Abstract
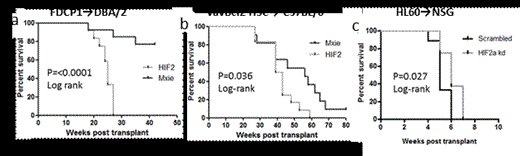
Percent survival of recipients of (a) FDCP1 cells retrovirally transduced with HIF-2α-MXIE vector or MXIE control empty vector, (b) vavBcl2 HSC transduced with HIF-2α-MXIE vector or MXIE empty vector, and (c) HL60 cells transduced with HIF-2α knocked-down or scrambled control lentiviral vectors.
Percent survival of recipients of (a) FDCP1 cells retrovirally transduced with HIF-2α-MXIE vector or MXIE control empty vector, (b) vavBcl2 HSC transduced with HIF-2α-MXIE vector or MXIE empty vector, and (c) HL60 cells transduced with HIF-2α knocked-down or scrambled control lentiviral vectors.
In a 2nd model, HSC from vavBcl2 transgenic mice were transduced with human HIF-2α-containing or empty MXIE retroviral vectors and subsequently transplanted into lethally irradiated wild-type recipients. Transduction of vavBcl2 HSC with HIF-2α resulted in the outgrowth of HIF-2α-expressing B cells which was not observed in recipients of vavBcl2 HSC transduced with empty vector. Consequently recipients of HIF-2α transduced vavBcl2 HSC succumbed more rapidly to spontaneous lymphoma compared to controls (Fig. 1b; p=0.036 log rank, hazard ratio = 2.971, MXIE median survival = 56 weeks, HIF2α median survival = 41 weeks).
Finally, HIF-2α was knocked-down in human leukemia cell lines U937 and HL60 using a shRNA lentiviral vector. HIF-2α knock-down resulted in a 2-fold decrease in proliferation in vitro. We next transplanted HL60-HIF-2a shRNA and HL60-scrambled shRNA cells into NOD/SCID/ IL2Rγ-/- (NSG) mice for each group. Notably, all recipients of HL60-HIF-2a shRNA cells succumbed to leukemia significantly later than recipients of HL60-scrambled shRNA cells (Fig. 1c; p=<0.027 Log-rank, hazard ratio = 0.1918, Scrambled median survival = 5 weeks, HIF-2α knock down median survival = 6 weeks). Together these data suggest that expression of HIF-2α in malignant hematopoietic cells provides a proliferative advantage in the hypoxic malignant BM enabling them to proliferate in the hypoxic leukemic BM while the proliferation of normal HSC, which do not express HIF-2α, is blocked by hypoxia-stabilized HIF-1α.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal