Luciferase-expressing (luc+) FVB/N L2G85 and C57BL/6 mice were generated as described and raised in the Stanford Animal Facility.13,14 Animal protocols were approved by the Institutional Animal Care and Use Committee of Stanford University.
Cell isolation
CD4/CD8 conventional T cells (Tcon) were prepared from splenocytes and peripheral lymph node cells and enriched with anti-CD4 magnetic-activated cell sorting (MACS; Miltenyi Biotec, Auburn, CA) and then anti-CD8 MACS; purity was assessed by fluorescence-activated cell sorter (FACS) on a LSR II or FACS Aria (BD Biosciences, San Jose, CA), and cells were mixed so CD4:CD8 = 2:1. T cell–depleted bone marrow (TCD-BM) was prepared by flushing bones and depleting T cells with anti-CD4 and anti-CD8 MACS beads. Tregs were prepared from pooled spleens and lymph nodes, by staining for CD25-allophycocyanin and CD4, enriching with anti-allophycocyanin MACS, and sorting for CD4+CD25hi cells on a FACS Aria or FACS Aria II (BD Biosciences).
Flow cytometric analysis
The following antibodies were purchased from BD Pharmingen (San Diego, CA), eBioscience (San Diego, CA), and Biolegend (San Diego, CA): CD4 (GK1.5), CD8 (53-6.7), CD25 (PC61), FcεR1α (MAR-1), CD117 (2B8), and Foxp3 (FJK-16s). Foxp3 staining was performed with an anti-mouse/rat Foxp3 Staining Set (eBiosciences). To stain dead cells, propidium iodide was used for freshly isolated cells, and ethidium monoazide (Molecular Probes, Carlsbad, CA) was used for fixed cells. Analysis was performed on a FACS Aria or LSR II (Becton-Dickinson, San Jose, CA).
Preparation and engraftment of bone marrow–derived cultured mast cells
Bone marrow–derived cultured mast cells (BMCMCs) were obtained by culture of bone marrow cells from femurs and tibia from WT, luc+, or IL-10−/− C57BL/6 mice and cultured for 4 to 6 weeks in 20% (v/v) medium conditioned by the growth of the WEHI-3 mouse myelomonocitic cell line (containing IL-3); at this time, >95% of the cells were identified as mast cells by flow cytometry (FcεRI+, c-Kit+). For mast cell engraftment studies, 5 × 106 BMCMCs were injected intraperitoneally into C57BL/6-KitW-sh/W-sh pups 4 to 5 weeks or age, and experiments were initiated 6 to 8 weeks later.
Transplantation model to evaluate GVHD
C57BL/6-Tyrc-2J/J or C57BL/6-KitW-sh/W-sh recipients were treated with total body irradiation (200-Kv X-ray source), consisting of 2 doses of 5 Gy, 4 hours apart. TCD-BM (C57BL/6) (5 × 106) were injected via tail vein on day 0, followed by WT or luc+ Tcon (FVB/N) to induce GVHD. Transplanted animals were kept in autoclaved cages with antibiotic water (sulfamethoxazole-trimethropim; Schein Pharmaceutical, Corona, CA).
In vivo and ex vivo bioluminescence imaging
Bioluminescence imaging was performed as described15 with an IVIS 7, IVIS 29, or IVIS Spectrum charge-coupled device imaging system (Xenogen, Alameda, CA). Images were analyzed with Living Image Software 2.5 (Xenogen) and Igor Pro Carbon (Wavemetrics, Lake Oswego, OR).
Mixed lymphocyte reaction
C57BL/6 Tcon (2 × 105) with or without varying doses of Tregs isolated from either C57BL/6 WT or C57BL/6-KitW-sh/W-sh mice were added per well of a 96-well flat-bottom plate containing cRPMI with 100 U/mL IL-2 (Chiron, Emeryville, CA) and 106 BALB/c splenocytes, which had received 30-Gy irradiation. Total volume per well was 200 μL. After culture at 37°C and 5% CO2 for 5 days, cells were pulsed with 1 µCi/well [3H]thymidine (GE Healthcare Piscataway, NJ) for the last 16 hours. Cells were harvested onto filter membranes using a Wallac harvester (Perkin-Elmer, Shelton, CT), and the amount of incorporated [3H]thymidine was measured with a Wallac Betaplate counter (Perkin-Elmer).
Histology and quantification of mast cells
Mice were killed, and tissues were fixed in 10% (v/v) buffered formalin and embedded in paraffin, and sections 4 mm in thickness were cut. Mesenteric windows were arranged onto slides and fixed for 1 hour in Carnoy’s solution (3:2:1 v/v/v of ethanol, chloroform, and acetic acid). All tissues were stained with Csaba stain for mast cell detection. Mast cells were quantified according to area (mm2) or expressed in numbers per millimeter horizontal field length using computer-generated image analysis (NIH Image J software, version 1.29).
Statistical analysis
Differences in animal survival (Kaplan-Meier survival curves) were analyzed by log-rank test. All other comparisons were performed with the 2-tailed Student t test, and P < .05 was considered statistically significant.
Results
GVHD is exacerbated and T-cell survival/proliferation is increased in C57BL/6-KitW-sh/W-sh mice
Because mast cell–deficient mice were reported to more readily reject skin grafts,10 we hypothesized that host mast cells also may play an anti-inflammatory or immunoreglatory role in GVHD. We used a major-mismatch model of acute GVHD in which TCD-BM and 2 × 106 MACS-enriched CD4/CD8 Tcon from FVB mice (H-2q) were transferred into allogeneic C57BL/6 recipients (H-2b) that had received myeloablative irradiation. To assess the influence of mast cells in disease induction, C57BL/6-KitW-sh/W-sh recipients (which virtually lack mast cells) were also used as recipients. We observed a severe exacerbation of GVHD and more rapid death in C57BL/6-KitW-sh/W-sh recipients, with a median survival time (MST) of 13 days compared with an MST of 60 days in C57BL/6 WT recipients (Figure 1; P < .0001). The difference in survival was most pronounced when Tcons were administered 4 days after transplantation, but also was observed if Tcons were administered on days 0, 2, or 7 of transplantation (day 2 shown below; others not shown).
Mast cell–deficient mice have exacerbated GVHD. Recipient mice, which were either C57BL/6 (H-2b) WT or mast cell–deficient C57BL/6-KitW-sh/Wsh mice, were lethally irradiated and transplanted with 5.0 × 106 TCD-BM, followed by 2.0 × 106 Tcon on day 4 (to induce GVHD) from FVB/N donors (H-2q). Data were pooled from 2 independent experiments performed, each of which gave similar results (n = 13 mice per group except for C57BL/6-KitW-sh/Wsh with irradiation + TCD-BM, n = 10). Significantly better survival was observed in the control mice containing mast cells than in the mast cell–deficient C57BL/6-KitW-sh/Wsh mice (P < .0001).
Mast cell–deficient mice have exacerbated GVHD. Recipient mice, which were either C57BL/6 (H-2b) WT or mast cell–deficient C57BL/6-KitW-sh/Wsh mice, were lethally irradiated and transplanted with 5.0 × 106 TCD-BM, followed by 2.0 × 106 Tcon on day 4 (to induce GVHD) from FVB/N donors (H-2q). Data were pooled from 2 independent experiments performed, each of which gave similar results (n = 13 mice per group except for C57BL/6-KitW-sh/Wsh with irradiation + TCD-BM, n = 10). Significantly better survival was observed in the control mice containing mast cells than in the mast cell–deficient C57BL/6-KitW-sh/Wsh mice (P < .0001).
To confirm that the differences in survival observed between C57BL/6-KitW-sh/W-sh recipients and WT mice were due to a lack of mast cells and not any other strain-specific factor, we repeated our GVHD survival study in a new mast cell–deficient model mouse: C57BL/6-Cpa3-Cre;Mcl-1fl/fl (supplemental Figure 1 on the Blood Web site). These mice have normal expression of c-Kit, but have a severe deficiency in mast cells (92-100%) and a marked deficiency in basophils (58-78%).12 In agreement with what was observed with C57BL/6-KitW-sh/W-sh recipients, there was an exacerbation of GVHD and more rapid death in C57BL/6-Cpa3-Cre;Mcl-1fl/fl recipients, with an MST of 8 days compared with an MST of 77 days in C57BL/6 WT recipients (supplemental Figure 1; P < .0001).
The exacerbation of GVHD in mast cell–deficient recipients suggested that mast cells might be exerting an immunosuppressive effect on Tcon survival and/or proliferation during GVHD induction. To explore this possibility, we used luciferase positive (luc+) Tcons where T cell populations can be assessed in vivo with bioluminescence imaging (BLI).16 Using BLI, which has been validated as a tool for assessing cell survival/proliferation, we observed significantly increased numbers of T cells in mast cell–deficient C57BL/6-KitW-sh/W-sh mice compared with WT recipients (Figure 2; P < .01). The differences in donor-derived T-cell survival/proliferation were most pronounced in total photons emitted from gut (twofold), liver (2.7-fold), and lymph nodes (3.3-fold).
Mast cell–deficient mice have increased amounts of Tcon survival/proliferation. Either WT C57BL/6 (H-2b) or mast cell–deficient (C57BL/6-KitW-sh/Wsh) hosts were transplanted with 5.0 × 106 TCD-BM, followed by 2.0 × 106luc+ Tcon on day 4. Bioluminescent images were taken 10 days after transplant (A: in vivo imaging; B: ex vivo imaging). (C) C57BL/6-KitW-sh/Wsh mice demonstrated significantly more T-cell survival/proliferation than the corresponding C57BL/6 WT mice in lymph nodes, liver, and gut of Tcon (n = 5). Shown is 1 experiment of the 4 performed, each of which gave similar results.
Mast cell–deficient mice have increased amounts of Tcon survival/proliferation. Either WT C57BL/6 (H-2b) or mast cell–deficient (C57BL/6-KitW-sh/Wsh) hosts were transplanted with 5.0 × 106 TCD-BM, followed by 2.0 × 106luc+ Tcon on day 4. Bioluminescent images were taken 10 days after transplant (A: in vivo imaging; B: ex vivo imaging). (C) C57BL/6-KitW-sh/Wsh mice demonstrated significantly more T-cell survival/proliferation than the corresponding C57BL/6 WT mice in lymph nodes, liver, and gut of Tcon (n = 5). Shown is 1 experiment of the 4 performed, each of which gave similar results.
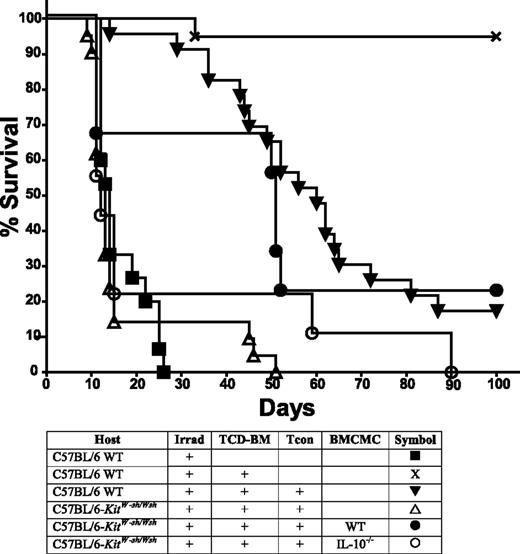
CD4+CD25+Treg numbers and function are similar in WT C57BL/6 and C57BL/6-KitW-sh/Wshmice and Tregs can suppress GVHD in the virtual absence of mast cells. (A) C57BL/6-KitW-sh/Wsh mice have similar numbers of Tregs compared with WT C57BL/6 mice as indicated by FACS analysis (n = 3). (B) In vitro proliferation of 2 × 105 C57BL/6 Tcon monitored by 3H-thymidine incorporation indicates Tcon proliferation can be equally suppressed by Tregs isolated from C57BL/6-KitW-sh/Wsh or WT C57BL/6 mice. White bar, unstimulated responders (nonproliferation control of 2 × 105 Tcon [CD4:CD8 = 2:1]); striped bar, Tcon responders with 4 × 105 irradiated BALB/c splenocytes as stimulators (proliferation control); black/gray bars, Tcon + stimulators in the presence of C57BL/6 WT or C57BL/6-KitW-sh/Wsh Treg as indicated. Bars indicate mean ± standard error (n = 3). (C) Highly purified CD4+CD25+ Tregs (5 × 105) from FVB/N donors (H-2q) were adoptively transferred into lethally irradiated C57BL/6 mice (H-2b) that had received TCD-BM and 1.0 × 106 Tcon from FVB/N donors (to induce GVHD). WT Tregs are equally capable of suppressing GVHD (P < .05 for both groups receiving Treg vs groups receiving Tcon alone in both WT and C57BL/6-KitW-sh/Wsh recipients, indicating mast cells are not involved in Treg suppression of GVHD; survival data are combined from 3 independent experiments (n = 10 for Treg groups; n = 15 for all other groups).
CD4+CD25+Treg numbers and function are similar in WT C57BL/6 and C57BL/6-KitW-sh/Wshmice and Tregs can suppress GVHD in the virtual absence of mast cells. (A) C57BL/6-KitW-sh/Wsh mice have similar numbers of Tregs compared with WT C57BL/6 mice as indicated by FACS analysis (n = 3). (B) In vitro proliferation of 2 × 105 C57BL/6 Tcon monitored by 3H-thymidine incorporation indicates Tcon proliferation can be equally suppressed by Tregs isolated from C57BL/6-KitW-sh/Wsh or WT C57BL/6 mice. White bar, unstimulated responders (nonproliferation control of 2 × 105 Tcon [CD4:CD8 = 2:1]); striped bar, Tcon responders with 4 × 105 irradiated BALB/c splenocytes as stimulators (proliferation control); black/gray bars, Tcon + stimulators in the presence of C57BL/6 WT or C57BL/6-KitW-sh/Wsh Treg as indicated. Bars indicate mean ± standard error (n = 3). (C) Highly purified CD4+CD25+ Tregs (5 × 105) from FVB/N donors (H-2q) were adoptively transferred into lethally irradiated C57BL/6 mice (H-2b) that had received TCD-BM and 1.0 × 106 Tcon from FVB/N donors (to induce GVHD). WT Tregs are equally capable of suppressing GVHD (P < .05 for both groups receiving Treg vs groups receiving Tcon alone in both WT and C57BL/6-KitW-sh/Wsh recipients, indicating mast cells are not involved in Treg suppression of GVHD; survival data are combined from 3 independent experiments (n = 10 for Treg groups; n = 15 for all other groups).
Mast cell–deficient C57BL/6-KitW-sh/W-sh mice have normal numbers and function of CD4+CD25+ Tregs
Based on the increased presence of both mast cells and Tregs in tolerated skin grafts, together with the production of IL-9 (a mast cell growth and activation factor) by Treg, Lu et al10 proposed a functional role for mast cells as intermediaries in Treg-induced tolerance. Furthermore, because C57BL/6-KitW-sh/W-sh mice exhibited increased Tcon numbers and consequently exacerbated GVHD, we investigated whether these mast cell–deficient mice may have a Treg deficiency or if Tregs isolated from such mast cell–deficient mice were less effective in suppressing T-cell proliferation. We observed similar numbers and percentages of FoxP3+ CD4 cells in both C57BL/6-KitW-sh/W-sh mice as in corresponding mast cell–containing WT mice in liver, spleen, and lymph node tissues, except for slightly more FoxP3+ cells in the spleen of C57BL/6-KitW-sh/W-sh mice, probably reflecting the larger size of the spleen in C57BL/6-KitW-sh/W-sh vs WT mice (Figure 3A). Furthermore, purified Tregs (CD4+CD25hi) from C57BL/6-KitW-sh/W-sh mice (FoxP3+-expressing cells > 98%) were equally as effective as Tregs from corresponding mast cell–containing control mice in exerting significant suppression of Tcon proliferation in vitro in a mixed-leukocyte reaction (Figure 3B; P < .05). Therefore, we found no indication that a deficiency in mast cells in C57BL/6-KitW-sh/W-sh mice results in either a decrease in numbers or function of Treg.
We know from previous studies that Tregs can effectively reduce the morbidity and mortality of GVHD in our transplant model.17-19 To further investigate a possible Treg–mast cell interaction, we induced GVHD in C57BL/6-KitW-sh/W-sh mice and in the corresponding mast cell–containing control WT mice and determined whether Treg would be as suppressive of GVHD in the absence of mast cells. Using Tregs purified from WT FVB donor mice, we observed that the addition of Tregs resulted in improved survival and a reduction in GVHD (Figure 3C). Adoptive transfer of Tregs into WT mice receiving Tcons improved the MST to 65.5 days vs an MST of 14 days in mice receiving Tcons only (P < .02). Similarly, transfer of Tregs into C57BL/6-KitW-sh/W-sh mice improved the survival to an MST of 34 days vs an MST of 10 days in C57BL/6-KitW-sh/W-sh mice, which received Tcons only (P < .001). When we take into account the exacerbation of GVHD induced by Tcons in C57BL/6-KitW-sh/W-sh recipients, the improvement in survival was even more pronounced when Tregs were added to C57BL/6-KitW-sh/W-sh recipients than to WT recipients. This indicates that, contrary to the hypothesis put forth by Lu et al10 based on their studies of skin grafts, we do not find any evidence that Treg function depends on or involves mast cells in our model of GVHD.
Engraftment of mast cells into C57BL/6-KitW-sh/W-sh mice improves survival in GVHD in a mechanism dependent on IL-10 production by the mast cells
Although GVHD is worsened in C57BL/6-KitW-sh/W-sh mice, indicating a potential role for mast cells, these data could also be due to other consequences of the large gene inversion in these mice. We therefore determined whether the impaired survival during GVHD observed in C57BL/6-KitW-sh/W-sh mice might be improved when mast cells were selectively engrafted into these mice. We prepared BMCMCs by in vitro incubation of bone marrow in the presence of IL-3. Because mast cell engraftment efficiency in various organs depends on the route of administration,11 we chose to engraft the mast cells in the peritoneal cavity as gut injury is a major cause of lethality in acute GVHD. BMCMCs were prepared from luc+C57BL/6 WT mice to allow for visualization of effective engraftment of mast cells in vivo in the peritoneal cavity and gut 8 weeks after adoptive transfer (Figure 4A). Furthermore, because IL-10 production contributed to mast cell–mediated suppression of inflammation in mast cell–engrafted mast cell–deficient mice in models of severe contact dermatitis and ultraviolet-induced skin pathology,20 we hypothesized that IL-10 production by mast cells also might play a critical role in suppression of GVHD. Therefore, we confirmed that BMCMCs from C57BL/6–IL-10−/− mice would also engraft into C57BL/6-KitW-sh/W-sh recipients (Figure 4B). We observed similar levels of engraftment of BMCMCs derived from WT or IL-10−/− donors in the mesenteric window, forestomach, and in the peritoneal cavity (as assessed by examining cells obtained by peritoneal lavage).
Engraftment of BMCMCs into C57BL/6-KitW-sh/Wshrecipients. (A) Engraftment of BMCMCs derived from luc+C57BL/6J mice was assessed 8 weeks after intraperitoneal injection. Ex vivo imaging of internal organs indicates that mast cell engraftment was primarily in the gut and stomach tissue and not in mesenteric lymph nodes or (from top left to right) kidney, cervical lymph nodes, liver, or spleen. (B) Toluidine blue staining confirmed the presence of mast cells in mesenteric windows, peritoneal cavity (by lavage), and forestomach of mice, whether mast cells were derived from WT C57BL/6 mice or IL-10−/− C57BL/6 mice.
Engraftment of BMCMCs into C57BL/6-KitW-sh/Wshrecipients. (A) Engraftment of BMCMCs derived from luc+C57BL/6J mice was assessed 8 weeks after intraperitoneal injection. Ex vivo imaging of internal organs indicates that mast cell engraftment was primarily in the gut and stomach tissue and not in mesenteric lymph nodes or (from top left to right) kidney, cervical lymph nodes, liver, or spleen. (B) Toluidine blue staining confirmed the presence of mast cells in mesenteric windows, peritoneal cavity (by lavage), and forestomach of mice, whether mast cells were derived from WT C57BL/6 mice or IL-10−/− C57BL/6 mice.
WT or IL-10–deficient BMCMCs were introduced intraperitoneally into C57BL/6-KitW-sh/W-sh mice 8 weeks before transplantation. The median survival of transplanted animals was significantly improved from 13 days in mast cell–deficient C57BL/6-KitW-sh/W-sh recipients to 51 days in C57BL/6-KitW-sh/W-sh mice, which had received WT BMCMCs (Figure 5; P < .01). However, if the engrafted BMCMCs were derived from IL-10−/− mice, the median survival was unchanged at 12 days, and thus no significant improvement in survival was observed. Together, these results support the conclusion that a mast cell deficiency contributes to, or possibly even fully accounts for, the exacerbation in GVHD in C57BL/6-KitW-sh/W-sh recipient mice and that IL-10 production by mast cells is involved in the mechanism by which mast cells modulate the immune response in this setting.
C57BL/6-KitW-sh/Wshmice that had received BMCMCs intraperitoneally have improved survival during GVHD induction. Recipient mice, which were C57BL/6J (H-2b) WT, mast cell–deficient C57BL/6-KitW-sh/Wsh, or C57BL/6-KitW-sh/Wsh engrafted with WT or IL-10−/− BMCMCs were given lethal irradiation and transplanted with 5.0 × 106 TCD-BM, followed by 2.0 × 106 Tcon on day 4 (to induce GVHD) from FVB/N donors (H-2q). Data were pooled from the 4 independent experiments performed, each of which gave similar results (n = 10 for mast cell–engrafted groups; n = 23 for all other groups). Significant improvement in survival was observed when C57BL/6-KitW-sh/Wsh recipients had been engrafted 8 weeks prior to transplant with WT BMCMCs (P < .01); however, significant improvement in survival was not observed if BMCMCs were derived from IL-10−/− mice. Furthermore, engraftment of WT BMCMCs into C57BL/6-KitW-sh/Wsh mice produced a survival curve not significantly different than that of WT C57BL/6J recipients.
C57BL/6-KitW-sh/Wshmice that had received BMCMCs intraperitoneally have improved survival during GVHD induction. Recipient mice, which were C57BL/6J (H-2b) WT, mast cell–deficient C57BL/6-KitW-sh/Wsh, or C57BL/6-KitW-sh/Wsh engrafted with WT or IL-10−/− BMCMCs were given lethal irradiation and transplanted with 5.0 × 106 TCD-BM, followed by 2.0 × 106 Tcon on day 4 (to induce GVHD) from FVB/N donors (H-2q). Data were pooled from the 4 independent experiments performed, each of which gave similar results (n = 10 for mast cell–engrafted groups; n = 23 for all other groups). Significant improvement in survival was observed when C57BL/6-KitW-sh/Wsh recipients had been engrafted 8 weeks prior to transplant with WT BMCMCs (P < .01); however, significant improvement in survival was not observed if BMCMCs were derived from IL-10−/− mice. Furthermore, engraftment of WT BMCMCs into C57BL/6-KitW-sh/Wsh mice produced a survival curve not significantly different than that of WT C57BL/6J recipients.
Discussion
This study provides evidence that mast cells can play an immunoregulatory role in GVHD. When mice were given allogeneic T cells, a dramatic increase in GVHD-associated mortality was observed in mast cell–deficient C57BL/6-KitW-sh/W-sh and C57BL/6-Cpa3-Cre;Mcl-1fl/fl mice compared with WT C57BL/6-Kit+/+ controls. This is in contrast to previous findings using a CD8-only GVHD model where another mast cell–deficient mouse strain (WBB6F1-KitW/W-v) demonstrated improved survival in the absence of mast cells.8 However, in the study by Murphy et al,8 GVHD induction was significantly weaker and survival improvement was slight. Also, the strain combinations used in the 2 studies may have influenced the finding that WBB6F1-KitW/W-v mice behaved differently than C57BL/6-KitW-sh/W-sh mice.
Because mast cells have been reported to have an important role in protection against infection,21 we cannot dismiss the possibility that the increased mortality observed in mast cell–deficient recipients that received allogeneic T cells could be partly due to an increased susceptibility to infection. However, the risk of infection was mitigated in all transplant recipients by the continuous administration of antibiotic in the drinking water of the mice. Also, as our major-mismatch model of GVHD involves significant inflammation and proliferation of T cells, we do not believe that these mice are significantly susceptible to infection compared with the WT animals. Furthermore, we demonstrated the novel finding that mast cells in fact are playing an immunoregulatory role in GVHD. The decrease in survival we observed in our model was associated with increased Tcon survival/proliferation in mice lacking mast cells. This finding indicates that the presence of mast cells resulted in a reduction in T-cell numbers. As the report by Lu et al10 indicated that mast cells can functionally interact with CD4+CD25+ Tregs, we hypothesized that perhaps the C57BL/6-KitW-sh/W-sh mice might have a deficiency or defect in Tregs, yet we found no evidence to support this hypothesis.
Because mast cell production of IL-10 was found to be an important mediator of mast cell–mediated suppression of inflammation in models of severe contact dermatitis or chronic ultraviolet irradiation,20 we investigated whether mast cell–derived IL-10 may be involved in the suppression of GVHD by mast cells. By using a mast cell engraftment model where bone marrow–derived cultured mast cells from either WT or IL-10−/−–deficient donor mice were engrafted pretransplant, we found that adoptive transfer of mast cells into mast cell–deficient animals prolonged survival and that IL-10 production by the mast cells contributed to reducing GVHD and improving survival. It remains to be investigated what may be triggering mast cells to produce IL-10 following the myeloablative conditioning of recipient mice and induction of GVHD. One possibility is lipopolysaccharide (LPS), which accumulates in the bloodstream during GVHD following gut injury.22,23 Masuda et al24 demonstrated that LPS increased production of Th2-associated cytokines by mast cells, including IL-10. Thus, 1 hypothesis is that LPS released during gastrointestinal injury in GVHD is activating the Toll-like receptor 4 receptors of mast cells, which in turn produce IL-10 and reduce T-cell proliferation.
In conclusion, our data provide evidence that further expands the roles of mast cells in inflammatory diseases and adds a novel immunoregulatory role to the complex repertoire of mast cell functions. As gastrointestinal injury is the likely culprit in the rapid mortality observed in mast cell–deficient C57BL/6-KitW-sh/W-sh mice, we do not feel that our results necessarily conflict with previous findings that mast cells can contribute to dermal fibrosis in other settings.7 It is therefore possible that mast cells can play both an inflammatory role in chronic GVHD associated with dermal fibrosis and an anti-inflammatory role in mucosal tissues and in systemic allogeneic T-cell proliferation. Mast cell function in GVHD appears to be dependent on the tissue they reside in and the factors and conditioning to which they are exposed. As a result, further investigation into modulation of mast cell function, and devising approaches to induce mast cells to exhibit enhanced production of IL-10, may lead to improved treatment of GVHD, as well as other inflammatory conditions.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all members of the Negrin and Galli laboratories for helpful discussions and technical assistance.
This work was supported by National Institutes of Health Research Program grants P01 HL075462 (National Heart, Lung, and Blood Institute) and P01 CA049605 (National Cancer Institute) (to R.S.N.), and National Institutes of Health grants AI023990 and AI070813 (National Institute of Allergy and Infectious Diseases), and CA072074 (National Cancer Institute) (to S.J.G.).
Authorship
Contribution: D.B.L.-G. designed and performed research, analyzed data, and wrote the manuscript; E.I.S., M.F., Y.P., and A.P. performed research; J.K. performed research and helped analyze data; and R.S.N. and S.J.G. designed experiments, provided overall guidance, and helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert S. Negrin, Stanford University, Center for Clinical Science Research Building, Room 2205, 269 W Campus Dr, Stanford, CA 94305; e-mail: negrs@stanford.edu; and Stephen J. Galli, Department of Pathology, L-235, Stanford University School of Medicine, 300 Pasteur Dr, Stanford, CA 94305; e-mail: sgalli@Stanford.edu.



![Figure 3. CD4+CD25+ Treg numbers and function are similar in WT C57BL/6 and C57BL/6-KitW-sh/Wsh mice and Tregs can suppress GVHD in the virtual absence of mast cells. (A) C57BL/6-KitW-sh/Wsh mice have similar numbers of Tregs compared with WT C57BL/6 mice as indicated by FACS analysis (n = 3). (B) In vitro proliferation of 2 × 105 C57BL/6 Tcon monitored by 3H-thymidine incorporation indicates Tcon proliferation can be equally suppressed by Tregs isolated from C57BL/6-KitW-sh/Wsh or WT C57BL/6 mice. White bar, unstimulated responders (nonproliferation control of 2 × 105 Tcon [CD4:CD8 = 2:1]); striped bar, Tcon responders with 4 × 105 irradiated BALB/c splenocytes as stimulators (proliferation control); black/gray bars, Tcon + stimulators in the presence of C57BL/6 WT or C57BL/6-KitW-sh/Wsh Treg as indicated. Bars indicate mean ± standard error (n = 3). (C) Highly purified CD4+CD25+ Tregs (5 × 105) from FVB/N donors (H-2q) were adoptively transferred into lethally irradiated C57BL/6 mice (H-2b) that had received TCD-BM and 1.0 × 106 Tcon from FVB/N donors (to induce GVHD). WT Tregs are equally capable of suppressing GVHD (P < .05 for both groups receiving Treg vs groups receiving Tcon alone in both WT and C57BL/6-KitW-sh/Wsh recipients, indicating mast cells are not involved in Treg suppression of GVHD; survival data are combined from 3 independent experiments (n = 10 for Treg groups; n = 15 for all other groups).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/22/10.1182_blood-2013-08-519157/4/m_3659f3.jpeg?Expires=1767704894&Signature=O7EPez8PcZU2jd8B7kq68uIMT8opfSHc5cSoQ6J8EDjDR28qeYMPBzsBR6hSpVHnDewrX3e1K0N18KjU7FyYBqZq4rKYsrtuBtg9cDCsPCHlWBrq7T3jXU6FG4yQp8N91oW-LqaOG9HJcnHL3hCLzoq6ZRuzTLhuHJWO4j~xDMLl-3-jQ4~i2058eVCJPmPa8McfWBQa12RD~WtfjWj9kb8ryP2lgMqSMRizxms-ShDYNvzKgJ4DowjI2FAvCDVf0V6wehsKVgIuHMpvCbmY-FdmafhvepKcl7wrmBQbyikMur6d9yaA2sclHsx8vye6r~DlUlPfRYuEcnfoCgW-Bw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal