Key Points
An anti-CD37 antibody-drug conjugate can kill B-lymphoma cells via direct inhibition, effector function, and payload delivery.
Targeting CD37 with an antibody-drug conjugate results in selective depletion of malignant human B cells.
Abstract
CD37 has gathered renewed interest as a therapeutic target in non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL); however, CD37-directed antibody-drug conjugates (ADCs) have not been explored. Here, we identified a novel anti-CD37 antibody, K7153A, with potent in vitro activity against B-cell lines through multiple mechanisms including apoptosis induction, antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and complement-dependent cytotoxicity. The antibody was conjugated to the maytansinoid, DM1, a potent antimicrotubule agent, via the thioether linker, N-succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC), and the resulting ADC, IMGN529, retained the intrinsic antibody activities and showed enhanced cytotoxic activity from targeted payload delivery. In lymphoma cell lines, IMGN529 induced G2/M cell cycle arrest after internalization and lysosomal processing to lysine-Nε-SMCC-DM1 as the sole intracellular maytansinoid metabolite. IMGN529 was highly active against subcutaneous B-cell tumor xenografts in severe combined immunodeficient mice with comparable or better activity than rituximab, a combination of cyclophosphamide, vincristine, and prednisone, or bendamustine. In human blood cells, CD37 is expressed in B cells at similar levels as CD20, and IMGN529 resulted in potent and specific depletion of normal and CLL B cells. These results support evaluation of the CD37-targeted ADC, IMGN529, in clinical trials in patients with B-cell malignancies including NHL and CLL.
Introduction
The CD37 antigen is a transmembrane protein of the tetraspanin superfamily that is highly expressed on B cells during the pre-B to peripheral mature B-cell stages, but is absent on early progenitor cells or terminally differentiated plasma cells.1-3 Although the exact physiological role of CD37 is unclear, it may play a role in immune cell proliferation and influences signaling via the Akt pathway.4-6 In normal tissues, CD37 expression is restricted to lymphoid tissues.1 However, CD37 is highly expressed on malignant B cells in non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL).1,7,8 This expression profile suggests that CD37 represents a promising therapeutic target for B-cell malignancies.
Anti-CD20 antibodies such as rituximab and ofatumumab have proven to be effective for treatment of NHL and CLL, either as single agents or in combination therapy, and a number of additional CD20-targeting therapeutic agents are in development. The intrinsic anticancer activities of these antibodies include direct signaling/proapoptotic activity and immune effector activities such as antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). However, many patients eventually experience relapse or experience resistance to available treatments, creating a need for additional therapeutic options. To date, a limited number of CD37-directed antibody therapeutic candidates have been evaluated in patients. Radioimmunotherapy with 131I-MB-1 in patients with NHL who experience relapse resulted in some limited clinical responses, but this agent was not further developed as a therapeutic drug.9,10 More recently, a CD37-binding small modular immunopharmaceutical protein advanced into clinical testing as a treatment of B-cell malignancies, and some clinical data have been reported.6,11,12 In addition, an Fc-engineered antibody to CD37 is in early development.13 Although these initial data suggest that CD37-directed therapies may be effective, there is still a need for agents with improved efficacy.
Antibody-drug conjugates (ADCs) have been developed that covalently link cytotoxic agents to tumor-targeting antibodies to enhance their antitumor potency.14,15 This approach is designed to allow for specific delivery of cytotoxic compounds to cells expressing the target antigen, through ADC binding, internalization, and intracellular payload release. Emerging clinical data have demonstrated the potential of ADCs to be novel, highly effective targeted cancer therapies. Brentuximab vedotin (Adcetris, SGN-35), an auristatin-based CD30-targeting conjugate, was granted accelerated approval as a treatment of relapsed Hodgkin lymphoma and systemic anaplastic large-cell lymphoma.16-18 Ado-trastuzumab emtansine (Kadcyla, T-DM1), an ADC consisting of the human epidermal growth factor receptor 2 (HER2)-targeting antibody, trastuzumab, coupled to the maytansinoid, DM1, was recently approved for the treatment of patients with HER2-positive metastatic breast cancer who had received prior treatment with trastuzumab (Herceptin) and a taxane chemotherapy.19-21
Here, we describe a novel CD37-targeting ADC, IMGN529, which combines the intrinsic proapoptotic and immune effector activities of its anti-CD37 antibody component with the cytotoxic potency of its DM1 maytansinoid payload. The resulting ADC demonstrates potent in vitro activity and in vivo efficacy against CD37-positive lymphoma cells, providing the rationale for its clinical development for treatment of B-cell NHL and CLL.
Methods
Cell lines
Ramos, Raji, Daudi, Namalwa, Farage, and RL lymphoma cells were obtained from ATCC. BJAB cells were provided by Dr Elliot Kieff (Harvard University). All other cell lines were obtained from DSMZ. Cells were maintained in RPMI-1640 with 10% fetal bovine serum, 2 mM of glutamine, and 1% penicillin-streptomycin (Life Technologies) at 37°C in a humidified 5% CO2 incubator.
Immunohistochemistry
Immunohistochemical (IHC) studies were conducted on microarrays with formalin-fixed, paraffin-embedded lymphoma tissue sections (US Biomax) after heat-induced antigen retrieval using murine anti-CD37 and anti-CD20 antibodies. Bound antibodies were detected using the avidin-biotin-peroxidase complex (Vector Laboratories) technique and diaminobenzidine (DAKO).
Antibody and conjugate generation
CD37-binding murine monoclonal antibodies were generated by standard hybridoma methodology after immunizations with murine cells transfected with the human CD37 antigen.22 Murine antibodies were humanized by variable domain resurfacing.23 Conjugation of antibodies to the DM1 maytansinoid was performed using the cross-linking agent N-succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) as described previously.24 Rituximab and alemtuzumab were obtained from commercial sources.
Apoptosis induction
Antibodies or conjugates were incubated with 2 × 105 cells/mL in complete medium (RPMI-1640, 10% fetal bovine serum, and 2 mM of l-glutamine) for 20 hours at 37°C. Cells were stained with 5 μL of annexin V-fluorescein isothiocyanate (both Life Technologies) for 15 minutes on ice. Samples were analyzed by flow cytometry on a FACSCalibur flow cytometer with CellQuest Pro (v5.2) for acquisition control and data analysis (both BD Biosciences). Sigmoidal dose-response curves were generated, and half-maximal effective concentration (EC50) values calculated using GraphPad Prism (GraphPad Software, San Diego, CA).
ADCC, ADCP, and CDC assays
Antibody-dependent cell-mediated cytotoxicity (ADCC) activity was measured with 5 × 104 target cells and purified human natural killer (NK) cells by lactate dehydrogenase (LDH) release (Cytotoxicity Detection Kit; Roche) after 4-hour incubation. The percentage of specific lysis was determined as % lysis = (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100.
Antibody-dependent cellular phagocytosis (ADCP) was evaluated using 2 × 105 PKH26-labeled Ramos target cells incubated with antibodies and isolated human monocyte–derived macrophages in round-bottom plates at 37°C for 90 minutes. Samples were subsequently stained with CD11b-APC to identify macrophages, and cell-associated fluorescence of PKH26 (FL2, target cells) and APC (FL4, macrophages) was measured by flow cytometry. The degree of phagocytosis was determined as % phagocytosis = 100 × (# of FL2+/FL4+ events)/(# of FL2+/FL4+ events + # of FL2+/FL4− events).
For complement-dependent cytotoxicity (CDC) assays, antibodies or conjugates were incubated with 5 × 104 target cells/well and 5% human complement (Sigma-Aldrich) for 2 hours. Cell viability was determined by an Alamar Blue assay (Life Technologies). Lipid raft assays were performed as described previously.25
Proliferation assays and cell cycle analysis
In vitro cytotoxicity was measured by incubating 5000 target cells with indicated agents in complete RPMI-1640 media for 5 days at 37°C. The viability of remaining cells was determined by a colorimetric WST-8 assay.26 For cell cycle analysis, cells were incubated with 10 nM of the indicated agents for 20 hours at 37°C followed by propridium iodide (PI) staining and flow cytometry analysis.27 Caspase activation was evaluated by incubating 5000 cells with the indicated agents for 1 day at 37°C followed by analysis using the Caspase-Glo 3/7 assay (Promega) according to the manufacturer’s protocol.
Expression analysis
Cells were incubated in phosphate-buffered saline (PBS), 0.1% sodium azide, and 0.5% bovine serum albumin (Sigma-Aldrich) at 2 × 105 cells/assay with 6 μg/mL of anti–CD37-PE (K7153A antibody labeled with R-phycoerythrin at a 1:1 ratio by standard methods) for 2 hours on ice. Samples were washed, fixed, and analyzed in conjunction with QuantiBRITE beads (BD Biosciences) by flow cytometry to obtain antibodies bound per cell (ABC) values.
In vitro processing
BJAB cells were exposed to IMGN529 prepared with [3H]-labeled DM1 (K7153A-SMCC-[3H]DM1) for 30 minutes at 37°C, washed extensively, and then incubated for 22 hours at 37°C. Cells were harvested by centrifugation and were subjected to acetone extraction to yield a protein-free cell extract. [3H]-Labeled catabolites were quantified by C-18 high-performance liquid chromatography analysis and liquid scintillation counting and were identified by liquid chromatography and mass spectrometry methods.27
In vivo xenograft models
Female CB.17 severe combined immunodeficient (SCID) mice (Charles River Laboratories, Wilmington, MA) were inoculated with 1 × 107 cells above the right flank. Treatment was initiated when tumors reached a volume of ∼100 mm3, and tumor volumes were measured twice weekly.
Cell depletion
All human blood samples were obtained after written informed consent, in accordance with the Declaration of Helsinki, under protocols approved by institutional review boards at each collection site. Fresh buffy coats or whole-blood samples from healthy donors were obtained from Research Blood Components (Brighton, MA). Peripheral blood mononuclear cells (PBMCs) were prepared by standard Ficoll-Paque centrifugation. Frozen PBMCs from patients with CLL were obtained from AllCells (Emeryville, CA). For each sample, 5 × 105 cells were stained for indicated blood cell markers (Miltenyi) together with 16 μg/mL of anti-CD20-PE (BD Biosciences) or 9 μg/mL of anti–CD37-PE for 1 hour on ice in PBS with 20% human AB serum (Sigma-Aldrich). Whole-blood samples were stained to identify granulocytes followed by red blood cell (RBC) lysis. Samples were analyzed in conjunction with QuantiBRITE beads as described for expression analysis.
For in vitro cell depletion, 90 µL of whole blood or PBMCs were incubated with 10 µg/mL of indicated agents for 1 hour at 37°C.28 Samples were costained for blood cell markers and were analyzed by flow cytometry with CountBright absolute counting beads (Life Technologies) to standardize cell counts. RBCs were lysed in whole-blood samples. Depletion was determined as Percent Depletion = 100 × (1 – cell-to-bead ratio of treated sample/cell-to-bead ratio of controls).
Results
CD37 expression in B-cell malignancies
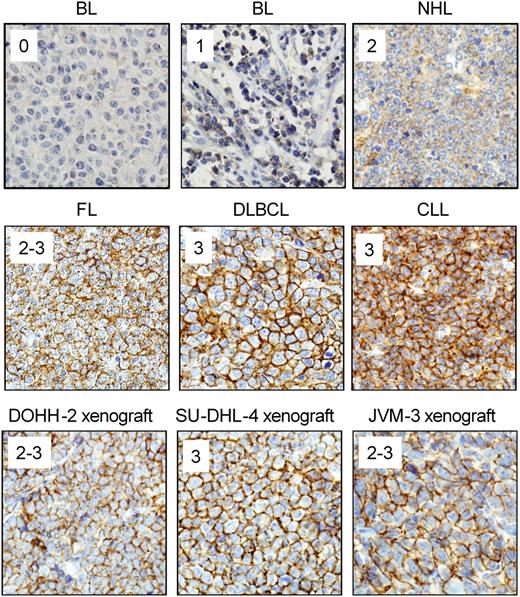
CD37 expression was compared with CD20 expression in tissue biopsies from patients with various B-cell malignancies by IHC. Strong positive staining for CD37 was seen in the vast majority of diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, Burkitt lymphoma, mantle cell lymphoma, and B-cell CLL samples tested (Table 1; Figure 1). In contrast, multiple myeloma and T-cell lymphoma biopsy samples did not show any positive staining for either CD37 or CD20, consistent with previous reports. Overall, NHL biopsy patient samples showed a similar prevalence for CD37 and CD20 staining, suggesting that CD37 is a promising target for development of a novel therapeutic.
CD37 and CD20 staining on paraffin-embedded lymphoma tissue cores by IHC
| Indication . | Histological subtype . | Percentage of positive cores . | n . | |
|---|---|---|---|---|
| CD37 . | CD20 . | |||
| B-cell NHL | Follicular lymphoma | 100 | 100 | 3 |
| DLBCL | 93 | 93 | 14 | |
| MALT lymphoma | 100 | 100 | 3 | |
| Burkitt lymphoma | 75 | 88 | 8 | |
| Mantle cell lymphoma | 90 | 100 | 29 | |
| Unspecified NHL | 95 | 95 | 22 | |
| CLL | 100 | 100 | 3 | |
| Multiple myeloma | 0 | 0 | 10 | |
| T-cell lymphoma | 0 | 0 | 3 | |
| Indication . | Histological subtype . | Percentage of positive cores . | n . | |
|---|---|---|---|---|
| CD37 . | CD20 . | |||
| B-cell NHL | Follicular lymphoma | 100 | 100 | 3 |
| DLBCL | 93 | 93 | 14 | |
| MALT lymphoma | 100 | 100 | 3 | |
| Burkitt lymphoma | 75 | 88 | 8 | |
| Mantle cell lymphoma | 90 | 100 | 29 | |
| Unspecified NHL | 95 | 95 | 22 | |
| CLL | 100 | 100 | 3 | |
| Multiple myeloma | 0 | 0 | 10 | |
| T-cell lymphoma | 0 | 0 | 3 | |
IHC studies were conducted with an anti-CD37 antibody (clone CT1, 4 µg/mL; Novocastra) and an anti-CD20 antibody (clone L26, 0.5 µg/mL; DAKO) compared with isotype controls (Beckman Coulter). Staining results were scored by a board certified pathologist. Staining intensity scoring: 0 = negative; 1 = weak; 2 = moderate; and 3 = strong. Positive staining was defined as a score of ≥2.
MALT, mucosa-associated lymphoid tissue.
Examples of CD37 staining on human lymphoma samples. Sections from paraffin-embedded lymphoma tissue and xenograft tumors were stained with anti-CD37 antibody as described for Table 1 and staining intensity was scored as follows: 0 = negative; 1 = weak; 2 = moderate; and 3 = strong. Lymphoma samples representing each intensity level and sections from xenograft tumor models used in efficacy studies are shown. All images were acquired on an Olympus BX51 light microscope at ×40 magnification with an Olympus DP71 camera and DP Controller software (v3.2.1.276).
Examples of CD37 staining on human lymphoma samples. Sections from paraffin-embedded lymphoma tissue and xenograft tumors were stained with anti-CD37 antibody as described for Table 1 and staining intensity was scored as follows: 0 = negative; 1 = weak; 2 = moderate; and 3 = strong. Lymphoma samples representing each intensity level and sections from xenograft tumor models used in efficacy studies are shown. All images were acquired on an Olympus BX51 light microscope at ×40 magnification with an Olympus DP71 camera and DP Controller software (v3.2.1.276).
Identification of a CD37 antibody with intrinsic activity
We first sought to identify CD37 antibodies with functional properties, similar to those described for therapeutic antibodies targeting other antigens on lymphoma cells. A large panel of CD37-binding murine monoclonal antibodies was generated, candidates were selected based on their functional properties, and subsequently humanized. One of these humanized anti-CD37 antibodies, designated K7153A, exhibited the strongest combined functional activity in apoptosis induction, ADCC, and CDC.
The proapoptotic activity of the K7153A antibody was compared with the anti-CD20 antibody rituximab in the absence of any cross-linking agents. K7153A exhibited similar or enhanced proapoptotic activity compared with rituximab in several lymphoma cell lines. K7153A had strong proapoptotic activity against Ramos, Raji, SU-DHL-6, DOHH-2, and Farage with 35% to 50% annexin V–positive cells (Figure 2A). Although rituximab had similar activity against DOHH-2 and Farage, it was notably less active against other cells.
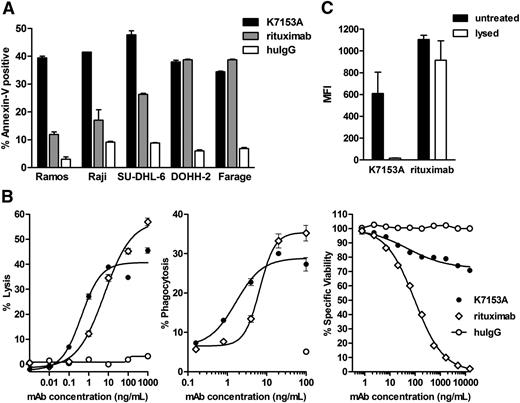
In vitro activity of K7153A antibody on lymphoma cell lines. (A) Induction of apoptosis measured by annexin V-fluorescein isothiocyanate staining after 20-hour incubation with 10 nM of K7153A antibody, rituximab, or nonbinding huIgG control. (B) Effector activity of K7153A compared with rituximab. ADCC activity (left) as determined by LDH release after 4-hour incubation using purified human NK effector cells and Daudi target cells at a 3:1 ratio. ADCP activity (middle) on PKH26-labeled Ramos cells incubated with isolated human monocyte–derived macrophages at a 1:1 ratio at 37°C for 90 minutes. CDC activity (right) against Ramos cells incubated in the presence of 5% human serum for 2 hours and viability measured by an AlamarBlue assay. (C) Lipid raft redistribution assay with Ramos cells incubated with 10 μg/mL of K7153 or rituximab followed by 0.5% Triton X-100 (lysed) for 15 minutes or left untreated. Mean fluorescence intensity of each sample was measured by flow cytometry.
In vitro activity of K7153A antibody on lymphoma cell lines. (A) Induction of apoptosis measured by annexin V-fluorescein isothiocyanate staining after 20-hour incubation with 10 nM of K7153A antibody, rituximab, or nonbinding huIgG control. (B) Effector activity of K7153A compared with rituximab. ADCC activity (left) as determined by LDH release after 4-hour incubation using purified human NK effector cells and Daudi target cells at a 3:1 ratio. ADCP activity (middle) on PKH26-labeled Ramos cells incubated with isolated human monocyte–derived macrophages at a 1:1 ratio at 37°C for 90 minutes. CDC activity (right) against Ramos cells incubated in the presence of 5% human serum for 2 hours and viability measured by an AlamarBlue assay. (C) Lipid raft redistribution assay with Ramos cells incubated with 10 μg/mL of K7153 or rituximab followed by 0.5% Triton X-100 (lysed) for 15 minutes or left untreated. Mean fluorescence intensity of each sample was measured by flow cytometry.
Immune effector activity is an important mechanism of antibody activity against cancer cells, and in particular, ADCC has been implicated in the clinical efficacy of rituximab.29,30 In vitro, K7153A exhibited significant ADCC activity against Daudi lymphoma target cells (Figure 2B, left), similar to the ADCC activity shown by rituximab. Similar results were obtained with Ramos cells (data not shown). K7153A also demonstrated significant ADCP activity via human macrophages against Ramos target cells, comparable to the ADCP activity observed with rituximab (Figure 2B, middle). K71531A exhibited CDC activity against Ramos cells, although to a lesser degree than rituximab (Figure 2B, right), and it did not induce CD37 redistribution to lipid rafts as seen for CD20 after rituximab treatment (Figure 2C).
Antitumor mechanisms of an anti-CD37 ADC
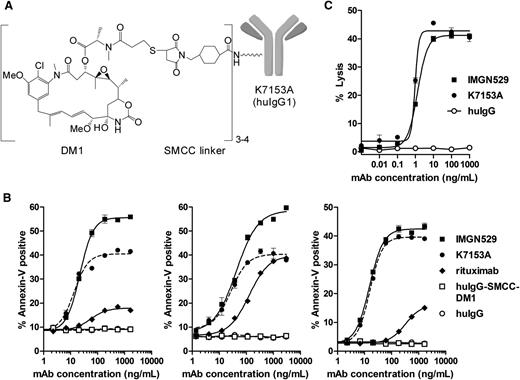
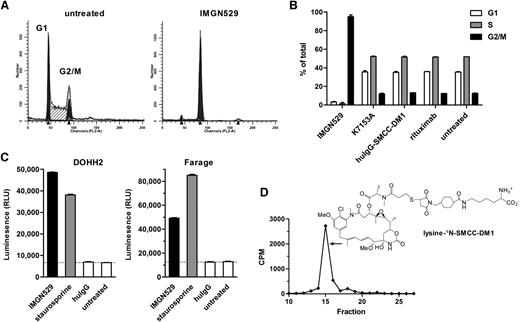
To produce an ADC with potentially enhanced anti-tumor activity, K7153A was coupled on amino groups of lysine residues to the maytansinoid DM1 via the SMCC linker, which forms a stable thioether bond with the sulfhydryl group of DM1 (Figure 3A). The resulting conjugate, termed IMGN529, contains 3 to 4 molecules of DM1 per antibody.
IMGN529 structure and in vitro activity. (A) Representation of the chemical structure of IMGN529, which was produced by conjugation of the DM1 maytansinoid via a thioether formed with the SMCC linker to the humanized CD37-binding K7153A antibody. Approximately 3 to 4 SMCC-DM1 moieties are linked per antibody. (B) Induction of apoptosis after 20-hour incubation with K7153A antibody, IMGN529, or nonbinding controls in Raji (left), DOHH2 (middle), and Ramos (right) cell lines. (C) ADCC activity as determined by LDH release after 4-hour incubation using purified human NK effector cells and Ramos target cells at a 3:1 ratio.
IMGN529 structure and in vitro activity. (A) Representation of the chemical structure of IMGN529, which was produced by conjugation of the DM1 maytansinoid via a thioether formed with the SMCC linker to the humanized CD37-binding K7153A antibody. Approximately 3 to 4 SMCC-DM1 moieties are linked per antibody. (B) Induction of apoptosis after 20-hour incubation with K7153A antibody, IMGN529, or nonbinding controls in Raji (left), DOHH2 (middle), and Ramos (right) cell lines. (C) ADCC activity as determined by LDH release after 4-hour incubation using purified human NK effector cells and Ramos target cells at a 3:1 ratio.
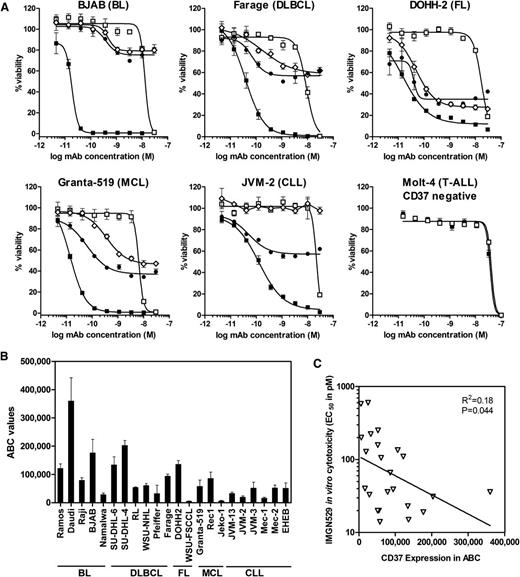
Both the K7153A antibody and the IMGN529 conjugate bound with a similar high affinity to CD37 antigen–positive cells with an EC50 of approximately 0.5 nM measured by flow cytometry (not shown), indicating that conjugation did not appreciably alter antibody-binding affinity. IMGN529 showed similar or enhanced proapoptotic activity relative to K7153A in annexin V assay using Ramos, Raji, and DOHH2 cells (Figure 3B). Nonbinding control antibody or control SMCC-DM1 conjugate had no effect, indicating that antigen binding is required for IMGN529 activity. In addition, IMGN529 showed equivalent ADCC (Figure 3C) and CDC (not shown) activity as K7153A against Ramos cells. These findings indicate that the strong proapoptotic and immune effector activities of K7153A are fully retained in the IMGN529 conjugate. The in vitro cytotoxicity of IMGN529 was tested against a panel of B-lymphoma and B-CLL cell lines compared with the unconjugated K7153A antibody, rituximab, and a nonbinding huIgG-SMCC-DM1 conjugate (Figure 4A). After a 5-day exposure, K7153A antibody treatment reduced cell viability for most cell lines tested with similar or greater direct cytotoxic potency than rituximab, despite the frequently higher expression level of CD20 on these cell lines (data not shown). The cytotoxic potency of the antibody was further enhanced by maytansinoid conjugation in IMGN529. Treatment with IMGN529 reduced cell viability in all antigen-positive cell lines tested in a dose-dependent manner with EC50 values in the range of 10 to 600 pM, whereas the nonbinding control conjugate had an EC50 of ≥10 nM. This corresponds to a specificity window between targeted and nontargeted conjugate activity of between 20- and 1000-fold. In contrast, there was no specificity window between IMGN529 and the nonbinding conjugate against antigen-negative Molt-4 cells (EC50 ≥10 nM for both conjugates), demonstrating that the potent IMGN529 in vitro cytotoxicity is antigen dependent (last panel in Figure 4A). In addition, no effect was seen on antigen-negative Molt-4 cells in coculture experiments with BJAB (data not shown).
IMGN529 has specific cytotoxicity against CD37+ lymphoma cell lines. (A) In vitro cytotoxicity measured by WST-8 assay after 5-day exposure to rituximab (⋄), K7153A antibody (●), IMGN529 (■), or nonbinding huIgG-SMCC-DM1 control conjugate (□). (B) CD37 expression by quantitative flow cytometry expressed as mean ABC values and standard deviation from several independent experiments. (C) EC50 of IMGN529 cytotoxicity plotted against CD37 mean ABC values for the various cell lines listed in panel B. Pearson correlation calculations were performed with 2-tailed P values, and correlation was significant (R2 = 0.18; P = .044).
IMGN529 has specific cytotoxicity against CD37+ lymphoma cell lines. (A) In vitro cytotoxicity measured by WST-8 assay after 5-day exposure to rituximab (⋄), K7153A antibody (●), IMGN529 (■), or nonbinding huIgG-SMCC-DM1 control conjugate (□). (B) CD37 expression by quantitative flow cytometry expressed as mean ABC values and standard deviation from several independent experiments. (C) EC50 of IMGN529 cytotoxicity plotted against CD37 mean ABC values for the various cell lines listed in panel B. Pearson correlation calculations were performed with 2-tailed P values, and correlation was significant (R2 = 0.18; P = .044).
To better understand the relationship between activity and antigen expression, cell lines were evaluated for CD37 levels. CD37 was expressed at significant levels, ranging from 6000 to 360 000 ABC, in the cell lines tested, which were derived from a variety of B-cell lymphomas (Figure 4B). IMGN529 showed potent and specific cytotoxicity against all cell lines within this relatively broad range of CD37 expression levels with a modest correlation between antigen levels and the degree of cytotoxicity in vitro (Figure 4C).
Cellular processing of IMGN529 and impact on cell cycle
Maytansinoids such as DM1 act as antimicrotubule agents, inhibiting tubulin polymerization and suppressing microtubule dynamic instability resulting in the growth arrest of cells in the G2/M phase of the cell cycle and, ultimately, cell death associated with caspase activation.31-33 Likewise, treatment of BJAB cells with IMGN529 led to an increase in the percentage of cells in the G2/M phase of the cell cycle (Figure 5A). This effect was dependent on antigen binding, as no change in cell cycle profile was seen after treatment with a nonbinding SMCC-DM1 conjugate (Figure 5B). Furthermore, this effect is dependent on the maytansinoid component of IMGN529, because the unconjugated K7153A antibody did not have an effect on cell cycle progression (Figure 5B). IMGN529 also induced significant levels of activated caspase-3/-7 in DOHH-2 and Farage cells, after 24-hour incubation (Figure 5C).
IMGN529 conjugate induces G2/M cell cycle arrest. (A) Cell cycle profiles of BJAB cells after treatment with 10 nM of IMGN529 for 20 hours followed by staining with PI. G1 and G2/M peaks indicated in black and S phase as a hatched area. (B) Percentage of BJAB cells in G1, S or G2/M phase after treatment with 10 nM of indicated agents for 20 hours followed by PI staining. Results are shown as mean and standard deviation obtained from duplicate samples. (C) Caspase-3/-7 activity measured in DOHH2 and Farage cells after 1 day of treatment with 10 nM of indicated agents. (D) High-performance liquid chromatography radiogram and structure of the in vitro catabolite of 3H-IMGN529 formed within cells after 22-hour incubation.
IMGN529 conjugate induces G2/M cell cycle arrest. (A) Cell cycle profiles of BJAB cells after treatment with 10 nM of IMGN529 for 20 hours followed by staining with PI. G1 and G2/M peaks indicated in black and S phase as a hatched area. (B) Percentage of BJAB cells in G1, S or G2/M phase after treatment with 10 nM of indicated agents for 20 hours followed by PI staining. Results are shown as mean and standard deviation obtained from duplicate samples. (C) Caspase-3/-7 activity measured in DOHH2 and Farage cells after 1 day of treatment with 10 nM of indicated agents. (D) High-performance liquid chromatography radiogram and structure of the in vitro catabolite of 3H-IMGN529 formed within cells after 22-hour incubation.
Consistent with results observed with other SMCC-DM1 conjugates, we identified a single major intracellular maytansinoid catabolite, lysine-Nε-SMCC-DM1, after incubation of BJAB cells with IMGN529 (Figure 5D).27,31,34 This result indicates that IMGN529 follows the expected pathway of target antigen binding and internalization, followed by lysosomal processing and antibody digestion, which leads to the release of the catabolite, lysine-Nε-SMCC-DM1.
IMGN529 activity in NHL and CLL xenograft models
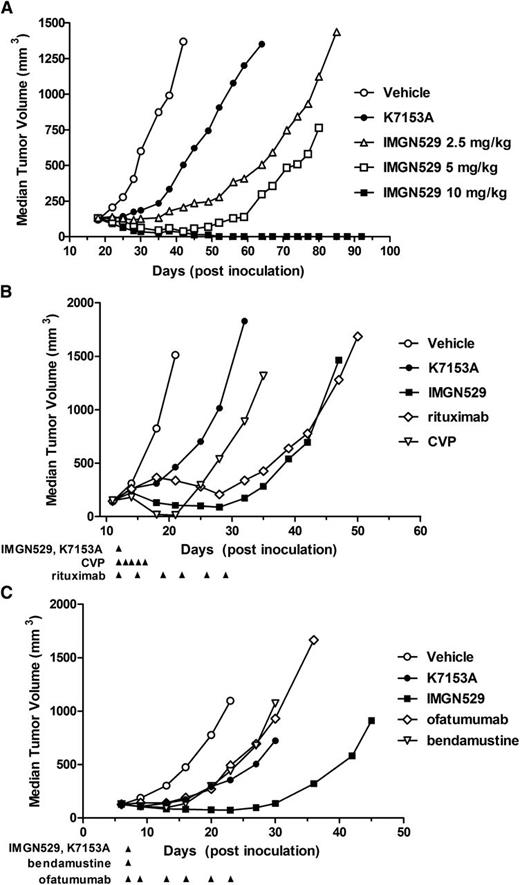
To assess the anti-tumor efficacy of IMGN529 compared with the unconjugated K7153A antibody, subcutaneous xenograft tumors of SU-DHL-4 cells, a DLBCL-derived model, were established in SCID mice (Figure 6A). A single dose of K7153A at 10 mg/kg caused tumor growth inhibition compared with vehicle treatment. IMGN529 caused a more pronounced tumor growth inhibition even at a single dose of 2.5 mg/kg. In addition, at study end (day 121), IMGN529 treatment resulted in 4 and 10 tumor-free survivors in the 5- and 10-mg/kg dose groups, respectively.
In vivo efficacy of IMGN529 in tumor xenograft models. (A) SCID mice (10 mice/group) bearing established SU-DHL-4 xenografts treated with a single intravenous administration on day 15 of PBS (○); 10 mg/kg of K7153A antibody (●); or IMGN529 at doses of 10 mg/kg (■), 5 mg/kg (□), or 2.5 mg/kg (△). (B) SCID mice (10 mice/group) bearing established DOHH-2 xenografts treated starting on day 12 with PBS (○), K7153A antibody (●) at 10 mg/kg × 1, IMGN529 (■) at 10 mg/kg × 1, rituximab (⋄) at 2 mg/kg, 2 qw × 3, or CVP (▽, cyclophosphamide 40 mg/kg × 1 intravenously, vincristine 0.5 mg/kg × 1 intravenously, and prednisone 0.2 mg/kg × 5 pdo). (C) SCID mice bearing established JVM-3 xenografts were treated as indicated starting on day 7 with PBS (○), K7153A antibody (●) at 5 mg/kg × 1), IMGN529 (▪) at 5 mg/kg × 1, ofatumumab (⋄) at 2 mg/kg, 2qw × 3, or bendamustine (▿) at 50 mg/kg. %T/C was calculated as the median tumor volume of each treated (T) group divided by the median tumor volume of the vehicle control (C) group, and activity was determined according to National Cancer Institute standards (%T/C ≤42% = active, %T/C value ≤12% = highly active).
In vivo efficacy of IMGN529 in tumor xenograft models. (A) SCID mice (10 mice/group) bearing established SU-DHL-4 xenografts treated with a single intravenous administration on day 15 of PBS (○); 10 mg/kg of K7153A antibody (●); or IMGN529 at doses of 10 mg/kg (■), 5 mg/kg (□), or 2.5 mg/kg (△). (B) SCID mice (10 mice/group) bearing established DOHH-2 xenografts treated starting on day 12 with PBS (○), K7153A antibody (●) at 10 mg/kg × 1, IMGN529 (■) at 10 mg/kg × 1, rituximab (⋄) at 2 mg/kg, 2 qw × 3, or CVP (▽, cyclophosphamide 40 mg/kg × 1 intravenously, vincristine 0.5 mg/kg × 1 intravenously, and prednisone 0.2 mg/kg × 5 pdo). (C) SCID mice bearing established JVM-3 xenografts were treated as indicated starting on day 7 with PBS (○), K7153A antibody (●) at 5 mg/kg × 1), IMGN529 (▪) at 5 mg/kg × 1, ofatumumab (⋄) at 2 mg/kg, 2qw × 3, or bendamustine (▿) at 50 mg/kg. %T/C was calculated as the median tumor volume of each treated (T) group divided by the median tumor volume of the vehicle control (C) group, and activity was determined according to National Cancer Institute standards (%T/C ≤42% = active, %T/C value ≤12% = highly active).
Next, we evaluated the antitumor activity of IMGN529 relative to existing lymphoma treatments using standard preclinical regimens in subcutaneous xenograft tumors of DOHH2 cells, a follicular lymphoma–derived model (Figure 6B). A multidose regimen of rituximab (2 mg/kg twice weekly × 3) delayed tumor growth in this model. The regimen of cyclophosphamide, vincristine, and prednisone (CVP) resulted in more complete but transient antitumor activity. A single dose of K7153A antibody (10 mg/kg) was moderately active, whereas a single 10-mg/kg dose of IMGN529 resulted in better tumor reduction than the multidose treatment with rituximab and a more durable delay in tumor growth than CVP.
In addition, we compared IMGN529 antitumor activity relative to existing CLL treatments in subcutaneous xenograft tumors of JVM-3 cells, a model derived from B-CLL in prolymphocytoid transformation (Figure 6C). K7153A, ofatumumab, and bendamustine treatment resulted in similar activity in this model. IMGN529 at 5 mg/kg showed greater efficacy with better tumor growth reduction and more sustained growth delay. There was 1 tumor-free survivor at the end of the study (day 111) in each of the groups treated with IMGN529 and ofatumumab.
CD37 antigen expression in these xenograft tumors was determined by IHC, with the same methodology used for lymphoma tumor biopsies to allow for direct comparison of antigen levels. All xenografts showed highly positive staining for CD37 at intensities that were in a similar range to those of lymphoma tumor biopsies (Figure 1), indicating that the levels of CD37 in these models are representative of the levels found in patients with NHL and patients with CLL.
IMGN529 depletes normal and malignant B cells in vitro
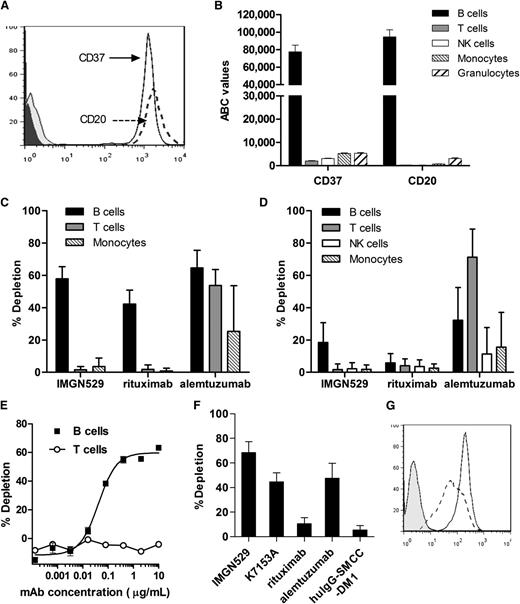
CD37 has been reported to be expressed in human B cells, with low level expression seen in other blood cell types. Because no direct comparison of antigen expression levels is available, we evaluated CD37 expression in peripheral blood cells more precisely by quantitative flow cytometry. CD37 showed a similar degree of staining on human B cells as CD20 (Figure 7A), with CD37 expression levels corresponding to approximately 77 000 ABC, whereas CD20 levels were similar at 95 000 ABC (Figure 7B). CD37 was expressed at much lower levels of ∼2000 to 5000 ABC in T cells, NK cells, monocytes, and granulocytes. No CD37 expression was observed in platelets or RBCs.
IMGN529 specifically depletes normal and malignant human B cells. (A) Histogram of human CD19+ B cells stained with anti-CD37-PE and anti-CD20-PE antibodies. (B) CD37 and CD20 expression levels in blood cells from healthy donors by quantitative flow cytometry (BD QuantiBRITE). Average values from 4 donors are plotted as ABC for B cells (CD19+), T cells (CD3+), NK cells (CD56+), monocytes (CD14+), and granulocytes (CD66abce+). (C) In vitro depletion of human blood cells after 1-hour incubation with 10 μg/mL of indicated compounds from purified PBMCs or (D) whole-blood samples derived from 5 healthy donors. (E) Dose-response curves for depletion of B cells (■) and T cells (○) from PBMCs treated with IMGN529. (F) In vitro depletion of CD19+CD5+ malignant B cells in PBMCs from 4 patients with CLL after 1-hour incubation with 10 μg/mL of indicated compounds (mean ± standard error of the mean is shown). (G) Histogram of CD19+CD5+ malignant B cells in PBMCs from a representative patient with CLL stained with anti-CD37-PE (solid line) and anti-CD20-PE (dashed line) antibodies compared with an isotype control (shaded).
IMGN529 specifically depletes normal and malignant human B cells. (A) Histogram of human CD19+ B cells stained with anti-CD37-PE and anti-CD20-PE antibodies. (B) CD37 and CD20 expression levels in blood cells from healthy donors by quantitative flow cytometry (BD QuantiBRITE). Average values from 4 donors are plotted as ABC for B cells (CD19+), T cells (CD3+), NK cells (CD56+), monocytes (CD14+), and granulocytes (CD66abce+). (C) In vitro depletion of human blood cells after 1-hour incubation with 10 μg/mL of indicated compounds from purified PBMCs or (D) whole-blood samples derived from 5 healthy donors. (E) Dose-response curves for depletion of B cells (■) and T cells (○) from PBMCs treated with IMGN529. (F) In vitro depletion of CD19+CD5+ malignant B cells in PBMCs from 4 patients with CLL after 1-hour incubation with 10 μg/mL of indicated compounds (mean ± standard error of the mean is shown). (G) Histogram of CD19+CD5+ malignant B cells in PBMCs from a representative patient with CLL stained with anti-CD37-PE (solid line) and anti-CD20-PE (dashed line) antibodies compared with an isotype control (shaded).
B-cell depletion is an indication of efficacy for B-cell targeting therapies such as rituximab. In vitro, rituximab has been shown to deplete B cells from human PBMCs.28,35 Likewise, CD52-targeting alemtuzumab (Campath-1H), can deplete lymphocytes in vitro.36,37 To further characterize IMGN529, we evaluated its ability to deplete human blood cells. In PBMC samples from healthy human donors, IMGN529 caused depletion of 60% of B cells but did not deplete T cells or monocytes (Figure 7C). The unconjugated K7153A antibody resulted in a similar effect on PBMC samples from the 2 donors tested (data not shown), suggesting that this activity is mediated at least in part by the intrinsic function of the antibody in this short-term depletion assay. Under identical conditions, rituximab depleted 40% of B cells, which is similar to reported values.35 Alemtuzumab depleted T cells, NK cells, and monocytes along with B cells. In whole-blood samples from 5 different donors, IMGN529 depleted 20% of B cells but had no effect on T cells, NK cells, or monocytes (Figure 7D). In the same assays, rituximab depleted less than 10% of B cells, which is in line with previous reports.35 Alemtuzumab depleted 30% of B cells along with 70% of T cells, 10% of NK cells, and 15% of monocytes. The B-cell depletion activity of IMGN529 was concentration dependent (Figure 7E), with an EC50 of 40 to 60 ng/mL. Importantly, strong B-cell depletion activity was also seen in PBMC samples from 4 patients with CLL, where IMGN529 caused depletion of an average of 70% of CD19+CD5+ malignant B cells, and the unconjugated antibody was able to deplete 45% of malignant B cells (Figure 7F). Malignant B cells in all CLL samples tested were uniformly CD37+ (Figure 7G). In the same experiments, alemtuzumab depleted an average of 48% of malignant B cells, but rituximab depleted only 10% of malignant B cells. This finding indicates that targeting of CD37 on B cells can lead to more potent in vitro B-cell depletion than targeting of CD20 with rituximab but still retains B-cell specificity.
Discussion
The addition of antibody-based therapeutics, such as CD20-targeting rituximab, to traditional chemotherapeutic agents has significantly improved clinical outcomes in B-cell malignancies. Like CD20, the CD37 antigen is widely expressed in all major subtypes of NHL and B-CLL, suggesting that the usefulness of antibody therapy targeting CD37 could be analogous to CD20-directed therapies.
Here, we describe the first CD37-targeting ADC, IMGN529, with potential as a novel therapeutic for B-cell malignancies. We demonstrate that this compound exhibits potent activity against B-cell lymphoma cell lines and xenograft tumors. Furthermore, it specifically and effectively depletes normal and malignant B cells from human blood samples in vitro. This conjugate possesses a unique combination of anticancer activities including proapoptotic and immune-effector functions provided by its anti-CD37 antibody component, and potent cytotoxicity enabled by targeted delivery of its maytansinoid payload. Benchmarked against rituximab, this novel anti-CD37 antibody exhibits similar ADCC and ADCP activity and superior direct proapoptotic activity and cytotoxicity against B-cell lymphoma cell lines. These antibody-directed activities are fully maintained in the context of the maytansinoid conjugate, which has 3 to 4 molecules of DM1 per antibody attached through a thioether linkage to antibody lysine residues. Similarly, ado-trastuzumab emtansine, which uses the same linker and method of DM1 attachment, retains all of the mechanisms of action of trastuzumab, including inhibition of HER2 signaling and ADCC activity.19,38 Thus, conjugation of DM1 by this approach is fully compatible with intrinsic antibody-directed activity and Fc-mediated immune effector function.
As shown for other SMCC-DM1–based conjugates,27 binding and internalization of IMGN529 results in intracellular release of a maytansinoid catabolite, lysine-Nε-SMCC-DM1, and ultimately leads to cell cycle arrest of CD37+ lymphoma cells. Cytotoxicity by IMGN529 was associated with caspase activation, consistent with an apoptotic mechanism of cell death. Although CD37 has not previously been pursued as an ADC target, potentially because of reports describing low antigen internalization,39,40 our results demonstrate that a CD37-targeting antibody-maytansinoid conjugate can indeed be active in vitro and in vivo and that this activity is a result of both antibody-mediated activity and conjugate-dependent functions.
Several B-cell surface antigens are being evaluated as targets for ADC therapy on the basis of their expression in B-cell malignancies but limited presence in normal tissues. SAR3419, an anti-CD19 antibody-maytansinoid conjugate, has shown promising activity in patients with NHL.41 Inotuzumab ozogamicin (CMC-544), an anti-CD22 antibody-calicheamicin conjugate, has advanced into phase 3 clinical trials for NHL and ALL.42,43 Antibody-auristatin conjugates targeting CD22 and CD79b have been reported.44-46 Together with the approval of brentuximab vedotin, this underscores the emerging potential of this class of targeted therapeutics for the treatment of leukemias and lymphomas. In particular, clinical experience with a variety of antibody-maytansinoid conjugates has shown that these conjugates can be safely administered at doses that approach those used for “naked” antibody therapies.47 For example, the maximum tolerated dose of ado-trastuzumab emtansine is 3.6 mg/kg given every 3 weeks, or 2.4 mg/kg weekly.48,49 Therefore, the tolerability of antibody-maytansinoid conjugates offers the prospect of using functional antibody attributes of an ADC candidate, as done here. This concept provides the potential of targeting actively dividing malignant cells via the maytansinoid component acting on microtubules and targeting nondividing malignant cells via antibody-mediated mechanisms.
Next-generation anti-CD20 antibodies have sought to improve efficacy over rituximab by enhancing existing antibody properties, including CDC, ADCC, or proapoptotic activity. In contrast, a therapeutic that incorporates a distinct mechanism of tumor cell killing, such as DM1-mediated inhibition of microtubule dynamics, may act independently of resistance mechanisms that tumor cells possess inherently or develop in response to CD20 antibody therapy.
We found that IMGN529 mediated potent in vitro B-cell depletion, described as a mechanism of action for rituximab. Depletion was restricted to B cells, suggesting that the very low levels of CD37 expression detected in other cells are below the threshold needed for this activity. Importantly, similar to the CD20 antigen, CD37 is not expressed on plasma cells or early progenitor cells, so these cells are most likely spared from any IMGN529-mediated activity as well.
IMGN529 was efficacious against established xenograft tumors derived from cell lines representing the major subtypes of B-cell NHL. As demonstrated by our IHC staining, these xenograft models have CD37 expression levels comparable to or below those found in patient tumor biopsies. In addition, these cell line models represent some of the cytogenetic changes of B-cell malignancies: SU-DHL-4 contains the t(14:18) BCL2-IGH rearrangement seen in DLBCL, DOHH-2 contains both t(14:18) and t(8:14) myc-IGH rearrangements present in “double-hit” lymphomas, and JVM-3 harbors trisomy12 found in CLL. The strong activity of IMGN529 in these models compares favorably to the activity of compounds used to treat patients with NHL and CLL such as rituximab, CVP, ofatumumab, and bendamustine. Overall, the potent activity exhibited by IMGN529 as a result of its multiple mechanisms of action supports its current phase 1 clinical development as the first CD37-targeting ADC therapeutic for the treatment of B-cell malignancies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood commentary on this article in this issue.
Acknowledgments
The authors are grateful to Dr John C. Byrd for helpful discussions. The authors thank all members of the ImmunoGen research team for their contributions to the work presented here.
Authorship
Contribution: J.D. designed the research, analyzed data, and wrote the manuscript; P.U.P. initiated the project and designed the research; S.C., Y.Y., M.L., K.C.L., and M.F.M. performed and analyzed the experiments; and C.N.C., H.K.E., J.P., R.J.L., T.C., and J.M.L. supervised the research and edited the manuscript.
Conflict-of-interest disclosure: P.U.P. is currently employed by Mersana Therapeutics, Inc. M.F.M. is currently employed by AstraZeneca. All remaining authors are employed by and have ownership interest in ImmunoGen, Inc.
The current affiliation for P.U.P. is Biology, Mersana Therapeutics, Inc., Cambridge, MA; and the current affiliation for M.F.M. is Oncology iMED, AstraZeneca, Waltham, MA.
Correspondence: Jutta Deckert, ImmunoGen, Inc., 830 Winter St, Waltham, MA 02451; e-mail: jutta.deckert@immunogen.com.