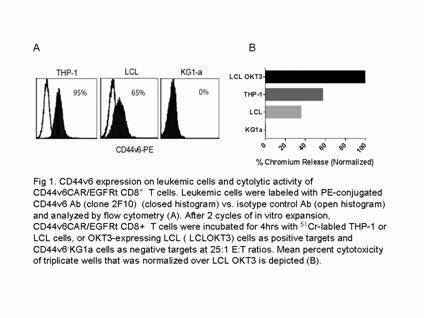
Leukemic stem cells (LSCs), are resistant to radio/chemotherapy and responsible for disease relapse. Therefore, prevention of leukemia recurrence relies on the eradication of LSCs, which remains challenging for the treatment of hematopoietic malignancies. CD44 and its ligand are critical components of LSC niches that contain crucial signals for LSCs self-renewal. A CD44-specific mAb (clone H90) has been shown to specifically eradicate LSCs in immunodeficient mouse models. Further, a splice variant of CD44, CD44 variant 6 (CD44v6) is over expressed on malignant hematopoietic cells including ALL, AML, CML, NHL and multiple myeloma and plays an important role for proliferation, homing, and metastasis of LSCs.
To study in vivo efficacy of the CD44v6CAR/EGFRt CD8+ T cells, a xenogeneic AML model was established by intravenous injection of 2x10^6 firefly luciferase expressing THP-1 cells (THP-1-ffluc) into NSG mice. Adoptive transfer of 5x10^6 CD44v6CAR/EGFRt CD8+ T cells dramatically reduced THP-1-ffluc tumors as evidenced by bioluminescent imaging 14 days post T cell infusion. To prove the specificity of targeting, CD19+LCL-ffluc cells that are 50% CD44v6 positive were systemically infused into NSG mice. As expected, CD44v6CAR/EGFRt CD8+ T exhibited less antitumor activity against LCL tumor than THP-1 tumor. Accordingly, irrelevant CE-7CAR+T cells (L1CAM specific) that were derived from the CD8+ TCM of the same donor had no antitumor activity. These results indicate that targeting CD44v6 with CAR-transduced CD8+ TCM cells has the potential to treat hematological malignancies. Moreover, CD44v6 redirected T cell therapy would apply to various hematopoietic malignancies that aberrantly over-express CD44v6.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal