Abstract
Inhibition of Brd4, a bromodomain and extra-terminal (BET) protein, results in antiproliferative effects and terminal differentiation in the MYC-driven B-cell malignancies multiple myeloma and Burkitt's lymphoma by selectively repressing MYC. Elevated MYC expression correlates with progression of B-cell chronic lymphocytic leukemia (B-CLL) marking Brd4 as a potential therapeutic target. 17p deletion or somatic TP53 mutations are the poorest prognostic factors in B-CLL, resulting refractoriness to conventional therapy. The recent identification of a Brd4-interacting protein as a validated target in CLL led us to evaluate the potential of BET inhibition, using jq1, for the treatment of B-CLL.
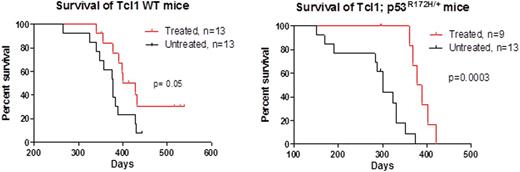
Jq1 remarkably reduced proliferation of the immortalized human B-CLL cell lines, Mec-1, Mec-2, and WaC3 (IC50 values: 1.12, 0.68, and 0.64 μM, respectively). These results were validated in 13 frozen and 7 fresh primary whole peripheral blood B-CLL samples (median EC50 0.66 μM [range, 0.051 to 2.169 μM]) using a FACS-based proprietary platform (Vivia Biothech). Eμ-TCL1 (n=13) & Eμ-TCL1:p53R172H/+ (n=9) mice treated with intraperitoneal (IP) injections of jq1 at 50 mg/kg/day for 21 consecutive days had a significant reduction in lymphocytes and white blood cells (WBC) (with excellent tolerance and no off-target effects on hemoglobin level or platelet counts) compared to vehicle-treated mice. The median lymphocyte count at baseline and after 2 weeks of jq1 therapy was 13.4 & 5.92 x10^3/μL (p=0.008) for Eμ-TCL1 mice and 13.2 & 7.2 x10^3/μL (p=0.009) for Eμ-TCL1:p53R172H/+ mice. jq1 therapy significantly prolonged survival in both Eμ-TCL1 (428 vs 377 days, p=0.05) and Eμ-TCL1:p53R172H/+ (382.5 vs 300 days, p=0.0003) mice compared to mice receiving vehicle. FACs analysis was performed on blood taken from Eμ-TCL1 (n=5) and Eμ-TCL1:p53R172H/+ (n=6) mice at baseline and after 7 daily IP jq1 doses. Eμ-TCL1:p53R172H/+ mice showed a significant reduction in CD5+/CD19+ lymphocytes (mean difference, 14.37 [95% CI, 2.4 to 26.3]; p=0.03), which was not observed in Eμ-TCL1 mice. Cell cycle analysis of cultured Eμ-TCL1 splenocytes treated with jq1 showed a significant increase (p<0.0005) in the number of sub-G1 cells compared to untreated splenocytes, regardless of p53 status. Sensitivity against fludarabine was also tested in these samples and we show that 10μM fludarabine did not elicit significant changes in the number of cells in sub-G1 compared to untreated cells but a significant difference (p=0.009) was observed between splenocytes treated with 10μM fludarabine and 1μM jq1. In depth analyses of RNA sequencing data of splenocytes from 10 matched-pairs samples (5 Eμ-TCL1 & 5 Eμ-TCL1:p53R172H/+) treated in culture with 0 or 1μM jq1 for 24 hours are currently underway to identify BET targets in B-CLL, but preliminary gene expression analysis reveal that the sensitivity of B-CLL to BET inhibition is not Myc-dependent despite elevated Myc levels. Notably, c-Fos was one of the most significant differentially expressed genes, regardless of p53 status. Interestingly, AP-1 complex, a heterodimer of c-Fos and c-Jun, is inhibited by direct interaction with Tcl-1. We are currently validating these findings in additional samples and performing functional assays to gain a better understanding of BET targets in B-CLL.
BET bromodomain inhibition markedly reduced proliferation in both human and mouse B-CLL samples. Furthermore, the loss of wild-type p53 activity did not desensitize CLL cells to jq1, which was also effective at inducing massive cell death in fludarabine resistant CLL cells, regardless of p53 status. Mice receiving jq1 exhibited a significant reduction in leukemic burden and prolonged survival compared to control mice, with a more pronounced effect observed in mice with mutant p53. These data demonstrate the potent effect of BET inhibition in B-CLL and more importantly, provide evidence as an effective treatment for aggressive forms of B-CLL with 17p deletion or TP53 mutation or chemoresistant refractory disease.
Primo:Vivia Biotech: Employment. Rojas:Vivia Biotech: Employment. Martinez:Vivia Biotech: Employment. Bradner:Tensha Therapeutics: Dr. Bradner is a scientific founder of Tensha Therapeutics, which is developing drug-like derivatives of the JQ1 bromodomain inhibitor as cancer therapeutics, through a license from the Dana-Farber Cancer Institute. Other. Ballesteros:Vivia Biotech: Employment.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal