Key Points
Presented are results from the phase 2 dose-expansion study of the combination of carfilzomib, lenalidomide, and dexamethasone (CRd).
CRd was well tolerated with robust, rapid, and durable responses.
Abstract
We previously reported a phase 1b dose-escalation study of carfilzomib, lenalidomide, and low-dose dexamethasone (CRd) in relapsed or progressive multiple myeloma where the maximum planned dose (MPD) was carfilzomib 20 mg/m2 days 1 and 2 of cycle 1 and 27 mg/m2 days 8, 9, 15, 16, and thereafter; lenalidomide 25 mg days 1 to 21; and dexamethasone 40 mg once weekly on 28-day cycles. Herein, we present results from the phase 2 dose expansion at the MPD, focusing on the 52 patients enrolled in the MPD cohort. Median follow-up was 24.4 months. In the MPD cohort, overall response rate (ORR) was 76.9% with median time to response of 0.95 month (range, 0.5-4.6) and duration of response (DOR) of 22.1 months. Median progression-free survival was 15.4 months. ORR was 69.2% in bortezomib-refractory patients and 69.6% in lenalidomide-refractory patients with median DOR of 22.1 and 10.8 months, respectively. A median of 9.5 (range, 1-45) carfilzomib cycles were started with 7.7% of patients requiring carfilzomib dose reductions and 19.2% discontinuing CRd due to adverse events (AEs). Grade 3/4 AEs included lymphopenia (48.1%), neutropenia (32.7%), thrombocytopenia (19.2%), and anemia (19.2%). CRd at the MPD was well tolerated with robust, rapid, and durable responses. This trial was registered at clinicaltrials.gov as #NCT00603447.
Introduction
Over the past 15 years, patients with multiple myeloma (MM) have experienced significant gains in survival, yet nearly all patients will eventually relapse and succumb to their disease.1 Triplet combinations with targeted agents (eg, bortezomib with thalidomide or lenalidomide and dexamethasone) have been shown to improve response rates, disease control, and survival outcomes compared with doublet combinations in patients with MM, including those with advanced or high-risk disease.2-5 These combinations, however, have also been associated with increased toxicity.
For instance, in recent phase 1/2 studies, the combination of bortezomib, lenalidomide, and high-dose dexamethasone (RVD) was shown to be highly efficacious in both the newly diagnosed and the relapsed/refractory settings, but there were high rates for some types of adverse events (AEs) that are associated with the individual agents, including neutropenia (lenalidomide) and peripheral neuropathy (bortezomib).6,7 Similarly, in a recent phase 3 study by the European Group for Blood and Marrow Transplantation (EBMT), the addition of bortezomib to the combination of thalidomide and dexamethasone (VTD) significantly improved the primary end point of time to progression compared with the combination of thalidomide and dexamethasone (TD) alone (19.5 vs 13.8 months; P = .001) in patients with relapsed or progressive MM, but was also associated with a higher incidence of grade 3/4 AEs (71% vs 57%; P = .024), particularly grade 3 peripheral neuropathy (29% vs 12%; P = .001) linked to both bortezomib and thalidomide.5
Peripheral neuropathy impacts a patient’s quality of life and can be difficult to manage, leading to dose reductions and/or early treatment discontinuation, resulting in suboptimal treatment. Thus, additional strategies to improve the tolerability and efficacy of myeloma therapy have been developed.8,9
Carfilzomib is a selective proteasome inhibitor that has demonstrated robust and durable activity and a favorable safety and tolerability profile as a single-agent treatment in heavily pretreated patients with relapsed and/or refractory MM in phase 1 and 2 trials.10-15 Grade 3/4 AEs are generally hematologic in nature and manageable with supportive measures or dose modifications. Importantly, across these studies there was no notable association between carfilzomib and neuropathic events. Carfilzomib is approved in the United States to treat patients with MM who received at least 2 prior lines of therapy, including bortezomib and an immunomodulatory agent, and who have experienced disease progression during or within 60 days of completing their last therapy.16
In view of the clinical experience with single-agent carfilzomib in advanced MM coupled with the compelling data from studies utilizing RVD,7 a phase 1b dose-escalation study (PX-171-006) was initiated to determine the safety profile and maximum tolerated dose (MTD) of carfilzomib in combination with lenalidomide and low-dose dexamethasone (CRd) in patients with relapsed or progressive MM (N = 40).17 Results from the dose-escalation study demonstrated that CRd was generally well tolerated at all dose levels and had encouraging activity with an overall response rate (ORR) of 62.5%. Therefore, a phase 2 dose-expansion study was initiated at the identified maximum planned dose (MPD) of CRd (carfilzomib 20/27 mg/m2, lenalidomide 25 mg, and dexamethasone 40 mg). Herein, we present efficacy and safety data of the PX-171-006 study after completion of the phase 2 dose-expansion portion (NCT00603447), including updated data for those patients enrolled in the dose-escalation portion (phase 1b), with a particular focus on the MPD cohort.
Patients and methods
Patients
Patients with symptomatic and measurable MM18 and evidence of relapsed or progressive disease (PD) after 1 to 3 prior lines of therapy were eligible. There were no restrictions on the type of prior treatment, but systemic therapies should have been discontinued for at least 3 weeks and treatment with radiation or immunotherapy for at least 4 weeks. Prior treatment with bortezomib, lenalidomide, or thalidomide was permitted but with certain caveats: for patients previously treated with bortezomib or lenalidomide and whose disease progressed while on therapy, the progression must have occurred 3 months after treatment initiation, and patients must not have discontinued lenalidomide treatment due to toxicity. Refractory disease was defined as achieving less than a minimal response (MR) or progression during prior therapy. Data on disease progression within 60 days of stopping prior treatment were not collected.
Eligibility criteria also included at least a MR to any prior therapy; Eastern Cooperative Oncology Group performance status 0 to 2; a life expectancy of >3 months; and adequate hepatic (bilirubin <2 times the upper limit of normal and alanine aminotransferase <3 times), bone marrow (absolute neutrophil count ≥1000/mm3, hemoglobin ≥8 g/dL, and platelet count ≥50 000/mm3) and renal function (creatinine clearance ≥50 mL/min). During phase 1b, patients previously treated with lenalidomide or bortezomib who had progressed during the first 6 months of treatment with bortezomib or lenalidomide were not eligible for enrollment.17 Additional eligibility criteria for phase 2 included at least a durable MR on any prior therapy. Other specific exclusion criteria included clinically significant neuropathy (grades 3, 4, or grade 2 with pain) at baseline or within 14 days of study entry; and history of significant cardiovascular disease, including congestive heart failure (New York Heart Association class III or IV), symptomatic ischemia, myocardial infarction within the preceding 6 months, or uncontrolled hypertension.
Study design
PX-171-006 was a multicenter, single-arm, open-label, phase 1b/2 study. The primary end point of the phase 1b portion of the study was safety and determination of the MTD or MPD, the results of which have been reported elsewhere.17 The objectives of the phase 2 portion of the study were to assess safety and secondary efficacy end points, including overall response rate (ORR; defined as ≥partial response [PR]), progression-free survival (PFS), and duration of response (DOR). Exploratory end points included the clinical benefit response rate (CBR; defined as ≥MR) and time to response (TTR), defined as time from the first dosing date to the first date of confirmed PR or better. Patients were not followed for survival outcomes after disease progression or after 2 years of follow-up if treatment was discontinued for reasons other than disease progression. The study was approved by review boards at all participating centers and conducted according to the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. All patients provided written informed consent.
Treatment schedule and study agents
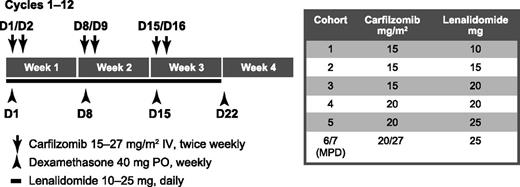
Patients were treated with CRd in 28-day cycles. Figure 1 depicts the dose cohorts of phase 1b and 2. At the MPD, carfilzomib was given intravenously as a 2- to 10-minute infusion on days 1, 2, 8, 9, 15, and 16 at an initial dose of 20 mg/m2 on days 1 and 2 of cycle 1 and then a target dose of 27 mg/m2 thereafter; lenalidomide was dosed orally at 25 mg/day on days 1 to 21; and dexamethasone was given at 40 mg once weekly. An optional 4-mg dose of dexamethasone could be given prior to carfilzomib administration on days 2, 9, and 16 if treatment-related fever or other events occurred any time after the preceding day’s carfilzomib administration. Dose modifications due to toxicity were permitted and could include dose delay until resolution or improvement of toxicity, or dose reductions for carfilzomib (20 and 15 mg/m2) and lenalidomide (20, 15, and 10 mg). Dexamethasone could be reduced to 20 mg once weekly, followed by once every other week, as needed. Once the phase 2 expansion was initiated at the MPD, patients enrolled in the lower-dose cohorts could have their doses escalated to the MPD at the discretion of the investigator and approval of the sponsor provided they had completed at least 4 cycles of CRd. Supportive measures included optional prophylactic allopurinol for patients who were at risk of tumor lysis syndrome. Required supportive measures included antibiotics (ciprofloxacin, amoxicillin, or trimethoprim/sulfamethoxazole during cycle 1 only), antiviral therapy (valacyclovir, famciclovir, or acyclovir), proton pump inhibitors, and anticoagulants (aspirin, low molecular weight heparin, or warfarin as indicated).
Treatment schema. Cycles 13 to 18 (maintenance) carfilzomib dosing modified to days 1, 2, 15, 16. In cohort 6/7, 20 mg/m2 was given on days 1 and 2 during cycle 1 and 27 mg/m2 was given thereafter. D, day; IV, intravenous; MPD, maximum planned dose; PO, orally.
Treatment schema. Cycles 13 to 18 (maintenance) carfilzomib dosing modified to days 1, 2, 15, 16. In cohort 6/7, 20 mg/m2 was given on days 1 and 2 during cycle 1 and 27 mg/m2 was given thereafter. D, day; IV, intravenous; MPD, maximum planned dose; PO, orally.
Patients who achieved at least stable disease (SD) after a total of 4 cycles (inclusive of the phase 1b study) were able to receive up to 8 additional cycles of treatment at their last tolerated doses. Patients who achieved at least SD after a total of 12 cycles could receive up to 6 additional maintenance treatment cycles with a modified carfilzomib dosing schedule (ie, administration on days 1, 2, 15, and 16) at their last tolerated doses. Patients who completed a total of 18 cycles of CRd could continue to receive CRd at the discretion of the investigator and approval of the sponsor or they could continue carfilzomib treatment by enrolling in an extension study (PX-171-010; NCT00884312).19
Assessments
Efficacy was assessed on day 15 of cycle 1 and on day 1 of all subsequent cycles according to disease response defined by the International Myeloma Working Group with the addition of MR per the EBMT.20,21 All response categories required confirmation with 2 consecutive assessments; the exception was patients with only 1 assessment of PD at the time of data cutoff who were assigned PD as best response. Safety was assessed by monitoring AEs, laboratory values, vital signs, and electrocardiograms throughout the study. Grading of AEs was performed according to Common Terminology Criteria for AEs (version 3.0).22 Serious AEs included those events that resulted in death or were life threatening, required hospitalization, or resulted in significant or persistent disability. Cytogenetic abnormalities were assessed from baseline samples by classic metaphase karyotyping and fluorescence in situ hybridization (FISH). High-risk abnormalities included deletion (del) 13 by metaphase cytogenetics and translocation t(4;14); t(14;16), or del(17p13) by FISH.23 All other abnormalities or the absence of abnormalities were considered standard risk.
Statistical analyses
Approximately 30 patients were planned for enrollment into the phase 2 dose-expansion portion of the study. Descriptive statistics were used to summarize categorical and continuous data variables. Analysis of efficacy and safety data were conducted for all enrolled patients who received at least 1 dose of carfilzomib, lenalidomide, or dexamethasone. Time-to-event end points were estimated by the Kaplan-Meier method with determination of medians and 95% confidence intervals. Follow-up was estimated by the reverse Kaplan-Meier method.24 Results are reported for the subgroup of patients who received the MPD (cohorts 6 and 7) and, for context, from the overall study population.
Results
Patients
This study was initiated in June 2008 with enrollment completed in February 2010. Data cutoff was May 8, 2013. Forty patients were enrolled in phase 1b with 8 treated at the MPD.17 Because of rapid, multicenter accrual, more patients were enrolled in the expansion phase than initially projected, with 44 patients enrolled in the dose-expansion portion of the phase 2 study and treated at the MPD. Taken together, the MPD cohort included a total of 52 patients, and the overall study population included a total of 84 patients.
In the MPD cohort, the median age was 63.0 years (Table 1). The median time since diagnosis was 3.1 years (range, 0-16) and the median number of prior therapies was 3 (range, 1-5). Of note, a small number of patients with 4 or 5 prior therapies (n = 9 overall) were enrolled due to an updated and refined application of the International Myeloma Working Group criteria.25 High-risk cytogenetics/FISH abnormalities were present in 21.2% of patients at baseline. Over three-quarters (80.8%) of patients had received prior treatment with bortezomib, while 73.1% had received lenalidomide. Furthermore, 25.0% were refractory (defined as ≤25% response or progression during therapy) to bortezomib, 44.2% were refractory to lenalidomide, and 26.9% were lenalidomide naïve. In addition, 55.8% had received transplantation (1 patient with an allogeneic transplant). Baseline characteristics of the MPD cohort were generally consistent with the overall study population.
Baseline characteristics
| . | MPD cohort (n = 52) . | Overall (N = 84) . |
|---|---|---|
| Age (y), median (range) | 63.0 (44-86) | 61.5 (43-86) |
| Gender, n (%) | ||
| Male | 31 (59.6) | 48 (57.1) |
| Female | 21 (40.4) | 36 (42.9) |
| ECOG Performance Status, n (%) | ||
| 0 | 25 (48.1) | 33 (39.3) |
| 1 | 23 (44.2) | 46 (54.8) |
| 2 | 4 (7.7) | 5 (6.0) |
| Heavy chain, n (%) | ||
| IgG | 29 (55.8) | 52 (61.9) |
| IgA | 13 (25.0) | 19 (22.6) |
| Time since diagnosis (y), median (range)* | 3.1 (0-16) | 3.1 (0-22) |
| Cytogenetics/FISH, n (%) | ||
| Standard risk | 40 (76.9) | 57 (67.9) |
| High risk† | 11 (21.2) | 22 (26.2) |
| Unknown | 1 (1.9) | 5 (6.0) |
| Prior lines of therapy, median (range) | 3 (1–5) | 2 (1–5) |
| Prior therapy, n (%)‡ | ||
| Corticosteroid | 50 (96.2) | 82 (97.6) |
| Bortezomib | 42 (80.8) | 65 (77.4) |
| Lenalidomide | 38 (73.1) | 59 (70.2) |
| Thalidomide | 24 (46.2) | 39 (46.4) |
| Bortezomib and lenalidomide | 31 (59.6) | 49 (58.3) |
| Bortezomib and lenalidomide or thalidomide | 38 (73.1) | 59 (70.2) |
| Alkylating agent | 37 (71.2) | 61 (72.6) |
| Anthracycline | 20 (38.5) | 29 (34.5) |
| Transplant | 29 (55.8)§ | 54 (64.3)§ |
| . | MPD cohort (n = 52) . | Overall (N = 84) . |
|---|---|---|
| Age (y), median (range) | 63.0 (44-86) | 61.5 (43-86) |
| Gender, n (%) | ||
| Male | 31 (59.6) | 48 (57.1) |
| Female | 21 (40.4) | 36 (42.9) |
| ECOG Performance Status, n (%) | ||
| 0 | 25 (48.1) | 33 (39.3) |
| 1 | 23 (44.2) | 46 (54.8) |
| 2 | 4 (7.7) | 5 (6.0) |
| Heavy chain, n (%) | ||
| IgG | 29 (55.8) | 52 (61.9) |
| IgA | 13 (25.0) | 19 (22.6) |
| Time since diagnosis (y), median (range)* | 3.1 (0-16) | 3.1 (0-22) |
| Cytogenetics/FISH, n (%) | ||
| Standard risk | 40 (76.9) | 57 (67.9) |
| High risk† | 11 (21.2) | 22 (26.2) |
| Unknown | 1 (1.9) | 5 (6.0) |
| Prior lines of therapy, median (range) | 3 (1–5) | 2 (1–5) |
| Prior therapy, n (%)‡ | ||
| Corticosteroid | 50 (96.2) | 82 (97.6) |
| Bortezomib | 42 (80.8) | 65 (77.4) |
| Lenalidomide | 38 (73.1) | 59 (70.2) |
| Thalidomide | 24 (46.2) | 39 (46.4) |
| Bortezomib and lenalidomide | 31 (59.6) | 49 (58.3) |
| Bortezomib and lenalidomide or thalidomide | 38 (73.1) | 59 (70.2) |
| Alkylating agent | 37 (71.2) | 61 (72.6) |
| Anthracycline | 20 (38.5) | 29 (34.5) |
| Transplant | 29 (55.8)§ | 54 (64.3)§ |
ECOG, Eastern Cooperative Oncology Group; IgA, immunoglobulin A; IgG, immunoglobulin G; MPD, maximum planned dose.
Data unavailable for 1 patient.
del 13 by metaphase cytogenetics or t(4;14); t(14;16), or del(17p13) by FISH.
Exposure to multiple drugs was not necessarily concurrent.
1 patient had received allogeneic transplant.
Study treatment disposition and dosing
In the MPD cohort, the median number of carfilzomib cycles started was 9.5 (range, 1-45), and a median of 8.5 and 9 cycles (range, 1-44) were started for lenalidomide and dexamethasone, respectively. Of the 52 patients in the MPD cohort, 19 (36.5%) completed more than 12 cycles of CRd and 13 (25%) completed more than 18 cycles, including maintenance treatment (Table 2). At data cutoff, 2 patients (3.8%) remained on CRd treatment. Four patients completed at least 18 cycles and continued treatment on PX-171-010. Twenty-six patients (50.0%) discontinued treatment due to disease progression, 10 (19.2%) due to AEs, 7 (13.5%) opted to proceed to stem cell collection/transplantation (n = 6) or maintenance (n = 1), and 5 (9.6%) because of patient/physician preference, 1 (1.9%) due to noncompliance, and 1 (1.9%) patient withdrew consent. The rates and reasons for discontinuations between the MPD cohort and overall study population were generally consistent.
Study treatment disposition
| . | MPD cohort (n = 52) . | Overall (N = 84) . |
|---|---|---|
| Number of CRd cycles received, n (%) | ||
| ≤12 | 33 (63.5) | 56 (66.7) |
| 13-18 | 6 (11.5) | 10 (11.9) |
| >18 | 13 (25.0) | 18 (21.4) |
| CRd discontinued, n (%) | 50 (96.2) | 81 (96.4) |
| Progressive disease | 26 (50.0) | 43 (51.2) |
| Adverse event | 10 (19.2) | 15 (17.9) |
| Withdrew consent | 1 (1.9) | 4 (4.8) |
| Completed treatment per protocol | — | 2 (2.4) |
| Other* | 13 (25.0) | 17 (20.2) |
| CRd active | 2 (3.8) | 3 (3.6) |
| . | MPD cohort (n = 52) . | Overall (N = 84) . |
|---|---|---|
| Number of CRd cycles received, n (%) | ||
| ≤12 | 33 (63.5) | 56 (66.7) |
| 13-18 | 6 (11.5) | 10 (11.9) |
| >18 | 13 (25.0) | 18 (21.4) |
| CRd discontinued, n (%) | 50 (96.2) | 81 (96.4) |
| Progressive disease | 26 (50.0) | 43 (51.2) |
| Adverse event | 10 (19.2) | 15 (17.9) |
| Withdrew consent | 1 (1.9) | 4 (4.8) |
| Completed treatment per protocol | — | 2 (2.4) |
| Other* | 13 (25.0) | 17 (20.2) |
| CRd active | 2 (3.8) | 3 (3.6) |
Includes patient/physician preference, patients proceeding to stem cell collection/transplantation or maintenance therapy, and noncompliance.
Dose reduction due to AEs was infrequent for carfilzomib (7.7%). The rate of dose reductions for any reason was 34.6% for lenalidomide and 42.3% for dexamethasone. Most patients in the MPD cohort missed at least one dose of carfilzomib (65.4%), lenalidomide (75.0%), and dexamethasone (63.5%). The average dose per administration among patients in the MPD cohort was a median of 26.7 mg/m2 for carfilzomib, 25.0 mg for lenalidomide, and 40.0 mg for dexamethasone (supplemental Table 1, available on the Blood Web site).
Efficacy
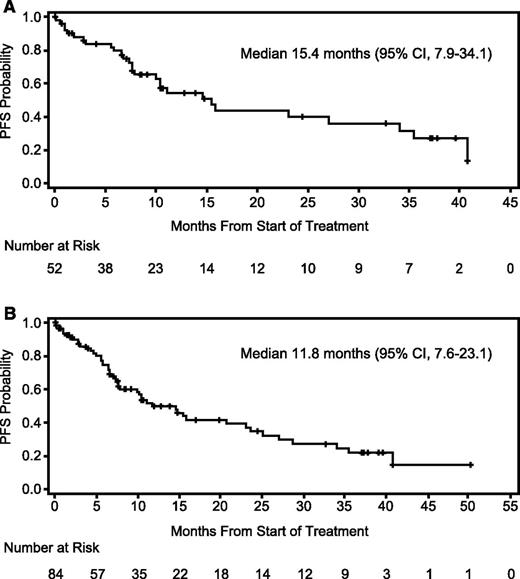
Response data are summarized in Table 3. In the MPD cohort, the ORR was 76.9% with a median time to response of 0.95 month (range, 0.5-4.6) and median DOR of 22.1 months (95% confidence interval [95% CI], 9.5-38.0). After a median follow-up of 24.4 months (95% CI, 10.6-37.3), median PFS was 15.4 months (95% CI, 7.9–34.1) (Figure 2). In post-hoc subset analyses of the MPD cohort, the ORR in bortezomib-refractory patients (n = 13) was 69.2% with a median DOR of 22.1 months (95% CI, 5.8-35.0) and median PFS of 15.4 months (95% CI, 1.2-27.0). In patients refractory to lenalidomide (n = 23), ORR was 69.6%, median DOR 10.8 months (95% CI, 6.1 to not estimable [NE]), and median PFS 7.9 months (95% CI, 6.6-34.1). Also of note, the ORR was 85.7% in the lenalidomide-naïve subgroup (n = 14), and the median DOR had not been reached (95% CI, 5.3-NE), nor had the median PFS (95% CI, 7.3-NE).
Response results
| . | MPD cohort (n = 52) . | Overall (N = 84) . |
|---|---|---|
| Best response, n (%) | ||
| Stringent complete response | 2 (3.8) | 3 (3.6) |
| Complete response | 1 (1.9) | 1 (1.2) |
| Very good partial response | 19 (36.5) | 30 (35.7) |
| Partial response | 18 (34.6) | 24 (28.6) |
| Minimal response | 0 (0) | 6 (7.1) |
| Stable disease | 3 (5.8) | 7 (8.3) |
| Progressive disease | 5 (9.6) | 6 (7.1) |
| Not evaluable | 4 (7.7) | 7 (8.3) |
| Overall response rate, n (%)* | 40 (76.9) | 58 (69.0) |
| Median time to response, months (range) | 0.95 (0.5-4.6) | 0.95 (0.5-29.9) |
| Median duration, months (95% CI)† | 22.1 (9.5-38.0) | 18.8 (9.7-33.4) |
| Clinical benefit rate, n (%)‡ | 40 (76.9) | 64 (76.2) |
| Median duration, months (95% CI)† | 22.6 (9.5-39.8) | 18.8 (9.7-26.1) |
| . | MPD cohort (n = 52) . | Overall (N = 84) . |
|---|---|---|
| Best response, n (%) | ||
| Stringent complete response | 2 (3.8) | 3 (3.6) |
| Complete response | 1 (1.9) | 1 (1.2) |
| Very good partial response | 19 (36.5) | 30 (35.7) |
| Partial response | 18 (34.6) | 24 (28.6) |
| Minimal response | 0 (0) | 6 (7.1) |
| Stable disease | 3 (5.8) | 7 (8.3) |
| Progressive disease | 5 (9.6) | 6 (7.1) |
| Not evaluable | 4 (7.7) | 7 (8.3) |
| Overall response rate, n (%)* | 40 (76.9) | 58 (69.0) |
| Median time to response, months (range) | 0.95 (0.5-4.6) | 0.95 (0.5-29.9) |
| Median duration, months (95% CI)† | 22.1 (9.5-38.0) | 18.8 (9.7-33.4) |
| Clinical benefit rate, n (%)‡ | 40 (76.9) | 64 (76.2) |
| Median duration, months (95% CI)† | 22.6 (9.5-39.8) | 18.8 (9.7-26.1) |
MPD, maximum planned dose.
Partial response or better.
Estimated by the Kaplan-Meier method.
Minimal response or better.
Progression-free survival. (A) PFS in the MPD cohort (n = 52). Twelve patients were censored for PFS because of alternate treatment (6 patients had achieved at least a partial response and decided to pursue other treatment). (B) PFS in the overall study population (N = 84). Nineteen patients were censored for PFS because of alternate treatment (7 patients had achieved at least a partial response and decided to pursue other treatment).
Progression-free survival. (A) PFS in the MPD cohort (n = 52). Twelve patients were censored for PFS because of alternate treatment (6 patients had achieved at least a partial response and decided to pursue other treatment). (B) PFS in the overall study population (N = 84). Nineteen patients were censored for PFS because of alternate treatment (7 patients had achieved at least a partial response and decided to pursue other treatment).
The efficacy data for the MPD cohort compared favorably to the overall population where the ORR was 69.0%, median DOR was 18.8 months (95% CI, 9.7-33.4), and median PFS was 11.8 months (95% CI, 7.6-23.1). In the lenalidomide-refractory subgroup (n = 30), the ORR was 60.0%, the median DOR was 13.8 months (6.1-NE) and the median PFS was 7.9 months (95% CI, 5.6-15.4). The ORR was 80.0% in the lenalidomide-naïve subgroup (n = 25), the median DOR was 21.4 months (95% CI, 21.1-NE), and the median PFS was 28.7 months (95% CI, 23.7-NE).
Safety
All patients in the MPD cohort experienced at least 1 treatment-emergent AE of any grade, 94.2% experienced a grade 3/4 event, and 28 patients (53.8%) experienced AEs that were deemed serious. The types and incidence of AEs were generally consistent between the MPD cohort and the overall study population. Table 4 summarizes the incidence of treatment-emergent AEs.
Treatment-emergent adverse events occurring in ≥25% of patients for any grade or ≥5% for grade 3/4 in the MPD cohort or overall study population
| . | MPD cohort n = 52 . | Overall N = 84 . | ||
|---|---|---|---|---|
| Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | |
| Hematologic events | ||||
| Neutropenia | 19 (36.5) | 17 (32.7) | 38 (45.2) | 32 (38.1) |
| Lymphopenia | 27 (51.9) | 25 (48.1) | 34 (40.5) | 32 (38.1) |
| Anemia | 17 (32.7) | 10 (19.2) | 32 (38.1) | 16 (19.0) |
| Thrombocytopenia | 16 (30.8) | 10 (19.2) | 29 (34.5) | 21 (25.0) |
| Leukopenia | 11 (21.2) | 6 (11.5) | 17 (20.2) | 11 (13.1) |
| Nonhematologic events | ||||
| Fatigue | 36 (69.2) | 6 (11.5) | 55 (65.5) | 6 (7.1) |
| Diarrhea | 30 (57.7) | 3 (5.8) | 47 (56.0) | 5 (6.0) |
| Cough | 21 (40.4) | 1 (1.9) | 35 (41.7) | 1 (1.2) |
| Upper respiratory tract infection | 23 (44.2) | 1 (1.9) | 34 (40.5) | 2 (2.4) |
| Pyrexia | 23 (44.2) | 0 (0) | 33 (39.3) | 0 (0) |
| Muscle spasms | 20 (38.5) | 2 (3.8) | 31 (36.9) | 2 (2.4) |
| Dyspnea | 19 (36.5) | 0 (0) | 30 (35.7) | 1 (1.2) |
| Peripheral edema | 19 (36.5) | 0 (0) | 28 (33.3) | 1 (1.2) |
| Back pain | 16 (30.8) | 1 (1.9) | 27 (32.1) | 2 (2.4) |
| Insomnia | 18 (34.6) | 0 (0) | 26 (31.0) | 0 (0) |
| Nausea | 18 (34.6) | 0 (0) | 26 (31.0) | 0 (0) |
| Hypophosphatemia | 20 (38.5) | 13 (25.0) | 25 (29.8) | 18 (21.4) |
| Hyperglycemia | 14 (26.9) | 4 (7.7) | 23 (27.4) | 11 (13.1) |
| Constipation | 14 (26.9) | 0 (0) | 22 (26.2) | 0 (0) |
| Hypokalemia | 13 (25.0) | 5 (9.6) | 22 (26.2) | 8 (9.5) |
| Arthralgia | 13 (25.0) | 1 (1.9) | 21 (25.0) | 1 (1.2) |
| Pain in extremity | 13 (25.0) | 0 (0) | 21 (25.0) | 0 (0) |
| Peripheral neuropathy* | 14 (26.9) | 1 (1.9)† | 18 (21.4) | 1 (1.2)† |
| Pneumonia | 9 (17.3) | 5 (9.6) | 13 (15.5) | 6 (7.1) |
| Hyponatremia | 8 (15.4) | 5 (9.6) | 14 (16.7) | 9 (10.7) |
| Alanine aminotransferase increased | 7 (13.5) | 4 (7.7) | 10 (11.9) | 5 (6.0) |
| Hemoglobin decreased | 4 (7.7) | 4 (7.7) | 6 (7.1) | 5 (6.0) |
| . | MPD cohort n = 52 . | Overall N = 84 . | ||
|---|---|---|---|---|
| Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | |
| Hematologic events | ||||
| Neutropenia | 19 (36.5) | 17 (32.7) | 38 (45.2) | 32 (38.1) |
| Lymphopenia | 27 (51.9) | 25 (48.1) | 34 (40.5) | 32 (38.1) |
| Anemia | 17 (32.7) | 10 (19.2) | 32 (38.1) | 16 (19.0) |
| Thrombocytopenia | 16 (30.8) | 10 (19.2) | 29 (34.5) | 21 (25.0) |
| Leukopenia | 11 (21.2) | 6 (11.5) | 17 (20.2) | 11 (13.1) |
| Nonhematologic events | ||||
| Fatigue | 36 (69.2) | 6 (11.5) | 55 (65.5) | 6 (7.1) |
| Diarrhea | 30 (57.7) | 3 (5.8) | 47 (56.0) | 5 (6.0) |
| Cough | 21 (40.4) | 1 (1.9) | 35 (41.7) | 1 (1.2) |
| Upper respiratory tract infection | 23 (44.2) | 1 (1.9) | 34 (40.5) | 2 (2.4) |
| Pyrexia | 23 (44.2) | 0 (0) | 33 (39.3) | 0 (0) |
| Muscle spasms | 20 (38.5) | 2 (3.8) | 31 (36.9) | 2 (2.4) |
| Dyspnea | 19 (36.5) | 0 (0) | 30 (35.7) | 1 (1.2) |
| Peripheral edema | 19 (36.5) | 0 (0) | 28 (33.3) | 1 (1.2) |
| Back pain | 16 (30.8) | 1 (1.9) | 27 (32.1) | 2 (2.4) |
| Insomnia | 18 (34.6) | 0 (0) | 26 (31.0) | 0 (0) |
| Nausea | 18 (34.6) | 0 (0) | 26 (31.0) | 0 (0) |
| Hypophosphatemia | 20 (38.5) | 13 (25.0) | 25 (29.8) | 18 (21.4) |
| Hyperglycemia | 14 (26.9) | 4 (7.7) | 23 (27.4) | 11 (13.1) |
| Constipation | 14 (26.9) | 0 (0) | 22 (26.2) | 0 (0) |
| Hypokalemia | 13 (25.0) | 5 (9.6) | 22 (26.2) | 8 (9.5) |
| Arthralgia | 13 (25.0) | 1 (1.9) | 21 (25.0) | 1 (1.2) |
| Pain in extremity | 13 (25.0) | 0 (0) | 21 (25.0) | 0 (0) |
| Peripheral neuropathy* | 14 (26.9) | 1 (1.9)† | 18 (21.4) | 1 (1.2)† |
| Pneumonia | 9 (17.3) | 5 (9.6) | 13 (15.5) | 6 (7.1) |
| Hyponatremia | 8 (15.4) | 5 (9.6) | 14 (16.7) | 9 (10.7) |
| Alanine aminotransferase increased | 7 (13.5) | 4 (7.7) | 10 (11.9) | 5 (6.0) |
| Hemoglobin decreased | 4 (7.7) | 4 (7.7) | 6 (7.1) | 5 (6.0) |
Data are n (%) of patients.
MPD, maximum planned dose.
Includes peripheral neuropathy, neuropathy, peripheral sensory neuropathy, peripheral motor neuropathy.
Grade 3.
In the MPD cohort, the most common nonhematologic AEs of any grade were fatigue (69.2%) and diarrhea (57.7%), and the most common hematologic AEs were lymphopenia (51.9%), neutropenia (36.5%), and anemia (32.7%). Grade 3/4 events were generally hematologic in nature and included lymphopenia (48.1%), neutropenia (32.7%), thrombocytopenia (19.2%), and anemia (19.2%). Rash was experienced by 19.2% of patients for any grade and 1.9% for grade 3/4. Four patients (7.7%) experienced grade 3/4 thromboembolic events. Treatment-emergent peripheral neuropathy occurred in 14 patients (26.9%), with 1 patient (1.9%) experiencing a grade 3 event and no grade 4 events. The most frequent serious AEs in the MPD cohort included pneumonia (5 patients, 9.6%) and gastrointestinal hemorrhage (2 patients, 3.8%). Serious AEs of a hematologic nature occurred in 5.8% of patients. The most common AEs associated with study drug discontinuation included abdominal pain, nausea, and fatigue (2 patients [3.8%] for each).
Collectively, cardiac AEs of any grade occurred in 10 patients (19.2%) in MPD cohort with 3 patients experiencing events ≥grade 3 in severity. Overall, 16 patients (19.0%) experienced cardiac AEs across cohorts with 6 patients (7.1%) experiencing events ≥grade 3. Individual types of AEs of grade 3/4 were infrequent and did not occur in more than 1 patient, including acute myocardial infarction, myocardial infarction, bradycardia, tachycardia, sick sinus syndrome, and coronary artery disease. One patient in the MPD cohort experienced a grade 5 cardiac arrest that was deemed unrelated to study treatment, and none of the ≥grade 3 cardiac events were considered related to carfilzomib. Overall, 1 patient experienced a ≥grade 3 cardiac event (grade 3 sick sinus syndrome) that was considered possibly related to carfilzomib.
Three deaths occurred during treatment or within 30 days of discontinuation, all in the MPD cohort, with progressive disease the primary cause of death in all 3. In 1 of these patients, a secondary cause of death (colonic stenosis) was deemed possibly related to study treatment by the investigator. This patient died 28 days after discontinuation of CRd and 2 days after surgery for colonic dilatation.
Discussion
Results from this study demonstrated that the CRd combination was highly active in patients with relapsed or progressive MM who had received 1-3 prior therapies. At the MPD of 20/27 mg/m2 of carfilzomib, more than three-quarters of patients (76.9%) achieved at least a partial response, despite 25.0% and 44.2% being refractory to bortezomib and lenalidomide, respectively. Importantly, these responses were both rapid and durable with 50% of patients in the MPD cohort achieving ≥PR within 0.95 month with a median DOR of 22.1 months. Additionally, the ORR of 80% and median PFS of 28.7 months from the lenalidomide subgroup is particularly encouraging. Notably, the efficacy results of the MPD cohort compared favorably to the overall population; in particular, the ORR of 76.9% at the MPD compared with 62.5% for the phase 1b portion (N = 40) of the study overall and 56.3% for patients in the phase 1b portion receiving CRd at doses lower than the MPD (n = 32).17
At the MPD, CRd was tolerable with manageable AEs, with the most common grade 3/4 AEs being hematologic in nature. Nonhematologic AEs, such as fatigue and diarrhea, were generally grade 1/2 in severity. Dose reductions of carfilzomib due to AEs were infrequent. Discontinuation of CRd due to an AE was moderate at 19.2% and not associated with a specific type of AE. Importantly, the low rate of grade 3 neuropathy and the absence of grade 4 neuropathic events during the present study are consistent with single-agent carfilzomib studies.10,12,13 Generally, the AE profile of the phase 2 portion of the study was consistent with the phase 1b portion17 and the types of AEs were consistent with studies of single-agent carfilzomib in advanced MM at similar dosing26 and studies of lenalidomide (25 mg) with high-dose dexamethasone (RD).27,28
The efficacy and safety data of CRd reported herein are generally consistent with findings from studies with other triplet combinations of a proteasome inhibitor, IMiD, and dexamethasone in the relapsed and/or refractory setting.5-7 As noted earlier, VTD significantly improved time to progression compared with TD in the phase 3 EBMT study but was also associated with increased rates of grade 3 peripheral neuropathy (29% vs 12%), grade 3/4 infection (14% vs 7%) and thrombocytopenia (17% vs 7%), and discontinuation due to AEs was higher (28% vs 9%). It is worth noting that prior treatment with bortezomib was relatively low (20%) as was prior use of IMiDs (8% for thalidomide), which may in part explain the CR rates in this study (25% for VTD vs 14% for TD; P = .024). In the current study, the CR rate was 4.8% overall (5.8% in the MPD cohort), but the majority of patients had prior treatment with bortezomib (77%), and 46% had received prior treatment with thalidomide and 70% with lenalidomide. Also, standard doses of bortezomib (1.3 mg/m2) and thalidomide (200 mg) were used for VTD, which likely explains the high rate of severe peripheral neuropathy with the triple combination.
The phase 1 and 2 studies that assessed the efficacy and safety of the RVD triplet as a salvage therapy used bortezomib at 1.0 mg/m2 and lenalidomide at 15 mg,6,7 based on the MTD of a phase 1 dose-escalation study,6 doses that are lower than standard treatment with the respective agents. In a single-arm phase 2 study in 64 patients with relapsed and/or refractory MM following 1 to 3 prior therapies, the ORR with RVD was 64% with a median time to response of 2.1 months and a median DOR of 8.4 months.7 Adverse event types and rates reported with RVD are consistent with those reported here for CRd, with the exception of lymphopenia and peripheral neuropathy. In the RVD study, the rate of grade 3/4 lymphopenia was 11% and 64% of patients experienced grade 1/2 peripheral sensory neuropathy and 3% experienced grade 3 peripheral motor neuropathy. In the current CRd study, the rate of grade 3/4 lymphopenia was 48.1% in the MPD cohort and 38.1% in the overall study population and peripheral neuropathy (peripheral neuropathy, neuropathy, peripheral sensory neuropathy, or peripheral motor neuropathy) in the MPD cohort was 26.9% for any grade and 1.9% for grade 3, with similar results in the overall study population (21.4% and 1.2%, respectively).
While the disease control reported here with the triplet CRd is encouraging, it is particularly promising when considering the patient population and historical studies with the doublet lenalidomide and dexamethasone.27,28 For instance, in a pooled analysis of 2 phase 3 studies, patients with relapsed or refractory MM treated with lenalidomide plus dexamethasone (n = 353) achieved an ORR of 60.6% and the median PFS of 11.1 months.29 While most of the patients had ≥2 prior therapies (81.6%), with prior treatments including stem cell transplant (58.4%), thalidomide (36.0%), and bortezomib (7.6%), none had prior treatment with lenalidomide. When considering the subgroup of patients who were lenalidomide naïve in the current study (n = 25 in the overall study population), treatment with CRd resulted in an ORR of 80% and a median PFS of 28.7 months. Granted, there are limitations to the data reported herein and comparisons across studies are confounded by differences in study design, number of patients studied, and patient populations, all of which will need to be confirmed in additional studies, preferably in the randomized setting.
To this, CRd is currently being investigated in the phase 3 trial ASPIRE (ClinicalTrials.gov NCT01080391), a randomized, controlled, phase 3 trial that will formally compare CRd vs RD in patients with relapsed MM and 1 to 3 prior therapies (estimated sample size of 780 patients).30 Additionally, 2 phase 2 studies evaluating CRd in patients with newly diagnosed MM are ongoing and have reported promising early findings,31,32 and encouraging results have recently been reported on the combination of pomalidomide, carfilzomib, and low-dose dexamethasone in the relapsed and refractory setting.33
In conclusion, the CRd regimen reported here demonstrated robust, rapid, and durable clinical activity with an acceptable tolerability profile in patients with relapsed or progressive MM, including patients who were refractory to lenalidomide and bortezomib. CRd appears to be a compelling option in relapsed MM that will require validation in the phase 3 randomized setting.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the patients and their families who contributed to this study, the staff of the participating study site, and the participating research nurses and data coordinators. Critical review was provided by Thomas Renau (Onyx Pharmaceuticals). Editorial and medical writing assistance was provided by Michael Raffin and Alan Saltzman (Fishawack Communications) and was funded by Onyx Pharmaceuticals. The study was sponsored by Onyx Pharmaceuticals. Study drugs were provided free of charge by Celgene Corporation (lenalidomide) and Onyx (carfilzomib and dexamethasone).
Authorship
Contribution: M.W. designed the research, performed research, collected data, and analyzed data; T.M. performed research and collected data; W.B. designed research, performed research, collected data, and analyzed data; M.A. designed research, performed research, and collected data; D.S.S. performed research and collected data; E.K. performed research, collected data, analyzed data, and performed statistical analysis; M.H. analyzed data and performed statistical analysis; R.Z.O. performed research, collected data, and analyzed data; R.N. designed research, performed research, collected data, and analyzed data; the investigators and representatives from Onyx Pharmaceuticals designed the study; the data were collected and analyzed by medical and statistical representatives from Onyx Pharmaceuticals in conjunction with the investigators; all authors had access to the primary data and participated in writing and editing this paper; and all participating institutions received support from Onyx Pharmaceuticals for the conduct of the study.
Conflict-of-interest disclosure: M.W. received research Funding from Onyx Pharmaceuticals. T.M. received honoraria from Celgene and is a consultant for Onyx Pharmaceuticals. W.B. is an advisory committee member of and received research funding from Celgene. M.A. is on the speakers bureau for Onyx Pharmaceuticals and is a member of the advisory committee at Celgene. D.S.S. received honoraria from and is a consultant, board of directors, or advisory committee member at Millennium and Celgene. E.K. is employed by and has equity ownership in Onyx Pharmaceuticals. M.H. is employed by and has equity ownership in Onyx Pharmaceuticals. R.Z.O. received honoraria from and is a board of directors or advisory committee member at Onyx Pharmaceuticals. R.N. received consultancy and research funding from and is on the speakers bureau at Celgene, Millennium, and Onyx Pharmaceuticals.
Correspondence: Michael Wang, Department of Lymphoma and Myeloma, MD Anderson Cancer Center, 1515 Holcombe Blvd, Box 429, Houston, TX 77030; e-mail: miwang@mdanderson.org.