Key Points
PD-L1 and PD-L2 expression were upregulated during GVHD, whereas PD-1/PD-L1 was more critical than PD-1/PD-L2 in downregulating GVHD.
Our data provide new insight into the differential roles of host PD-L1 and PD-L2 and associated mechanisms in controlling GVHD.
Abstract
Programmed death 1 (PD-1) and its ligands, PD-L1 and PD-L2, play an important role in the maintenance of peripheral tolerance. We explored the role of PD-1 ligands in regulating graft-versus-host disease (GVHD). Both PD-L1 and PD-L2 expression were upregulated in the spleen, liver, colon, and ileum of GVHD mice. Whereas PD-L2 expression was limited to hematopoietic cells, hematopoietic and endothelial cells expressed PD-L1. PD-1/PD-L1, but not PD-1/PD-L2, blockade markedly accelerated GVHD-induced lethality. Chimera studies suggest that PD-L1 expression on host parenchymal cells is more critical than hematopoietic cells in regulating acute GVHD. Rapid mortality onset in PD-L1-deficient hosts was associated with increased gut T-cell homing and loss of intestinal epithelial integrity, along with increased donor T-cell proliferation, activation, Th1 cytokine production, and reduced apoptosis. Bioenergetics profile analysis of proliferating alloreactive donor T-cells demonstrated increased aerobic glycolysis and oxidative phosphorylation in PD-L1-deficient hosts. Donor T-cells exhibited a hyperpolarized mitochondrial membrane potential, increased superoxide production, and increased expression of a glucose transporter in PD-L1-deficient hosts. Taken together, these data provide new insight into the differential roles of host PD-L1 and PD-L2 and their associated cellular and metabolic mechanisms controlling acute GVHD.
Introduction
T-cell fate after T-cell receptor ligation is determined in part by the balance between the costimulatory and coinhibitory pathways, which serve to keep the immune system in check. During graft-versus-host disease (GVHD), coinhibitory pathways can be upregulated, reducing injury to the host during acute GVHD.1 Programmed death 1 (PD-1; CD279), a member of the B7:CD28 superfamily, is an inhibitory receptor that attenuates T-cell receptor signaling by recruitment of phosphatases.2,3 Interactions between PD-1 and its ligands PD-L1 (B7-H1, CD274) and PD-L2 (B7–dentritic cell [DC], CD273) deliver inhibitory signals that regulate T-cell activation, tolerance, and immune-mediated tissue damage.4-7 PD-L1 and PD-L2 differ in expression patterns, with PD-L2 being more restricted than PD-L1 expression.7 PD-L1 is expressed constitutively on hematopoietic cells and nonhematopoietic cells and upregulates after activation.8 PD-L2 expression is restricted primarily to DCs, macrophages, and cultured bone marrow (BM)-derived mast cells.9 Broad PD-L1 expression suggests an important role in inhibiting immune responses in lymphoid and nonlymphoid organs.
PD-L1 is a key mediator of T-cell tolerance in tissues, shielding target organs such as islets from diabetogenic T effectors10,11 or neural tissue in experimental autoimmune encephalomyelitis.12 Patients with multiple sclerosis treated with interferon β (IFN-β) have elevated PD-L1 mRNA, suggesting part of the anti-inflammatory effect of IFN-β treatment is a result of PD-L1.13 PD-L1 autoantibodies have been found in patients with rheumatoid arthritis and correlate with active disease.14 Blocking anti-PD-L1 monoclonal antibody (mAb) enhanced alloantigen-specific T-cell expansion and T helper cell 1 (Th1) differentiation and accelerated solid organ graft rejection.15 PD-L1-immunoglobulin fusion protein given with anti-CD154 Ab or rapamycin prevents solid organ allograft rejection and facilitates tolerance induction.16 Despite its importance in peripheral tolerance, PD-1/PD-1 ligand interactions in regulating acute GVHD have not been studied in detail. Previously, we reported that blockade or absence of PD-1 on donor cells accelerates GVHD, which is associated with increased IFN-γ production.17 Here, we investigated the functional significance of PD-1 ligands expressed on host tissues in regulating immune responses in acute GVHD and explored the mechanism of PD-1-ligand-mediated regulation of alloimmune responses.
Methods
Information concerning mice, BM transplant (BMT), tissue histology, immunohistochemistry, immunofluorescence, fluorescein isothiocyanate (FITC)-dextran permeability assay, bioluminescence imaging studies, flow cytometry, cytokine enzyme-linked immunosorbent assay, serum lipopolysaccharide detection, metabolism assays, and statistical analyses is detailed in the supplemental Methods on the Blood Web site.
Results
Enhanced expression of PD-1 ligands in acute GVHD
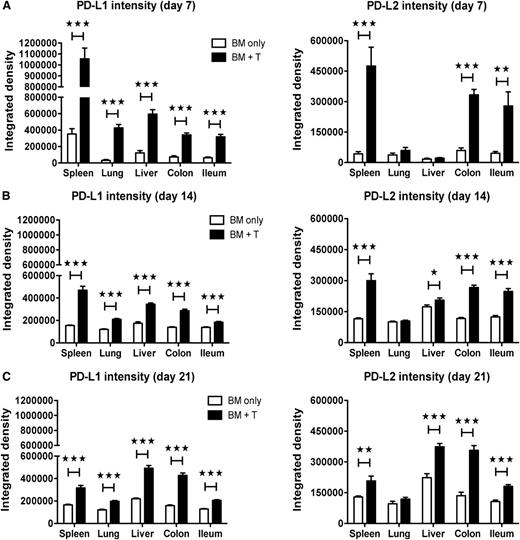
To investigate the expression pattern of PD-1 ligands in acute GVHD, PD-L1 and PD-L2 expression was analyzed by immunohistochemistry. Lethally irradiated C57BL/6 (B6) recipients were transplanted with allogeneic BALB/c BM with or without purified T-cells to induce GVHD, and tissues were analyzed on day 7, day 14, and day 21 after BMT. PD-L1 expression was more abundant in spleen, lung, liver, colon, and ileum in GVHD vs non-GVHD mice, which waned in all but the colon by day 21 after BMT (supplemental Figure 1A-B). PD-L2 expression was more pronounced in spleen, colon, and ileum in GVHD vs non-GVHD mice, and heightened expression in colon and ileum persisted through day 21 after BMT (supplemental Figure 1C-D). PD-L1 and PD-L2 expression was confirmed in a different model (B6→BALB/c; data not shown). Analysis of PD-L1 and PD-L2 intensity by immunofluorescence staining confirmed elevated expression of PD-L1 in spleen, lung, liver, colon, and ileum and PD-L2 in spleen, liver, colon, and ileum during GVHD (Figure 1).
Enhanced expression of PD-1 ligands in acute GVHD. Lethally irradiated BALB/c recipients were given 107 B6 BM cells alone or with 2 × 106 B6 T-cells. Immunofluorescence staining was performed on day 7 (A), day 14 (B), and day 21 (C) for PD-L1 and PD-L2 (4 mice/group). Images were captured at ×200 magnification, using an Olympus FluoView 500 or Olympus FluoView 1000 BX2 confocal laser scanning microscope and analyzed with Adobe Photoshop CS3 (version 10) for determination of the relative fluorescence staining intensity. Data are representative of 2 independent experiments. *P < .05; **P < .01; ***P < .001.
Enhanced expression of PD-1 ligands in acute GVHD. Lethally irradiated BALB/c recipients were given 107 B6 BM cells alone or with 2 × 106 B6 T-cells. Immunofluorescence staining was performed on day 7 (A), day 14 (B), and day 21 (C) for PD-L1 and PD-L2 (4 mice/group). Images were captured at ×200 magnification, using an Olympus FluoView 500 or Olympus FluoView 1000 BX2 confocal laser scanning microscope and analyzed with Adobe Photoshop CS3 (version 10) for determination of the relative fluorescence staining intensity. Data are representative of 2 independent experiments. *P < .05; **P < .01; ***P < .001.
To discern cell types expressing PD-L1 and PD-L2, immunofluorescence costaining was performed. During GVHD, PD-L1 was strongly expressed on CD45+ hematopoietic cells and CD31+ endothelial cells, whereas PD-L2 expression was limited to hematopoietic cells (supplemental Figure 2). PD-L1 expression was observed on Lyve-1+ lymphatic vessels and gp38/podoplanin+ stromal cells in ileum. PD-L1 and PD-L2 were expressed on macrophages and DCs, and PD-L1, but not PD-L2, was expressed on T-cells. PD-L1 and PD-L2 expression was confirmed in anti-major histocompatibility complex class I, H-2Kd+ host and H-2Kb+ donor cells (supplemental Figure 2; data not shown).
PD-1/PD-L1, but not PD-1/PD-L2, blockade exacerbates acute GVHD
To explore differential contributions of PD-L1 vs PD-L2 in GVHD, lethally irradiated BALB/c recipients of supplemental B6 T-cells were given isotype-matched control or anti-PD-L1 and/or anti-PD-L2 blocking mAbs. GVHD-induced lethality was significantly accelerated with anti-PD-L1 vs control Ab (P < .0001; Figure 2A), whereas anti-PD-L2 mAb did not accelerate GVHD lethality at this T-cell dose (P = .055). The magnitude of GVHD acceleration induced by anti-PD-L1 and anti-PD-L2 mAbs was similar to that observed with anti-PD-L1 mAb alone. Increased GVHD severity induced by blocking mAb against PD-L1 was seen using 2 distinct mAbs (MIH-7; 10F.9G2), and no differences were seen between these reagents (supplemental Figure 3A). Lethality was accelerated in a second model in which B10.BR recipients given allogeneic B6 BM, plus splenocytes, were treated with anti-PD-L1 mAb (P < .0001; Figure 2B). GVHD-induced lethality was accelerated further when B10.BR recipients were given a 3-fold higher spleen cell dose (supplemental Figure 3B). Anti-PD-L2 mAb treatment did not accelerate lethality (P = .56). Thus, anti-PD-L1, but not anti-PD-L2, mAb markedly accelerates GVHD lethality.
PD-1/PD-L1, but not PD-1/PD-L2, blockade exacerbates acute GVHD. (A) Lethally irradiated BALB/c recipients were given 107 B6 BM cells alone or with 2 × 106 B6 T-cells and treated with isotype-matched control antibody, anti-PD-L1, anti-PD-L2, or anti-PD-L1 and anti-PD-L2 mAbs (n = 10–18/group). Kaplan–Meier survival curve of transplanted mice (isotype control vs anti-PD-L1 [P < .0001]; isotype control vs anti-PD-L2 [P = .055]; isotype control vs anti-PD-L1 and anti-PD-L2 [P = .0001]). (B) Lethally irradiated B10.BR recipients were given 107 B6 BM cells alone or with 5 × 106 B6 splenocytes and treated with isotype-matched control antibody, anti-PD-L1, or anti-PD-L2 mAb (n = 8–14/group). Kaplan–Meier survival curve of transplanted mice (isotype control vs anti-PD-L1 [P < .0001]; isotype control vs anti-PD-L2 [P = .356]). (C) Lethally irradiated wt B6 recipients or PD-L1−/−PD-L2−/− recipients were given 107 BALB/c BM cells alone or with 5 × 106 BALB/c splenocytes (n = 14–34/group). Kaplan–Meier survival curve of transplanted mice (BM + splenocytes: wt vs PD-L1−/−PD-L2−/− recipients; P < .0001). (D) Lethally irradiated wt B6 recipients, PD-L1−/− recipients, or PD-L2−/− recipients were given 107 BALB/c BM cells alone or with 5 × 106 BALB/c splenocytes (n = 8–17/group). Kaplan–Meier survival curve of transplanted mice (BM + splenocytes: wt vs PD-L1−/− recipients [P = .0001]; BM + splenocytes: wt vs PD-L2−/− recipients [P = .003]; BM + splenocytes: PD-L1−/− vs PD-L2−/− recipients [P = .029]). (E) Lethally irradiated wt B6 recipients or PD-L1−/−PD-L2−/− recipients were given 107 BALB/c BM cells alone or with 1 × 106 BALB/c CD25-depleted T-cells or with 2 × 106 BALB/c CD25-depleted T-cells (n = 8/group). Kaplan–Meier survival curve of transplanted mice (BM + 1 × 106 CD25− T: wt vs PD-L1−/−PD-L2−/− recipients [P = .0006]; BM + 2 × 106 CD25− T: wt vs PD-L1−/−PD-L2−/− recipients [P = .0006]). (F) Lethally irradiated BALB/c recipients were given 107 B6 BM cells alone or with 0.75 × 106 B6 T-cells or with 0.75 × 106 B6 CD25-depleted T-cells and treated with isotype-matched control antibody or anti-PD-L1 mAbs (n = 8–10/group). Kaplan–Meier survival curve of transplanted mice (BM + T cells: isotype control vs anti-PD-L1 [P < .0001]; BM + CD25-depleted T cells: isotype control vs anti-PD-L1 [P = .074]; BM + T cells [isotype control] vs BM + CD25-depleted T cells [isotype control] [P = .0008]).
PD-1/PD-L1, but not PD-1/PD-L2, blockade exacerbates acute GVHD. (A) Lethally irradiated BALB/c recipients were given 107 B6 BM cells alone or with 2 × 106 B6 T-cells and treated with isotype-matched control antibody, anti-PD-L1, anti-PD-L2, or anti-PD-L1 and anti-PD-L2 mAbs (n = 10–18/group). Kaplan–Meier survival curve of transplanted mice (isotype control vs anti-PD-L1 [P < .0001]; isotype control vs anti-PD-L2 [P = .055]; isotype control vs anti-PD-L1 and anti-PD-L2 [P = .0001]). (B) Lethally irradiated B10.BR recipients were given 107 B6 BM cells alone or with 5 × 106 B6 splenocytes and treated with isotype-matched control antibody, anti-PD-L1, or anti-PD-L2 mAb (n = 8–14/group). Kaplan–Meier survival curve of transplanted mice (isotype control vs anti-PD-L1 [P < .0001]; isotype control vs anti-PD-L2 [P = .356]). (C) Lethally irradiated wt B6 recipients or PD-L1−/−PD-L2−/− recipients were given 107 BALB/c BM cells alone or with 5 × 106 BALB/c splenocytes (n = 14–34/group). Kaplan–Meier survival curve of transplanted mice (BM + splenocytes: wt vs PD-L1−/−PD-L2−/− recipients; P < .0001). (D) Lethally irradiated wt B6 recipients, PD-L1−/− recipients, or PD-L2−/− recipients were given 107 BALB/c BM cells alone or with 5 × 106 BALB/c splenocytes (n = 8–17/group). Kaplan–Meier survival curve of transplanted mice (BM + splenocytes: wt vs PD-L1−/− recipients [P = .0001]; BM + splenocytes: wt vs PD-L2−/− recipients [P = .003]; BM + splenocytes: PD-L1−/− vs PD-L2−/− recipients [P = .029]). (E) Lethally irradiated wt B6 recipients or PD-L1−/−PD-L2−/− recipients were given 107 BALB/c BM cells alone or with 1 × 106 BALB/c CD25-depleted T-cells or with 2 × 106 BALB/c CD25-depleted T-cells (n = 8/group). Kaplan–Meier survival curve of transplanted mice (BM + 1 × 106 CD25− T: wt vs PD-L1−/−PD-L2−/− recipients [P = .0006]; BM + 2 × 106 CD25− T: wt vs PD-L1−/−PD-L2−/− recipients [P = .0006]). (F) Lethally irradiated BALB/c recipients were given 107 B6 BM cells alone or with 0.75 × 106 B6 T-cells or with 0.75 × 106 B6 CD25-depleted T-cells and treated with isotype-matched control antibody or anti-PD-L1 mAbs (n = 8–10/group). Kaplan–Meier survival curve of transplanted mice (BM + T cells: isotype control vs anti-PD-L1 [P < .0001]; BM + CD25-depleted T cells: isotype control vs anti-PD-L1 [P = .074]; BM + T cells [isotype control] vs BM + CD25-depleted T cells [isotype control] [P = .0008]).
In support of the idea that blocking function of anti-PD-1 ligand mAbs accounted for accelerated GVHD lethality, studies were performed in PD-L1−/−PD-L2−/− BMT recipients. GVHD lethality in B6 PD-L1−/−PD-L2−/− recipients of BALB/c BM plus splenocytes was accelerated compared with wild-type (wt) recipients (P < .0001; Figure 2C). Allogeneic PD-L1−/− recipients had marked augmentation of lethality compared with wt (P = .0001) or PD-L2−/− (P = .029; Figure 2D) recipients. In contrast to anti-PD-L2 mAb, PD-L2−/− vs wt recipients had significantly increased GVHD lethality (P = .003; Figure 2D), suggesting anti-PD-L2 mAb did not adequately block PD-1/PD-L2 interactions or anti-PD-L2 mAb blocked an inhibitory effect of a donor T-cell subset in GVHD lethality response.
Because donor CD4+25+ regulatory T-cell (Treg) depletion accelerates GVHD-induced lethality,18 we sought to determine whether GVHD acceleration by PD-1/PD-1 ligand blockade was a result of impaired donor Treg function. We compared the survival of wt, PD-L1−/−, PD-L2−/−, and PD-L1−/−PD-L2−/− mice receiving grafts containing Treg-depleted T-cells. BALB/c donor CD25-depleted T-cells caused accelerated GVHD lethality in PD-1 ligand deficient vs wt recipients (Figure 2E; data not shown). Lethality acceleration rates were similar to that observed with donor Treg-replete T-cells. Differences between anti-PD-L2 mAb administration and PD-L2−/− recipients cannot be accounted for by adverse effects of anti-PD-L2 mAb on donor Tregs. In another study, BALB/c recipients given a low dose of Treg-replete or Treg-deplete B6 T-cells were treated with isotype-matched control or anti-PD-L1 mAb (Figure 2F). GVHD-induced lethality was significantly accelerated in recipients of Treg-deplete vs Treg-replete T-cells treated with control Ab (P = .0008), and Treg-replete T-cells given anti-PD-L1 vs control mAb (P < .0001). No significant survival differences were seen in recipients of Treg-deplete T-cells given anti-PD-L1 vs control Ab (P = .074), and GVHD lethality was comparable in recipients of Treg-replete vs Treg-deplete T-cells treated with anti-PD-L1 mAb.
PD-L1 expression on parenchymal cells is critical for suppression of acute GVHD
Our data indicate that hematopoietic cells and endothelial cells express PD-L1 (supplemental Figure 2). To define which cell type is critical for PD-L1-mediated GVHD suppression, we created BM chimeras (PD-L1−/−→wt) in which tissue cells were PD-L1+/+ and BM-derived cells were PD-L1−/−, wt→PD-L1−/− chimeras where tissue cells were PD-L1−/− and BM-derived cells were PD-L1+/+, and control chimeras (wt→wt). After 3 months, we analyzed peripheral blood lymphocytes and spleen for engraftment; nearly 100% of Ag-presenting cells were of BM donor origin (data not shown). Chimeras were reirradiated and infused with allogeneic BALB/c BM with splenocytes to induce GVHD. As expected, wt→PD-L1−/− chimeras died significantly faster than wt→wt chimeras (P < .0001; Figure 3). Compared with wt→PD-L1−/− chimeras, survival was significantly prolonged in PD-L1−/−→wt chimeras (P = .0009), suggesting that PD-L1 expression by parenchymal cells was more critical in attenuating acute GVHD. Survival was prolonged in wt→wt vs PD-L1−/−→wt chimeras (P = .06), suggesting a role of PD-L1 expression by host Ag-presenting cells. These data suggest that PD-L1 expression by parenchymal cells contributes to GVHD prevention, consistent with data suggesting that PD-L1 expression by parenchymal cells is required for tolerizing infiltrating T-cells and preventing GVHD persistence19 and the importance of PD-L1 expression by parenchymal cells in an autoimmune diabetes model.11
PD-L1 expression on parenchymal cells is critical for suppression of acute GVHD. Lethally irradiated wt B6 or PD-L1−/− recipients were given BM cells from PD-L1−/− or wt B6 mice, respectively, to create chimeras. We also created control chimeras (wt→wt). After 3 months, these chimeras were reirradiated and infused with allogeneic BALB/c BM cells with 10 × 106 BALB/c splenocytes. Kaplan–Meier survival curve of transplanted mice (n = 8–11/group). wt→ wt vs PD-L1−/−→wt chimeras [P = .06]; wt→wt vs wt→PD-L1−/− chimeras [P < .0001]; PD-L1−/−→wt vs wt→ PD-L1−/− chimeras [P = .0009]). Data are representative of 2 independent experiments.
PD-L1 expression on parenchymal cells is critical for suppression of acute GVHD. Lethally irradiated wt B6 or PD-L1−/− recipients were given BM cells from PD-L1−/− or wt B6 mice, respectively, to create chimeras. We also created control chimeras (wt→wt). After 3 months, these chimeras were reirradiated and infused with allogeneic BALB/c BM cells with 10 × 106 BALB/c splenocytes. Kaplan–Meier survival curve of transplanted mice (n = 8–11/group). wt→ wt vs PD-L1−/−→wt chimeras [P = .06]; wt→wt vs wt→PD-L1−/− chimeras [P < .0001]; PD-L1−/−→wt vs wt→ PD-L1−/− chimeras [P = .0009]). Data are representative of 2 independent experiments.
Blockade of PD-1/PD-1 ligand interactions induced preferential tissue damage by donor T-cells
To determine the effect of PD-1/PD-1 ligand blockade on GVHD target tissues, BALB/c recipients given supplemental allogeneic B6 T-cells were treated with isotype-matched control Ab or anti-PD-L1 mAb. The composite pathology score and individual target organ score for liver was significantly higher in recipients given anti-PD-L1 vs control Ab, and the GVHD score for ileum was higher in the anti-PD-L1-treated group (supplemental Figure 4A). In addition, B10.BR recipients were given supplemental allogeneic B6 T-cells and treated with isotype-matched control Ab or anti-PD-L1 and anti-PD-L2 mAbs. GVHD-induced lethality was accelerated in recipients given anti-PD-L1 and anti-PD-L2 vs control Ab (P < .0001, data not shown), and GVHD score for colon was significantly higher on day 7 after BMT (supplemental Figure 4B). Histopathologic examination of day 7 tissues showed more severe tissue damage in spleen (supplemental Figure 4C). CD4 T-cell numbers increased significantly in colon on day 7 and day 10, along with increased CD8 T-cells in spleen on day 7 and in ileum on day 10 in recipients treated with anti-PD-L1 and anti-PD-L2 vs control Ab (Figure 4A). Modest to no differences were observed in CD4 or CD8 T-cell numbers in other GVHD organs at either time (Figure 4A; data not shown). By immunohistochemistry, increased T-cell infiltration in colon and ileum was seen in recipients given anti-PD-L1 and anti-PD-L2 vs control Ab (supplemental Figure 4D).
Blockade of PD-1/PD-1 ligand interactions induced preferential tissue damage by donor T-cells. (A) Lethally irradiated B10.BR recipients were given 107 B6 BM cells with 2 × 106 B6 T-cells and treated with isotype-matched control antibody or anti-PD-L1 and anti-PD-L2 mAbs. Immunohistochemistry staining for CD4 (clone RM4-5) and CD8 (clone 53-6.7) T-cells on day 7 and day 10 after BMT. Cell numbers in spleen, liver, colon, and ileum are shown. Cells were quantified by counting the number of antibody-binding-positive cells in a 100 mm2 field of view under the microscope and obtaining an average of counts from 4 representative fields (n = 4 mice/group). (B) Lethally irradiated B10.BR recipients were given 107 B6 BM cells alone or with 10 × 106 B6 splenocytes and treated with isotype-matched control antibody or anti-PD-L1 mAb. Plasma FITC-dextran concentration was measured on day 7 after BMT (n = 5 mice/group). (C) Lethally irradiated BALB/c recipients were given 107 B6 BM cells alone or with 1.5 × 106 B6 luciferase transgenic T-cells and treated with isotype-matched control antibody or anti-PD-L1 mAb. On day 4 and day 6 after BMT, mice were injected intraperitoneally with luciferin, and after 5 minutes, mice were imaged using a Xenogen IVIS imaging system for 2 minutes (n = 7–9 mice/group). On day 6 after BMT, mice were killed, and isolated organs were imaged for 1 minute in the presence of luciferin (n = 7 mice/group). (A−C) Data are representative of 2 independent experiments. *P < .05; **P < .01; ***P < .001.
Blockade of PD-1/PD-1 ligand interactions induced preferential tissue damage by donor T-cells. (A) Lethally irradiated B10.BR recipients were given 107 B6 BM cells with 2 × 106 B6 T-cells and treated with isotype-matched control antibody or anti-PD-L1 and anti-PD-L2 mAbs. Immunohistochemistry staining for CD4 (clone RM4-5) and CD8 (clone 53-6.7) T-cells on day 7 and day 10 after BMT. Cell numbers in spleen, liver, colon, and ileum are shown. Cells were quantified by counting the number of antibody-binding-positive cells in a 100 mm2 field of view under the microscope and obtaining an average of counts from 4 representative fields (n = 4 mice/group). (B) Lethally irradiated B10.BR recipients were given 107 B6 BM cells alone or with 10 × 106 B6 splenocytes and treated with isotype-matched control antibody or anti-PD-L1 mAb. Plasma FITC-dextran concentration was measured on day 7 after BMT (n = 5 mice/group). (C) Lethally irradiated BALB/c recipients were given 107 B6 BM cells alone or with 1.5 × 106 B6 luciferase transgenic T-cells and treated with isotype-matched control antibody or anti-PD-L1 mAb. On day 4 and day 6 after BMT, mice were injected intraperitoneally with luciferin, and after 5 minutes, mice were imaged using a Xenogen IVIS imaging system for 2 minutes (n = 7–9 mice/group). On day 6 after BMT, mice were killed, and isolated organs were imaged for 1 minute in the presence of luciferin (n = 7 mice/group). (A−C) Data are representative of 2 independent experiments. *P < .05; **P < .01; ***P < .001.
Next we assessed whether increased intestinal injury in recipients treated with anti-PD-1 ligand mAb may have contributed to accelerated lethality. We tested epithelial barrier function by oral administration of FITC-dextran that enters into the bloodstream if the epithelial barrier has been compromised.20 Significantly increased GVHD-related epithelial damage and loss of epithelial integrity was observed in recipients given anti-PD-L1 vs control Ab (Figure 4B).
A hallmark of acute GVHD is alloreactive donor T-cell expansion in a proinflammatory environment, induced by a conditioning regimen and deregulated immune mechanisms. To evaluate the effect of donor T-cell expansion in the absence of host PD-1 ligand expression, BALB/c recipients were given supplemental allogeneic B6 luciferase transgenic (luc+) T-cells and treated with isotype-matched control Ab or anti-PD-L1 mAb. Expansion of luc+ T-cells was quantified by total photon flux on day 4 and day 6 after BMT, and bioluminescence imaging signal intensity significantly increased on day 6 in anti-PD-L1 vs control Ab-treated recipients (Figure 4C; supplemental Figure 5). Donor luc+ T-cells signals in secondary lymphoid organs and GVHD target tissues was significantly higher in recipients given anti-PD-L1 mAb (Figure 4C; supplemental Figure 5), consistent with migration to and proliferation of donor T-cells in acute GVHD.
These results suggest that blocking of host PD-1 ligand expression can cause increased infiltration and expansion of donor T-cells into GVHD target tissues, especially intestine, leading to loss of epithelial cell integrity and increased mortality.
PD-1/PD-1 ligand blockade increases donor T-cell proliferation, activation, and effector function while reducing apoptosis in GVHD mice
To elucidate potential contributory mechanisms responsible for severe GVHD-induced lethality, donor T-cell proliferation was evaluated post-BMT. Allogeneic splenocytes labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) dye to track cell division were transferred into lethally irradiated recipients treated with control Ab or anti-PD-L1 and anti-PD-L2 mAbs. Responder frequency and proliferation capacity of donor CD4 and CD8 T-cells were significantly higher in spleen and mesenteric lymph nodes (MLNs) of recipients given anti-PD-L1 and anti-PD-L2 vs control Ab (Figure 5A). Although no significant differences in the average number of divisions of proliferating CD4 T-cells was seen, there was a significantly increased number of CD8 T-cells that had undergone cellular division in recipients given anti-PD-L1 and anti-PD-L2 vs control Ab (data not shown). Frequencies of annexin V+ apoptotic donor CD4 and CD8 T-cells on day 5 were significantly lower in spleen of mice given anti-PD-L1 and anti-PD-L2 vs control Ab (Figure 5B). Similar results were obtained for donor CD8 T-cells in MLNs.
Host PD-1 ligand expression affect proliferation and survival of allogeneic donor T-cells. Lethally irradiated B10.BR recipients were given 107 B6 BM cells with 50 × 106 CFSE-labeled B6 splenocytes and treated with isotype-matched control antibody or anti-PD-L1 and anti-PD-L2 mAbs. (A) Mice were killed on day 5 after BMT (n = 5 mice/group), and splenocytes and MLNs were analyzed by flow cytometry for CFSE dilution. Cells were gated on H-2Kb+ and analyzed for CD4+ or CD8+ events. (B) Spleen and MLNs were harvested (n = 5 mice/group) on day 5 and analyzed by flow cytometry for annexin V-positive donor CD4 and CD8 T-cells. (A-B) Data are representative of 2 independent experiments. *P < .05; **P < .01; ***P < .001.
Host PD-1 ligand expression affect proliferation and survival of allogeneic donor T-cells. Lethally irradiated B10.BR recipients were given 107 B6 BM cells with 50 × 106 CFSE-labeled B6 splenocytes and treated with isotype-matched control antibody or anti-PD-L1 and anti-PD-L2 mAbs. (A) Mice were killed on day 5 after BMT (n = 5 mice/group), and splenocytes and MLNs were analyzed by flow cytometry for CFSE dilution. Cells were gated on H-2Kb+ and analyzed for CD4+ or CD8+ events. (B) Spleen and MLNs were harvested (n = 5 mice/group) on day 5 and analyzed by flow cytometry for annexin V-positive donor CD4 and CD8 T-cells. (A-B) Data are representative of 2 independent experiments. *P < .05; **P < .01; ***P < .001.
To determine whether loss of host PD-1 ligand influenced donor T-cell effectors, lethally irradiated B10.BR recipients were given B6 BM with spleen cells and blocking anti-PD-L1 mAb or control Ab. On day 7 after BMT, increased infiltration of donor CD4 and CD8 T-cells was observed in spleen, accompanied by a higher frequency of integrin lymphocyte Peyer's patch adhesion molecule-1 (LPAM-1) (α4β7) expression on CD4 and CD8 T-cells and a PD-1hi subset in recipients given anti-PD-L1 vs control Ab (Figure 6A; data not shown). The mucosal homing integrin α4β7 is expressed by T- and B-cells that circulate from blood to mucosal sites and is frequently found on T- and B-cells entering the intestinal lamina propria,21,22 which may indicate direct trafficking of these cells into intestinal tissues. Both donor CD4 and CD8 T-cells in recipients given anti-PD-L1 mAb had an activation phenotype indicated by significantly higher expression of CD25, PD-1 and/or significantly lower expression of CD62L (Figure 6B; data not shown). Fas ligand expression on donor CD4 and CD8 T-cells was significantly lower in recipients given anti-PD-L1 vs control Ab, and donor CD4 and CD8 T-cells from GVHD mice had significantly higher coexpression of PD-1 and a second inhibitory receptor, T-cell immunoglobulin mucin 3 (Tim-3), in PD-L1−/− vs wt recipients (data not shown). Analysis of lysosomal marker CD107a and cytolytic effector molecule granzyme B indicated increased activation of donor CD4 and CD8 T-cells in PD-L1−/− vs wt recipients (Figure 6D).
GVHD acceleration induced by PD-1/PD-1 ligand blockade resulted in increased activation and effector function of donor T-cells. (A-C) Lethally irradiated B10.BR recipients were given 107 B6 BM cells with 5 × 106 B6 splenocytes and treated with isotype-matched control antibody or anti-PD-L1 mAb. (A) Mice were killed on day 7 after BMT, and splenocytes (n = 5 mice/group) were analyzed by flow cytometry for LPAM-1 (α4β7) expression on donor CD4 and CD8 T-cells. (B) Common activation markers (CD25 and CD62L) on donor CD4 and CD8 T-cells were analyzed on day 7 (n = 4–5 mice/group) by flow cytometry. (C) Intracellular cytokine staining was performed on day 7 (n = 5 mice/group) and analyzed by flow cytometry to detect the percentage of donor CD4 and CD8 T-cells producing IFN-γ, IL-17, TNF-α, IL-2, IL-4, and IL-10 in spleen. (D) Lethally irradiated wt B6 recipients or PD-L1−/− recipients were given 107 BALB/c BM cells with 5 × 106 BALB/c splenocytes. Mice were killed on day 7 after BMT (n = 5 mice/group), and splenocytes were analyzed by flow cytometry for intracellular expression of CD107a or granzyme B on donor CD4 and CD8 T-cells. Data are presented as mean fluorescence intensity (MFI). (A−D) Data are representative of 2 independent experiments. *P < .05; **P < .01; ***P < .001.
GVHD acceleration induced by PD-1/PD-1 ligand blockade resulted in increased activation and effector function of donor T-cells. (A-C) Lethally irradiated B10.BR recipients were given 107 B6 BM cells with 5 × 106 B6 splenocytes and treated with isotype-matched control antibody or anti-PD-L1 mAb. (A) Mice were killed on day 7 after BMT, and splenocytes (n = 5 mice/group) were analyzed by flow cytometry for LPAM-1 (α4β7) expression on donor CD4 and CD8 T-cells. (B) Common activation markers (CD25 and CD62L) on donor CD4 and CD8 T-cells were analyzed on day 7 (n = 4–5 mice/group) by flow cytometry. (C) Intracellular cytokine staining was performed on day 7 (n = 5 mice/group) and analyzed by flow cytometry to detect the percentage of donor CD4 and CD8 T-cells producing IFN-γ, IL-17, TNF-α, IL-2, IL-4, and IL-10 in spleen. (D) Lethally irradiated wt B6 recipients or PD-L1−/− recipients were given 107 BALB/c BM cells with 5 × 106 BALB/c splenocytes. Mice were killed on day 7 after BMT (n = 5 mice/group), and splenocytes were analyzed by flow cytometry for intracellular expression of CD107a or granzyme B on donor CD4 and CD8 T-cells. Data are presented as mean fluorescence intensity (MFI). (A−D) Data are representative of 2 independent experiments. *P < .05; **P < .01; ***P < .001.
Intracellular staining for cytokine production in spleen, liver, and MLNs revealed 1.5- to 2-fold higher IFN-γ-producing donor CD4 and CD8 T-cells in mice given anti-PD-L1 vs control Ab (Figure 6C; supplemental Figure 6). Frequencies of tumor necrosis factor α (TNF-α)–producing donor CD4 and CD8 T-cells in liver and MLNs, and IFN-γ- or TNF-α-producing T-cells coexpressing PD-1, were significantly higher in recipients given anti-PD-L1 (supplemental Figure 6; data not shown). No significant difference was observed in interleukin 2 (IL-2) production. Increased frequencies of IL-17–, IL-4–, or IL-10–producing donor T-cells were variably seen in spleen, liver, and MLN of mice given anti-PD-L1 vs control Ab, although frequencies of cells expressing either IFN-γ or TNF-α was significantly higher. Frequencies of donor CD4 and CD8 T-cells producing both IFN-γ and TNF-α, or the frequencies of IFN-γ production by CD107a expressing donor CD4 and CD8 T-cells, were significantly higher in PD-L1−/− vs wt recipients (data not shown). Analysis of serum samples on day 7 after BMT indicated significantly elevated levels of IFN-γ (2.4-fold) and TNF-α (2-fold), and 2-fold higher levels of endotoxin in mice given anti-PD-L1 vs control Ab (supplemental Figure 7).
Taken together, these results suggest loss of PD-L1 expression in host can induce increased donor T-cell infiltration, proliferation, activation, and production of proinflammatory cytokines, which can promote greater GVHD severity in target tissues.
GVHD acceleration induced by PD-1/PD-L1 blockade resulted in increased donor T-cell metabolic stress
Allogeneic donor T-cells become activated post-BMT and rapidly proliferate in response to histocompatibility antigens expressed on host tissues. T-cell activation induces glucose uptake and glycolysis to produce more adenosine triphosphate (ATP) to maintain the cellular ATP/adenosine 5′-diphosphate ratio under conditions of high energy demand. Studies have shown that compared with syngeneic T-cells, allogeneic T-cells increase aerobic glycolysis and oxidative phosphorylation to maintain cellular ATP.23 We have verified and extended these findings in wt vs PD-L1−/− recipients.
Lethally irradiated wt B6, PD-L1−/−, or Thy1.2+ BALB/c recipients were given supplemental Thy1.2+ BALB/c BM cells with CFSE-labeled Thy1.1+ BALB/c splenocytes. We compared the bioenergetics of proliferating donor T-cells on day 5 after BMT. Naive Thy1.1+ BALB/c mice were included as controls. Lactate production, which reflects rate of aerobic glycolysis, increased up to 2-fold in syngeneic BMT vs naive T-cells; lactate production by allogeneic T-cells increased further with significantly higher production in PD-L1−/− vs wt recipients (Figure 7A). Notably, intracellular amounts of pyruvate in allogeneic vs syngeneic T-cells were significantly less (Figure 7B), as pyruvate is converted into lactate to generate ATP. We measured oligomycin-inhibited O2 consumption (reflecting the rate of oxidative phosphorylation, OXPHOS) by donor T-cells. The O2 consumption rate was significantly higher in PD-L1−/− vs wt allogeneic vs syngeneic recipients (Figure 7C; supplemental Figure 8A). We observed a significant increase in GLUT1 (the major glucose transporter on hematopoietic cells24 ) expression in proliferating (CFSElo) donor T-cells in PD-L1−/− vs wt allogeneic vs syngeneic recipients (Figure 7D), contrasting with the basal expression in resting naive T-cells (supplemental Figure 8B).
GVHD acceleration induced by PD-1/PD-L1 blockade resulted in increased metabolic stress in donor T-cells. Lethally irradiated wt B6, PD-L1−/−, or Thy1.2+ BALB/c recipients were given 107 Thy1.2+ BALB/c BM cells with 30 × 106 CFSE-labeled Thy1.1+ BALB/c splenocytes. (A-H) Mice were killed on day 5 after BMT, and experiments were performed as described. Thy1.1+ donor T-cells were purified, and lactate (A) and pyruvate (B) production and oxygen consumption (C) by donor T-cells were measured. Naive Thy1.1+ BALB/c mice (n = 4) were included as control. Mice splenocytes were analyzed by flow cytometry for GLUT1 (D), TMRM (E), dihydroethidium (F), and annexin V (H) expression in undivided (CFSEhi) and divided (CFSElo) donor T-cells. MFI; mean fluorescence intensity. (G) Splenocytes were also analyzed by flow cytometry for CFSE dilution. Cells were gated on Thy1.1+ and analyzed for CD4+ or CD8+ events. (A-H) Data are representative of 5–9 mice/group from 2-3 independent experiments. *P < .05; **P < .01; ***P < .001.
GVHD acceleration induced by PD-1/PD-L1 blockade resulted in increased metabolic stress in donor T-cells. Lethally irradiated wt B6, PD-L1−/−, or Thy1.2+ BALB/c recipients were given 107 Thy1.2+ BALB/c BM cells with 30 × 106 CFSE-labeled Thy1.1+ BALB/c splenocytes. (A-H) Mice were killed on day 5 after BMT, and experiments were performed as described. Thy1.1+ donor T-cells were purified, and lactate (A) and pyruvate (B) production and oxygen consumption (C) by donor T-cells were measured. Naive Thy1.1+ BALB/c mice (n = 4) were included as control. Mice splenocytes were analyzed by flow cytometry for GLUT1 (D), TMRM (E), dihydroethidium (F), and annexin V (H) expression in undivided (CFSEhi) and divided (CFSElo) donor T-cells. MFI; mean fluorescence intensity. (G) Splenocytes were also analyzed by flow cytometry for CFSE dilution. Cells were gated on Thy1.1+ and analyzed for CD4+ or CD8+ events. (A-H) Data are representative of 5–9 mice/group from 2-3 independent experiments. *P < .05; **P < .01; ***P < .001.
We measured mitochondrial membrane potential (ΔΨm), as increased oxygen consumption by alloreactive T-cells could increase trichloracetic acid cycle activity and nicotinamide adenine dinucleotide hydrate production, which could hyperpolarize ΔΨm. Mitochondrial activity in donor T-cells was measured with tetramethylrhodamine (TMRM), a dye accumulating within mitochondria in proportion to ΔΨm. TMRM fluorescence intensity was significantly higher in wt allogeneic vs syngeneic recipients, with the highest intensity in PD-L1−/− recipients (Figure 7E). TMRM fluorescence intensity was significantly less in CD4 T-cells in resting naive vs syngeneic recipients (supplemental Figure 8C). We measured superoxide production by activated donor T-cells, as higher ΔΨm can induce superoxide production from mitochondrial respiratory chains. Cells were stained with dihydroethidium, a redox-sensitive dye specific for superoxide. Proliferating donor CD4 and CD8 T-cells contained more superoxide in allogeneic PD-L1−/− vs wt recipients than resting donor cells (Figure 7F; supplemental Figure 8D). Analysis of the proliferation capacity of donor T-cells suggested increased proliferation of both CD4 and CD8 T-cells in PD-L1−/− vs wt recipients, and alloreactive donor T-cells proliferated significantly faster than donor T-cells in syngeneic recipients (Figure 7G). Frequencies of annexin V+ apoptotic donor CD4 and CD8 T-cells were significantly lower in PD-L1−/− vs wt recipients (Figure 7H).
Discussion
The PD-1/PD-1 ligand pathway plays an important role in regulating alloimmune responses and in the induction and maintenance of peripheral tolerance. We investigated the role of PD-1 ligands in regulating acute GVHD. Antibody-blocking experiments demonstrated that anti-PD-L1, but not anti-PD-L2, mAb significantly accelerated GVHD lethality, which was also observed in PD-L1−/− or PD-L2−/− recipients. The greater GVHD magnitude using genetic vs pharmacologic approaches to block PD-1/PD-L2 interaction might be a result of the ensured loss of PD-L2 expression vs inadequate blocking at the tissue level or a relatively shorter duration of blockade by anti-PD-L2 mAb administration. However, GVHD lethality was comparable in recipients treated with anti-PD-L1 vs anti-PD-L1 plus anti-PD-L2 mAbs and in PD-L1−/− vs PD-L1−/−PD-L2−/− recipients, and GVHD lethality was accelerated in PD-L1−/− vs PD-L2−/− recipients, indicating that PD-L1 is the dominant ligand for PD-1 during GVHD. These data are consistent with non-GVHD studies demonstrating that the PD-1/PD-L1 pathway is more relevant than the PD-1/PD-L2 pathway in delivering inhibitory signals to alloreactive T-cells expressing PD-1.7,25
PD-L1 suppresses T-cell activation.7 The importance of PD-L1 as a negative regulator of T-cell activation has been shown in autoimmune disease10-12 and organ transplantation15,16 models. PD-L1 expressed by recipient hematopoietic and parenchymal cells induces alloreactive CD8 T-cell exhaustion and reduced graft-versus-leukemia effects in radiation-conditioned recipients.26-28 In a murine asthma model, PD-L2, but not PD-L1, played a dominant role in controlling tissue damage.29 The predominant role of PD-L2 in regulating short-term alloreactive CD8 T-cell response in irradiated DBA/2 recipients30 suggests the effects of PD-L1 and PD-L2 may be strain- and model-dependent.
PD1 and PD-L1 are highly expressed on Tregs, and PD-L1 and PD-1 play important roles in Tregs development and function.31-33 Human T-cells overexpressing PD-L1 can induce suppressor activity that can prevent xenogeneic GVHD.34 In contrast, the negative regulatory role of PD-L1 in expansion and function of Tregs has been also reported.35 We considered the possibility that blockade of PD-1/PD-1 ligand interaction may impair the generation of Tregs in vivo, enhancing alloimmune responses and accelerating GVHD. Although infusion of donor T-cells caused accelerated GVHD in PD-L1−/−, PD-L2−/−, or PD-L1−/−PD-L2−/− vs wt recipients, the rate of acceleration was independent of CD25-depletion of donor T-cells, suggesting that rapid acceleration of GVHD lethality in PD-1 ligand knockout recipients was independent of donor thymus-derived Tregs. In preliminary studies to determine whether decreased peripheral-derived Tregs might be observed in recipients treated with anti-PD-L1 mAb, recipients of CD25-depleted T-cells were noted to have a low frequency of forkhead box P3+ cells (<1.5%), and no significant difference was observed in recipients treated with anti-PD-L1 vs control Ab (data not shown).
A likely contributory mechanism for accelerated GVHD-induced lethality in anti-PD-L1 vs control Ab-treated recipients is increased donor T-cell infiltration in GVHD tissues, especially the gastrointestinal tract and secondary lymphoid organs, along with decreased annexin V+ donor T-cells. Chimera studies have shown that PD-L1 expressed on nonhematopoietic cells can serve as a key mediator of T-cell tolerance within tissues, protecting target organs from potentially pathogenic self-reactive T-cells,11,14 and PD-L1 expression may be required for apoptosis of activated T-cells.10,36 Lymphatic endothelial cells can induce peripheral tolerance via PD-L1.37 We have shown that in GVHD, PD-L1 is highly expressed on hematopoietic cells and endothelial cells, including Lyve-1+ cells, and PD-L1 expression by parenchymal cells is more critical in regulating acute GVHD. We speculate that loss of PD-L1 expression in tissues resulted in increased infiltration of alloreactive donor T-cells, causing more severe tissue damage.
IFN-γ production by Th1 cells plays an important role in Th1-mediated tissue damage during GVHD.38 IFN-γ may upregulate host major histocompatibility complex, costimulatory molecules, or it may stimulate production of other inflammatory cytokines39 that may function as a GVHD effector mechanism. IFN-γ can also downregulate Th1 inflammatory response by inducing donor T-cell apoptosis and upregulating PD-L1 tissue expression that can mediate anergy and apoptosis of activated infiltrating T-cells.40 The frequency of IFN-γ producing donor T-cells in spleen, MLN, and liver was significantly higher in recipients treated with anti-PD-L1 mAb. Elevated LPAM-1 (α4β7) expression by activated donor T-cells coexpressing PD-1 suggest that increased IFN-γ production by PD-1/PD-L1 blockade may have resulted in upregulation of LPAM-1 expression and increased homing into intestine, causing more tissue damage. Studies have reported that α4β7+ vs α4β7− donor T-cells induced more severe intestinal GVHD,41 and donor CD4 T-cells localized in intestine and associated lymphoid tissues expressed α4β7 and produced IFN-γ early during acute graft-versus-host reaction.42 The higher pathologic GVHD score, increased infiltration of donor T-cells, and loss of epithelial integrity suggest that blockade of PD-1/PD-L1 interaction induced significantly higher frequency of IFN-γ-producing allogeneic T-cells that resulted in severe gastrointestinal injury. These data are consistent with findings by others suggesting that IFN-γ can cause tissue damage in gut and liver during acute GVHD.38,43 TNF-α production by alloreactive T-cells is also important in the induction of GVHD,44 and blockade of TNF-α is used clinically to treat GVHD.45 Blocking of PD-1/PD-L1 interaction induced significantly higher levels of TNF-α in the circulation and a higher frequency of TNF-α–producing allogeneic T-cells in liver and MLNs, which may have contributed to tissue damage and exacerbation of the disease. Intestinal damage during acute GVHD from TNF-α can cause increased leakage of inflammatory stimuli, including lipopolysaccharide, into the systemic circulation, which then triggers additional TNF-α production, making the intestine a pivotal target organ in the pathophysiology of GVHD.46
In GVHD, donor T-cells proliferate in response to host alloantigenic disparities and mediate lethal immune response. Proliferating donor T-cells in PD-L1−/− recipients increased OXPHOS by 2.5-fold and lactate production up to 4.5-fold vs resting T-cells, and GLUT1 expression was increased by 60% to 70%, suggesting that alloreactive T-cells used both aerobic glycolysis and OXPHOS for ATP synthesis. Our results are consistent with the findings that uptake of glucose analog is increased in mice and humans during GVHD.47 Signaling through inhibitory receptors on T-cells (CTLA-4 or PD-1) decreases protein kinase B activity and glycolysis,48 suggesting that blockade of PD-1/PD-L1 interaction will increase metabolic activity and function of proliferating donor T-cells. Mitochondrial electron transport chain is a major source of reactive oxygen species (ROS) in mammalian cells in the form of superoxide.49 Mitochondrial ROS production also increases in proportion with ΔΨm, as hyperpolarization of ΔΨm can prolong the half-life of reactive intermediates, which increases the formation of superoxide.50 Therefore, it is not surprising that increased OXPHOS by alloreactive T-cells would exhibit increased ROS production. Our results also suggest that donor T-cells in PD-L1−/− recipients exhibited both hyperpolarization of ΔΨm and increased superoxide production.
In summary, we demonstrated the important function of PD-L1 in regulating acute GVHD. Key findings were: PD-L1 and PD-L2 upregulation in GVHD target organs, that PD-1/PD-L1 was more critical than PD-1/PD-L2 in downregulating GVHD, and that rapid mortality onset in PD-L1−/− hosts were associated with increased proliferation, activation, Th1 cytokine-production, and metabolic stress of donor T-cells, along with increased homing in GVHD target tissues including gut, as well as loss of intestinal epithelial integrity. Tissue expression of PD-L1 was important in regulating alloreactive donor T-cell responses after BMT. Taken together, these data point to the critical role of PD-L1, but not PD-L2, in the prevention of GVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr David A. Bernlohr and Rocio Foncea (Department of Biochemistry, Molecular Biology and Biophysics, University of Minnesota, Minneapolis, MN) for assistance in Seahorse experiments, Jason Mitchell (Department of Laboratory Medicine and Pathology, University of Minnesota) and Ryan Flynn for technical assistance, and Klara Noble for animal handling.
This work was supported in part by grants from the National Institutes of Health Research Program (P01), National Institute of Allergy and Infectious Diseases (AI056299) (to M.H.S., N.N., R.A., G.J.F., A.H.S., and B.R.B.); Research Project Grant Program (R01), Institute of Allergy and Infectious Diseases (AI034495), Heart, Lung and Blood Institute (HL056067, HL049997), and National Cancer Institute (CA072669) (to B.R.B.). The use of confocal microscope was made available through a National Center for Research Resources Shared Instrumentation Grant (1 S10 RR16851).
Authorship
Contribution: A.S. designed and performed experiments, analyzed data, designed figures, and wrote the paper; K.A., B.H.K., and R.G.V. performed experiments; P.A.T. designed and performed experiments and analyzed data; A.P.-M. assigned pathology scoring and designed and interpreted tissue staining; D.H.M. and W.J.M. discussed experimental design and edited the paper; M.A., H.Y., M.H.S., N.N., G.J.F., and A.H.S. provided reagents and edited the paper; B.T.F. and R.A. discussed and analyzed data, shared unpublished data, and edited the paper; G.S. discussed and shared unpublished data and edited the paper; and B.R.B. designed, discussed, and analyzed data and edited the paper.
Conflict-of-interest disclosure: R.A. has a patent and receives patent royalties on the PD-1 pathway. G.J.F. and A.H.S. have patents and receive patent royalties on the PD-1 pathway and are scientific founders of Costim Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, University of Minnesota Cancer Center and Department of Pediatrics, Division of Blood and Marrow Transplantation, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.

![Figure 2. PD-1/PD-L1, but not PD-1/PD-L2, blockade exacerbates acute GVHD. (A) Lethally irradiated BALB/c recipients were given 107 B6 BM cells alone or with 2 × 106 B6 T-cells and treated with isotype-matched control antibody, anti-PD-L1, anti-PD-L2, or anti-PD-L1 and anti-PD-L2 mAbs (n = 10–18/group). Kaplan–Meier survival curve of transplanted mice (isotype control vs anti-PD-L1 [P < .0001]; isotype control vs anti-PD-L2 [P = .055]; isotype control vs anti-PD-L1 and anti-PD-L2 [P = .0001]). (B) Lethally irradiated B10.BR recipients were given 107 B6 BM cells alone or with 5 × 106 B6 splenocytes and treated with isotype-matched control antibody, anti-PD-L1, or anti-PD-L2 mAb (n = 8–14/group). Kaplan–Meier survival curve of transplanted mice (isotype control vs anti-PD-L1 [P < .0001]; isotype control vs anti-PD-L2 [P = .356]). (C) Lethally irradiated wt B6 recipients or PD-L1−/−PD-L2−/− recipients were given 107 BALB/c BM cells alone or with 5 × 106 BALB/c splenocytes (n = 14–34/group). Kaplan–Meier survival curve of transplanted mice (BM + splenocytes: wt vs PD-L1−/−PD-L2−/− recipients; P < .0001). (D) Lethally irradiated wt B6 recipients, PD-L1−/− recipients, or PD-L2−/− recipients were given 107 BALB/c BM cells alone or with 5 × 106 BALB/c splenocytes (n = 8–17/group). Kaplan–Meier survival curve of transplanted mice (BM + splenocytes: wt vs PD-L1−/− recipients [P = .0001]; BM + splenocytes: wt vs PD-L2−/− recipients [P = .003]; BM + splenocytes: PD-L1−/− vs PD-L2−/− recipients [P = .029]). (E) Lethally irradiated wt B6 recipients or PD-L1−/−PD-L2−/− recipients were given 107 BALB/c BM cells alone or with 1 × 106 BALB/c CD25-depleted T-cells or with 2 × 106 BALB/c CD25-depleted T-cells (n = 8/group). Kaplan–Meier survival curve of transplanted mice (BM + 1 × 106 CD25− T: wt vs PD-L1−/−PD-L2−/− recipients [P = .0006]; BM + 2 × 106 CD25− T: wt vs PD-L1−/−PD-L2−/− recipients [P = .0006]). (F) Lethally irradiated BALB/c recipients were given 107 B6 BM cells alone or with 0.75 × 106 B6 T-cells or with 0.75 × 106 B6 CD25-depleted T-cells and treated with isotype-matched control antibody or anti-PD-L1 mAbs (n = 8–10/group). Kaplan–Meier survival curve of transplanted mice (BM + T cells: isotype control vs anti-PD-L1 [P < .0001]; BM + CD25-depleted T cells: isotype control vs anti-PD-L1 [P = .074]; BM + T cells [isotype control] vs BM + CD25-depleted T cells [isotype control] [P = .0008]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/17/10.1182_blood-2013-05-500801/4/m_3062f2.jpeg?Expires=1765896635&Signature=HJmlVkCcSUP~Cyac7p2rTOy4Zfdtl6Bee7zGZHKclXslUgpOFSVhUcStaN10cURLGKYnbSvpZx5HAsxM5SSobGHZeSbp8RWnBE2--w~whOyKv4ELSfF3xDvxmfPKE75~z2oxJQ89AeQFqoHqJeTuUfkrNF2KXCGrufq3722HvLob6ulSmHVJBAWE-wl~C9TzDFRXTDDhnMl~uP40a~jRJ75YEVWmWC0AS5CvR~kEJhht68tk6yXUxnoWrVYM9XWpGAT-16C1T5-dfkZdyJ9I3B7ZMWYNZVUMpdftQlQuiT88Wx2SlW~lGWLn1Qv~gI6UArwLRW6yOJFx71BAhvDiJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. PD-L1 expression on parenchymal cells is critical for suppression of acute GVHD. Lethally irradiated wt B6 or PD-L1−/− recipients were given BM cells from PD-L1−/− or wt B6 mice, respectively, to create chimeras. We also created control chimeras (wt→wt). After 3 months, these chimeras were reirradiated and infused with allogeneic BALB/c BM cells with 10 × 106 BALB/c splenocytes. Kaplan–Meier survival curve of transplanted mice (n = 8–11/group). wt→ wt vs PD-L1−/−→wt chimeras [P = .06]; wt→wt vs wt→PD-L1−/− chimeras [P < .0001]; PD-L1−/−→wt vs wt→ PD-L1−/− chimeras [P = .0009]). Data are representative of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/17/10.1182_blood-2013-05-500801/4/m_3062f3.jpeg?Expires=1765896635&Signature=noNM13639Bd3PeCW38s1pceQin7nSADJC32DCyDMl7tSsTC37s6vHO88kwNSU223DbNoIFpJPQjyLzmgepX6Dr9CxLTgVfioL3iByRJlm8GyPxq8GLJnlBv~DaYkpez8EilJUIYRTNBESM-f5Lf6KbAs0M6dvEscLynLzHWlZ36qac7Ne3NboAFkCb7dHX15BB~AUynmGITEIXNsAHOhuC0FKVOU3mtK664Um4A3wIoW~qd7hx2oGDq32a7DxY74RcuC4YbTHNbclNhvPu6YdiCOJzdTfG5yM~xLgEz0N4aZGX31o-KpxsME0vLc~kWDsUSV45hP9H18xIOyTeHCbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



