Abstract
The JAK1/2 dual kinase inhibitor INC424 (ruxolitinibª, Jakafi¨) has been recently approved for the treatment of intermediate or high-risk myelofibrosis (MF) and is in late-stage clinical trials for polycythemia vera (PV). Although JAK inhibitors have been shown to improve patient constitutional symptoms and reduce splenomegaly, JAK inhibitor monotherapy does not significantly reduce mutant allele burden in the majority of MPN patients. Furthermore, the therapeutic window of JAK inhibitors is limited due to the essential role of the JAK-STAT signaling pathway in normal hematopoiesis, which has been observed in the clinic where these inhibitor have been associated with dose limiting toxicities including anemia and thrombocytopenia. Therefore, there is a need to identify additional pathways that might be involved in the development and maintenance of MPN mutant clones, which could then be targeted in combination with JAK2 for improved therapeutic benefit. Recent studies have shown that the hedgehog (Hh) signaling pathway has important roles in normal hematopoiesis as well as in the pathogenesis of myeloid malignancies. We therefore investigated whether the Hh pathway was activated in MPN patients and whether it represented a therapeutic target in MPN preclinical models.
We observed 20-100 fold increase in expression of Hh target genes including Gli1 and Ptch1 by quantitative PCR in granulocytes isolated from MPN patients as compared to normal controls. We also observed activation of the pathway in a murine bone marrow transplant (BMT) model of PMF using a Gli-luciferase reporter system. These data demonstrate the Hh pathway is active in primary MPN patient samples and in preclinical, models of PMF.
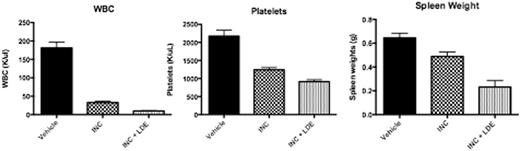
Reduction in blood counts and spleen weights observed in combination of INC424 and LDE225 as compared to INC424 monotherapy.
Rose:Novartis Pharmaceutical: Employment. Amakye:Novartis Pharmaceutical: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal