Abstract
Pyruvate kinase deficiency (PKD) is an autosomal recessive enzymopathy that is the most common cause of hereditary nonspherocytic hemolytic anemia (HNSHA). PKD is a rare disease characterized by a life-long chronic hemolysis with severe co-morbidities. It is hypothesized that insufficient energy production to maintain red cell membrane homeostasis promotes the chronic hemolysis. Treatment is generally palliative, focusing on the resultant anemia, and there are no approved drugs that directly target mutated pyruvate kinase.
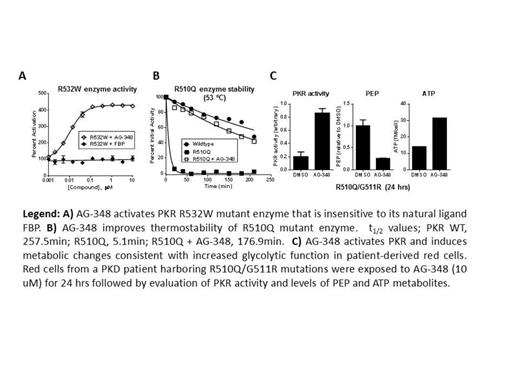
Here, we describe the mechanism of action and cellular effects of AG-348, an allosteric activator of the red cell isoform of pyruvate kinase (PKR). Hundreds of mutant alleles of PKR have been identified and are known to have deleterious effects on catalytic activity, protein stability, or protein expression. We demonstrate that AG-348 can potently activate a spectrum of recombinantly expressed PKR mutant proteins, including mutations that span distinct subdomains of the enzyme. The R532W mutation is quite sensitive to AG-348 modulation, with over 4-fold activation of the enzyme activity, even as the mutation renders PKR insensitive to stimulation by its endogenous allosteric regulator fructose 1,6-bisphosphate (FBP) (Figure A). Crystallographic analysis reveals that very few mutations associated with PKD occur within the AG-348 binding pocket, accounting for its broad activity. The binding of AG-348 attenuates the thermostability defect of several mutant alleles of PKR, including the commonly observed R510Q mutant that has a half-life of ∼2% of the half-life of wild-type PKR when incubated at 53°C. Pre-incubation of the R510Q protein with AG-348 restores the half-life to ∼70% that of the wild-type enzyme (Figure B).
PKD red cells are characterized by changes in metabolism associated with defective glycolysis, including a build-up of the PKR substrate phosphenolpyruvate (PEP) and deficiency in the PKR product adenosine triphosphate (ATP). PKD red cells from several patients with distinct compound heterozygous PKR mutations exposed to AG-348 had increased PKR enzyme activity (up to 4-fold over control) and showed consistent dose and time-dependent metabolic responses (Figure C), including sharp reductions in PEP (up to 70% compared to control) and increases in ATP levels (up to 100% over control). These responses were observed in patient samples harboring PKR mutations that we had studied biochemically (including R486W and R510Q), but also in an instance where the mutation had not previously been biochemically characterized (A495V). In these ex-vivo settings, ATP levels in AG-348 treated cells can reach levels that are typical of normal, non-PKD red cells.
Kung:Agios Pharmaceuticals: Employment, Equity Ownership. Hixon:Agios Pharmaceuticals: Employment, Equity Ownership. Kosinski:Agios Pharmaceuticals: Employment, Equity Ownership. Histen:Agios Pharmaceuticals: Employment, Equity Ownership. Hill:Agios Pharmaceuticals: Employment, Equity Ownership. Si:Agios Pharmaceuticals: Employment, Equity Ownership. Kernytsky:Agios Pharmaceuticals: Employment, Equity Ownership. Chen:Agios Pharmaceuticals: Employment, Equity Ownership. DeLaBarre:Agios Pharmaceuticals: Employment, Equity Ownership. Clasquin:Agios Pharmaceuticals: Employment, Equity Ownership. Ho:Agios Pharmaceuticals: Employment, Equity Ownership. Salituro:Agios Pharmaceuticals: Employment, Equity Ownership. Popovici-Muller:Agios Pharmaceuticals: Employment, Equity Ownership. Agresta:Agios Pharmaceuticals: Employment, Equity Ownership. Silverman:Agios Pharmaceuticals: Employment, Equity Ownership. Dang:Agios Pharmaceuticals: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal