Abstract
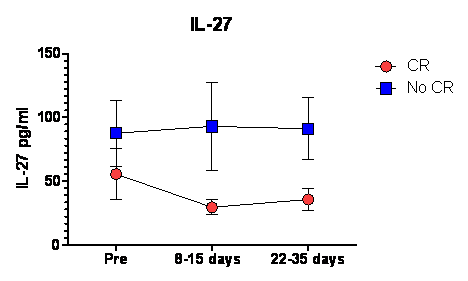
Between presentation and remission of AML, loss of leukemia burden and the recovery of normal hematopoiesis are likely to be associated with major changes in cytokine profiles which could inform pathophysiology of hematopoiesis and immune recovery and may be predictive for outcome. However cytokine fluctuations in AML before and after induction chemotherapy are not well characterized. To profile the cytokine signatures of patients with AML, we analyzed 57 cytokines, chemokines, and growth factors in blood of 11 patients with AML (mean age 58 years; 31-69) undergoing conventional remission induction chemotherapy enrolled into an investigational study (VICCHEM 1073). Plasma was obtained from heparinized peripheral blood collected at onset of leukemia, 8-14 days, and 22-35 days after the initiation of induction chemotherapy and also from 12 healthy donors. Cytokine levels were measured in duplicate using magnetic beads based Luminex assay (Affymetrix, CA, USA). Compared with normal controls, 5 cytokine patterns were observed. i) levels significantly lower at onset of leukemia, and lowest 8-14 days after induction correlating with lymphocyte count: GM-CSF, M-CSF, PDGF-AA, EGF, FGF basic, IL1b, IL-2,IL4, IL10, IL12p40, IL12p70, IL13, IL15, IL17a, IL22, IL23p19, TNF beta, TNF alpha, IFN alpha, IFN gamma, TGF alpha, MCP3, LIF, Granzyme B, sFAS ligand, TRAIL, (p<0.05). Most of these cytokines are predominantly produced by T cells or other immune cells. ii) levels significantly higher in AML through chemotherapy induction and recovery: IL-27 (p=0.002), MPO, IL2Ra, IL-21, , IP-10, MIG, MIP1 alpha, SDF-1, MCP1(p<0.05), HGF (p=0.05), VEGF (p=0.07), IL1Ra (p=0.058). These cytokines are predominantly produced by stromal cells. iii) levels significantly higher at the onset of leukemia and correlating with lymphocyte count: CD40 ligand (p<0.05). iv) levels significantly lower at onset of leukemia but inversely correlated with lymphocyte count; Flt3-ligand, sFAS (p<0.05). v) No significant differences and fluctuation: NGF, GRO alpha, IL1a, IL3, IL5, IL6, IL7, IL8, IL9, MIP1b, SCF. Among the cytokines persistently elevated in AML, IL-27 was significantly higher in patients who did not achieve complete remission after induction chemotherapy (p=0.03). To investigate the biological consequences of elevated IL-27 in the AML microenvironment, we examined the effect of IL-27 on T-cell function. Previous studies in mice show that IL-27 rapidly induces PD-L1 expression on naïve CD4+ T-cells. Human CD4+ naïve and CD4+ cells were isolated from healthy volunteers (n=4) according to RoboSep protocols (Stemcell Technologies, Vancouver, Canada), then incubated with IL-27 or IL-6 for up to 72 hours. IL-27 was found to induce PD-L1 expression in a time and dose-dependent manner especially in CD4+ naïve and central memory populations. These findings support other findings that AML suppresses protective antileukemic immune responses and cause T cell exhaustion. IL-27 production induced by AML cells may explain exhaustion of CD4+ T-cells through increased PD-L1 expression. Targeting IL-27 may improve immune function in AML and lead to better survival.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal