In this issue of Blood, Willems et al describe the dependence of acute myeloid leukemia (AML) cells on glutamine for maintaining protein synthesis downstream of mammalian target of rapamycin (mTOR) and show that the enzyme asparaginase can be used to target this dependence.1 Using various AML cell lines, primary samples, and CD34+ stem cells from healthy donors, the authors support the notion that asparaginase may offer a therapeutic benefit in AML—not from its well-known enzymatic activity, but from its “off-target” effects on glutamine levels that result in inhibition of downstream mTOR signaling, inhibition of protein synthesis, and ultimately loss of viability.
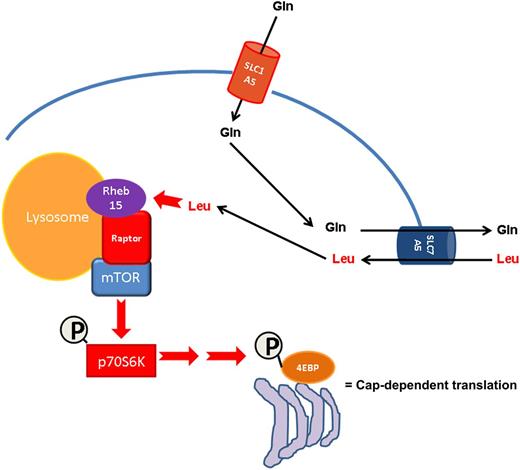
Glutamine depletion by asparaginase indirectly inhibits mTOR activity via decreased leucine uptake in AML. AML cells accumulate intracellular glutamine through the activity of the SLC1A5 transporter. Intracellular glutamine in turn promotes leucine uptake via the SLC7A5 amino acid translocator. Leucine then facilitates Rheb15-dependent mTOR activation at the lysosomal surface resulting in increased cap-dependent mRNA translation. Asparaginase can efficiently inhibit this pathway via deamination of glutamine.
Glutamine depletion by asparaginase indirectly inhibits mTOR activity via decreased leucine uptake in AML. AML cells accumulate intracellular glutamine through the activity of the SLC1A5 transporter. Intracellular glutamine in turn promotes leucine uptake via the SLC7A5 amino acid translocator. Leucine then facilitates Rheb15-dependent mTOR activation at the lysosomal surface resulting in increased cap-dependent mRNA translation. Asparaginase can efficiently inhibit this pathway via deamination of glutamine.
Albeit not without side effects, both clinically available forms of asparaginase (Kydrolase and Erwinase) have demonstrated good pharmacokinetics and documented success for the treatment of pediatric2,3 and adult acute lymphocytic leukemia (ALL)4 as well as some pediatric AML.5 Traditionally, it has been assumed that the therapeutic effects of the enzyme are mediated by reduction of asparagine plasma levels via deamination, although glutamine deamination is also observed clinically.6 Notably, some of the significant side effects of asparaginase therapy such as acute pancreatitis, thrombotic complications, and immunosuppression have been attributed to its glutaminase activity, leading to the pharmaceutical efforts to generate an enzyme that does not affect the metabolism of glutamine.7 Hence, one of the important implications of the study by Willems et al is that, at least in AML cells, glutaminase activity of asparaginase is necessary for its antitumor activity, substantiating the importance to characterize its molecular mechanisms of action.
In light of the reported importance of glutamine for fueling solid tumor growth via its anaplerotic contribution to Krebs cycle oxoglutarate (reviewed in Hensley et al8 ), the findings of Willems et al add valuable insight into another possible mechanism by which glutamine promotes malignant cell survival, namely the regulation of mTOR downstream signaling and protein translation. Although asparaginase has long been reported to inhibit protein synthesis, the mechanism was assumed to depend strictly on asparagine deamination6 —and to a lesser extent, on its glutaminase activity—which would in turn inhibit protein synthesis because of limiting amounts of asparaginyl transfer RNA. The work presented in this issue of Blood supports the intriguing notion that before substrate limitation could take place, the machinery for cap-messenger RNA (mRNA) translation is inactivated via inhibition of the amino acid/mTOR sensing machinery at the lysosomal surface and dephosphorylation of p70S6 kinase (see schematic below). Although it is unclear if glutamine is an ipso facto signal on the amino acid sensing machinery, convincing evidence is provided that reduced intracellular glutamine severely limits leucine uptake, which would in turn hinder Rheb-mediated mTOR activation at the lysosomal surface.9 It is tempting to speculate that mTOR inhibition is triggered to prevent the futile translation of mRNA into polypeptide fragments that would otherwise accumulate and lead to toxicity. In support of this, Willems et al demonstrate that protective autophagy is activated in response to glutamine depletion, and that genetic interference with key autophagic proteins (beclin and ATG5) results in the rapid onset of apoptosis. This key finding may lay the groundwork for future combination studies of asparaginase with inhibitors of autophagy such as the antimalarial agent chloroquine.
Willems et al also identified the glutamine transporter SLC1A5 as the main transporter for glutamine uptake in most AML cell lines and upregulation of glutamine synthetase as a potential resistance mechanism to glutamine ablation in AML cell lines and primary samples. It is unclear if SLC1A5 is the main transporter for glutamine uptake in AML blasts in vivo and if glutamine synthetase will also be upregulated in AML patients that may received asparaginase therapy. Nonetheless, recent evidence suggests that the bone marrow microenvironment—in particular adipocytes—may contribute to asparaginase resistance via, in part, asparagine and glutamine release, and that this resistance mechanism may be more evident in obese patients (at least it is in obese mice).10 This observation may support the notion that patient selection via body mass index could be an important consideration for future selection of the patients who might benefit from introduction of asparaginase into current treatment paradigms for AML.
In conclusion, the results presented by Willems et al in this issue of Blood uncover a novel, anaplerotic-independent role of glutamine in leukemic cells, and support the development of asparaginase—or agents that target glutamine transport into leukemic cells—as a novel therapeutic strategy to inhibit mTOR and downstream cap-dependent mRNA translation in AML. Given the satisfactory clinical track record of Kydrolase and Erwinase in ALL and pediatric AML, these results warrant an evaluation of their clinical efficacy and pharmacodynamic action on mTOR signaling in the context of adult AML. Further preclinical studies are warranted to characterize most potent and safe combinations of asparaginase with chemotherapeutic and targeted clinical modalities in AML to assure its success in this difficult-to-treat disease with depressingly low cure rates.
Conflict-of interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal