In this issue of Blood, Hottz et al provide compelling evidence that dengue virus (DV) induces (1) platelet synthesis of interleukin-1β (IL-1β); (2) platelet-derived IL-1β–containing microvesicles (MVs) that increase vascular permeability; and (3) DV-triggered inflammasome activation in platelets.1
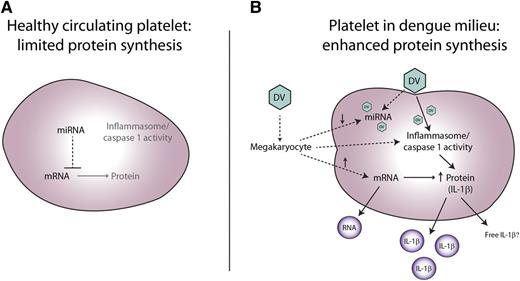
Model of dengue-mediated platelet IL-1β synthesis and release. (A) Model proposes that platelets in healthy state have relatively modest levels of the components of the inflammasome and caspase 1 activity, and limited messenger RNA (mRNA) translation into protein. The low level of translation may be due to microRNA (miRNA) inhibition of translation and/or low mRNA levels. (B) DV induces in vivo platelet translation of mRNA into IL-1β. DV mediates inflammasome-mediated caspase 1 activation, enabling processing into active IL-1β with subsequent release into MVs for systemic transport. Activated platelets also release RNA in vesicles. Because DV replication involves silencing host miRNA production, megakaryocytes may deliver less miRNA for inhibiting platelet mRNA translation, or DV could compete with endogenous miRNAs. In addition, DV may induce increased megakaryocyte delivery of IL-1β mRNA to the platelet. Dashed lines indicate the uncertainty of the early events in the model.
Model of dengue-mediated platelet IL-1β synthesis and release. (A) Model proposes that platelets in healthy state have relatively modest levels of the components of the inflammasome and caspase 1 activity, and limited messenger RNA (mRNA) translation into protein. The low level of translation may be due to microRNA (miRNA) inhibition of translation and/or low mRNA levels. (B) DV induces in vivo platelet translation of mRNA into IL-1β. DV mediates inflammasome-mediated caspase 1 activation, enabling processing into active IL-1β with subsequent release into MVs for systemic transport. Activated platelets also release RNA in vesicles. Because DV replication involves silencing host miRNA production, megakaryocytes may deliver less miRNA for inhibiting platelet mRNA translation, or DV could compete with endogenous miRNAs. In addition, DV may induce increased megakaryocyte delivery of IL-1β mRNA to the platelet. Dashed lines indicate the uncertainty of the early events in the model.
Dengue is a viral disease spread by mosquitos. Although most commonly occurring in the tropics, there has been a worldwide increasing geographic expansion and the World Health Organization considers half the world at risk for infection (http://www.who.int/mediacentre/factsheets/fs117/en/). Severe dengue (previously known as dengue haemorrhagic fever) is relatively common (∼500 000 cases each year), and is characterized by severe bleeding, thrombocytopenia, vascular permeability with plasma leakage and shock, and severe organ impairment. It is the leading cause of death among children in some Asian and Latin American countries. The responsible molecular mechanisms are not well understood, but the proinflammatory cytokine IL-1β is increased in the plasma of patients2 and levels are associated with disease severity. The authors have previously shown that DV causes platelet activation and mitochondrial dysfunction. This manuscript tests the hypothesis that DV-mediated platelet activation induces platelet synthesis and processing of IL-1β, which is capable of disrupting the endothelial cell barrier function.
Using flow cytometry, the authors found that compared with platelets from healthy age- and gender-matched controls, a higher percentage of platelets from patients with dengue were positive for IL-1β. Importantly, when leukocyte-depleted platelets from normal individuals were incubated with DV in vitro, IL-1β levels increased in both platelets and the platelet supernatant. The authors found DV also induced platelet release of IL-1β-containing MVs. The simplest interpretation of these data are that DV induces both platelet translation of IL-1β mRNA into protein and platelet release of IL-1β. The authors collected 36 patients with serologically/molecularly confirmed dengue. Among these, the 16 with clinical signs of vascular permeability had significantly higher IL-1β levels in platelet-derived MVs. When the authors exposed cultured human microvascular endothelial cells to MVs recovered from DV-exposed platelets, they observed a significant increase in permeability to albumin, and this increase was inhibited by IL-1 receptor blockade.
IL-1β is synthesized as an inactive precursor that is cleaved to an active form by caspase 1, but little is known about caspase 1 in platelets. The authors show that compared with control platelets, the platelets from dengue patients have enhanced caspase 1 activation and DV induces caspase 1 processing and activation in normal platelets. How might DV do this? Caspase 1 is activated by inflammasomes, which are multimolecular complexes involved in innate immunity.3 The nucleotide-binding domain, leucine-rich repeat-containing (NLR) proteins define one class of inflammasomes that are activated by diverse agonists, including pathogen, environmental, and self-derived activators. The NRLP3 (also known as cryopyrin) inflammasome requires the adaptor apoptosis-associated speck-like protein containing a caspase recruitment domain to recruit procaspase-1. Although DV is known to activate NLR protein-3 inflammasome in macrophages,4 there had been no information regarding inflammasomes in platelets. The authors not only provide evidence for an NLR protein-3 inflammasome in platelets, but also show DV induces caspase 1 activation and IL-1β activation in a caspase 1-dependent manner. In addition, the DV-induced caspase 1 activation requires functional mitochondria, consistent with reactive oxygen species-mediated NLR protein-3 inflammasome activation in monocytes.5
This interesting study has at least 3 potentially important findings as summarized in the figure. First, these results provide insights into the mechanisms of the most serious complication of a globally important disease, and the data suggest systemic delivery of IL-1β via platelet MVs may contribute, in part, to the abnormal vascular permeability in dengue. Second, these are the first data suggesting the inflammasome is present and functional in human platelets. The authors have just scratched the surface in this regard, and it will be important to advance these findings using additional experimental approaches, since most of the authors’ conclusions are based on the use of small molecule inhibitors and probes. Numerous questions remain, including the following: What is the significance of delivering IL-1β in vesicles as opposed to free in the plasma? How does the IL-1 receptor participate in this process? Does DV induce megakaryocytes to transcribe more mRNA or to repress microRNA biogenesis? Regarding the latter, DV replication involves silencing microRNA biogenesis pathways6 and it may be of interest that platelets express high levels of miR-495, which is predicted to target IL-1β mRNA.
The third important finding pertains to platelet mRNA translation. It is well-established that platelets synthesize IL-1β and release it into MVs when stimulated by LPS in vitro.7 Nevertheless, it has been challenging to show platelet translation occurs in in vivo situations, in part because it is difficult to uncouple translation occurring in a platelet from that occurring in a megakaryocyte. Furthermore, a traditional and conservative view of platelet physiology focuses primarily on hemostasis and pathologic thrombosis – processes in which the major function is over in minutes. This limited view has difficulty integrating in vitro translation experiments that appear to require 1 or more hours. However, infections or other causes of inflammation subject the in vivo platelet to potentially activating stimuli for days on end – ample time for the platelet translation machinery to synthesize proteins. This notion is further supported by the recent demonstration that platelets have increased IL-1β content and appear to be a major source of plasma IL-1β in mice infected with Plasmodium berghei.8 Thus, when considering that platelets from patients with dengue have increased IL-1β content and that DV induces accumulation of IL-1β when incubated with pure platelets, then the most logical explanation is that platelets translate IL-1β mRNA into protein in vivo. Because megakaryocytes and leukocytes were eliminated from the experiments of Hottz et al1 and other potential explanations are far less straightforward, one is reminded of a quote from Sir Arthur Conan Doyle’s greatest creation: “Eliminate all other factors, and the one which remains must be the truth.” Sherlock Holmes, The Sign of the Four.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal