Abstract
Primary plasma cell leukemia (pPCL) is a rare form of plasma cell malignancy with poor prognosis (usually less than 12 months survival with conventional chemotherapy). Only one prospective study with Lenalidomide-Dex before high dose therapy has been recently reported1. Bortezomib-based regimens and high dose Melphalan/ autologous stem cell transplantation (HDM/ASCT) have shown promising results in small retrospective studies. We report here the first prospective multicenter phase II trial for pPCL patients (pts) treated with Bortezomib-Doxorubicine-Dexamethasone (PAD) / Bortezomib-Cyclosphosphamide-Dexamethasone (VCD) as induction before HDM/ASCT. With the aim to improve response and survival, allograft or second ASCT plus consolidation/maintenance with Lenalidomide-Bortezomib-Dex (VRD) were proposed.
Non-previously treated pts with a diagnosis of pPCL were enrolled in this phase II study of the IFM group from April 2010 to July 2013. PAD (dexa 40 mg + bortezomib 1,3 mg/m2 on day 1, 4, 8, 11 + doxorubicine 30 mg/m2 day 4) and VCD (dexa + bortezomib + cyclophosphamide 300 mg/m2 day 1, 8) were administrated alternatively each 21 days for 4 cycles. For responding patients with circulating plasma cell < 1%, peripheral stem cells were collected after Cyclophosphamide + G-CSF. Either (i) double HDM/ASCT was performed followed by consolidation/maintenance íVDR (dexa 40mg + bortezomib 1,3 mg/m2 on day 1, 4, 8, 11 + lenalidomide 15 mg day 1-15) each 3 months and Lenalidomide 15 mg 21/28 days on others months, for 1 yearý or (ii) tandem HDM/ASCT-reduced intensity conditioning-allograft if there were < 66 years-old. Primary end-point was progression free survival (PFS); secondary end-points were responses rates, overall survival (OS), feasibility, and toxicity. We evaluated the disease response to therapy with the IMWG criteria and the percentage of circulating plasma cells; minimal residual disease (MRD) was evaluated during follow-up. Peripheral circulating plasma cells and bone marrow plasmocytes were collected at diagnosis for centralized FISH and SNP array analysis.
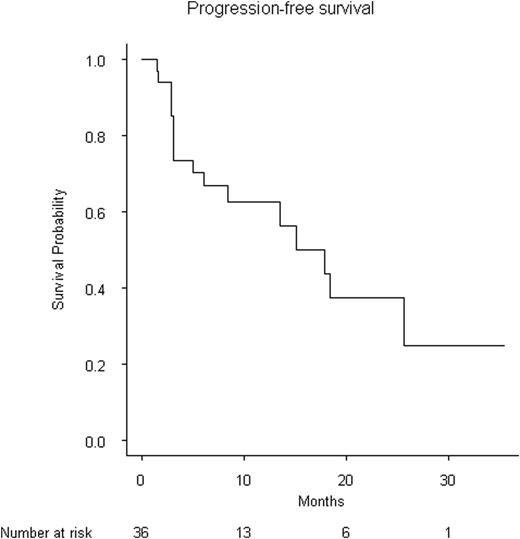
40 pts were enrolled with a median age of 55y (27-71). Median follow-up was 12.6 months (7.6-24.8). At diagnosis, the median number of circulating plasma cell was 5.2 G/L (1.3-66). Twenty-two percent had creatinine clearance < 50 ml/min, and 11% < 30 ml/min, 44% had an ISS score of 2 and 37% ISS 3. After induction 35 pts are evaluable. In the intent to treat analysis, 25 (72%) responded (VGPR+CR 37%, PR 28%, SD 5%) and 10/35 (28%) were refractory (pts having circulating plasma cell ≥ 1%) and did not continue the study. Twenty-three of the 25 responding pts underwent HDM/auto and 20 pts are evaluable at 3 months: CR+VGPR 14/20 (70%), PR 4/20 (20%), 1/20 (5%) and PD 1/20 (5%). Second HDM/auto was performed in 6 pts and allograft in 12. Ten of the 14 (72%) evaluable pts achieved VGPR or better and 4/14 (28%) PR. Six pts are currently on consolidation/maintenance phase with VRD. Median PFS was 17.8 months. Median OS was not reached. Genomic data are shown in Table I. MDR evaluation will be presented later.
Major toxicities were hematological and infections. Eight pts died: 5 during induction, 1 at HDM/ASCT and 2 post allograft while in relapse. Of note, 2 pts had meningeal involvement at relapse.
| FISH . | n . | % . |
|---|---|---|
| t(4 ;14) | 3/28 | 11 |
| t(14 ;16) | 4/25 | 16 |
| del 17p | 7/28 | 25 |
| del 13 | 11/28 | 40 |
| SNP array | ||
| hyperdiploidy (≥3Ch) | 3/28 | 11 |
| anomalies ≥ 20 | 12/28 | 43 |
| anomalies < 6 | 5/28 | 18 |
| whole/partial loss 13 | 16/28 | 57 |
| gain 1q | 16/28 | 57 |
| gain/amp CCND1 | 11/28 | 39 |
| gain/amp cMYC | 9/28 | 32 |
| loss/del TP53 | 8/28 | 28 |
| CDKN2C homozygous del | 4/28 | 14 |
| FISH . | n . | % . |
|---|---|---|
| t(4 ;14) | 3/28 | 11 |
| t(14 ;16) | 4/25 | 16 |
| del 17p | 7/28 | 25 |
| del 13 | 11/28 | 40 |
| SNP array | ||
| hyperdiploidy (≥3Ch) | 3/28 | 11 |
| anomalies ≥ 20 | 12/28 | 43 |
| anomalies < 6 | 5/28 | 18 |
| whole/partial loss 13 | 16/28 | 57 |
| gain 1q | 16/28 | 57 |
| gain/amp CCND1 | 11/28 | 39 |
| gain/amp cMYC | 9/28 | 32 |
| loss/del TP53 | 8/28 | 28 |
| CDKN2C homozygous del | 4/28 | 14 |
This first large study for pPCL patients with PAD/VCD as induction and HDM/ASCT is effective and induce high responses rates. Consolidation with Allograft or bortezomib-lenalidomide-dex is currently investigated.
1Musto P et al. Blood (ASH Annual Meeting Abstracts) 2011; 118 Abstract2925Table I: genomic
Leleu:CELGENE: Honoraria; JANSSEN: Honoraria. Moreau:Celgene: Honoraria, Speakers Bureau. Avet-Loiseau:CELGENE: Honoraria, Speakers Bureau; JANSSEN: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal