Key Points
Platelet β1 integrin−mediated signals control granule secretion and hemostasis
β1 integrin−mediated outside-in signaling is independent of direct kindlin-integrin interaction
Abstract
Integrins are critical for platelet adhesion and aggregation during arterial thrombosis and hemostasis. Although the platelet-specific αIIbβ3 integrin is known to be crucial for these processes, the in vivo role of β1 integrins is a matter of debate. Here we demonstrate that mice expressing reduced levels of β1 integrins or an activation-deficient β1 integrin show strongly reduced platelet adhesion to collagen in vitro and in a carotis ligation model in vivo. Interestingly, hypomorphic mice expressing only 3% of β1 integrins on platelets show normal bleeding times despite reduced platelet adhesion. The residual 3% of β1 integrins are able to trigger intracellular signals driving Rac-1−dependent granule release required for platelet aggregation and hemostasis. Our findings support a model, in which platelet β1 integrins serve as an important signaling receptor rather than an adhesion receptor in vivo and therefore promote β1 integrins as a promising and so far clinically unemployed antithrombotic target.
Introduction
The rupture of atherosclerotic plaques leads to the exposure of matrix proteins, which in turn trigger arterial thrombosis, leading to stroke and cardiac infarction. Different platelet receptors sense the exposed matrix and induce platelet activation and secretion of prothrombotic molecules, leading to further platelet recruitment and finally thrombus formation. Integrins represent the major family of cell-adhesion receptors on platelets. They are heterodimeric transmembrane molecules consisting of α and β subunits. The αIIbβ3 integrin is exclusively expressed on platelets and can bind von Willebrand factor (vWF), fibrinogen, and fibronectin. In addition to αIIbβ3, platelets express three β1 integrins, α2β1, α5β1, and α6β1, albeit at lower levels, which bind to collagen, fibronectin, and laminin, respectively. Circulating platelets express integrins in a conformation with a low affinity for the ligand. At sites of vascular injury, extracellular stimuli, such as collagen binding to glycoprotein VI (GPVI), induce the formation of active integrins with high affinity for ligands. This process is termed integrin activation or integrin inside-out signaling and requires the direct interaction of talin and kindlins with the cytoplasmic domain of the β integrin subunit.1,2
Although the fundamental role of the αIIbβ3 integrin for platelet-mediated hemostasis is indisputable, the in vivo relevance of β1 integrins on platelets remains controversial because of either different approaches to target β1 integrin function in platelets, including the use of α2 or β1 integrin-deficient mice,3,4 β1 integrin−blocking antibodies, synthetic collagen-derived peptides, or small-molecule inhibitors,5-7 or to the different experimental methods used to model arterial thrombosis8,9 and to assay bleeding tendency.3,10 Depending on the assay, β1 integrins have been reported to be both crucial and dispensable for platelet accumulation in vivo. Several in vitro studies indicated an important role of α2β1 integrin in the activation of various signaling molecules that are known to induce integrin αIIbβ3 activation, platelet spreading, and aggregation.11,12 In one study with two patients, investigators reported that the loss of α2β1 integrin is associated with minor bleeding13,14 attributable to defective α2β1 integrin function, resulting in impaired platelet adhesion and aggregation to collagen or collagen-derived peptides under shear conditions.10,15 Overall, the exact role of β1 integrins remains a matter of debate.
In the present study we therefore investigated the role of β1 integrins on platelets during arterial thrombosis by using mouse mutants that express different levels of β1 integrins or an activation-deficient β1 integrin. We report that β1-null mice or an activation-deficient β1 integrin mutant show prolonged bleeding times because of insufficient activation of Rac-1, actin dynamics, granule secretion, and platelet aggregation. Interestingly, platelets expressing 3% of wild-type β1 integrin show reduced adhesion to sites of vascular injury; however, bleeding times, Rac-1 activation, and degranulation are not affected. The relevance of these findings is discussed.
Materials and methods
Reagents and antibodies
Thrombin and heparin were purchased from Sigma-Aldrich and convulxin (CVX), adenosine 5′-diphosphate, and U46619 were from Alexis. Horm-collagen was purchased from Nycomed. The following antibodies were used for flow cytometry: PE-integrin β1 (CD29), PE-IgG hamster isotype (Biolegend), PE-integrin β3 (CD61; eBioscience), FITC-integrin α2 (CD49b; Emfret), PE-integrin α5 (CD49e) and PE-integrin α6 (CD49f; PharMingen), FITC-9EG7 (active integrin β-1), IgG1 rat isotype control (BD Biosciences); FITC-GPIa, FITC-GPVI, FITC-GPIX, PE-CD62P, PE-JON/A (active Integrin αIIbβ3; all from Emfret), FITC-IgG2a rat isotype control, FITC-IgG2b rat isotype control (NatuTec), and PE-IgG2a rat isotype control (PharMingen).
The following antibodies were used for western blotting: antikindlin-3,16 anti-Talin-1 (Sigma-Aldrich), antifibrinogen (γ-chain) (Abcam), antithrombospondin-1, anti-VEGF, anti-vWF (Santa Cruz Biotechnologies, Inc), anti-GAPDH (Calbiochem), anti-Rac-1 (Cell Biolabs), anti-PAK1 (phosphorylated-Thr432)/PAK2 (phosphorylated-Thr402), anti-MLC (phosphorylated-Ser19), anti-MLC (Cell Signaling), anti-FAK (phosphorylated-Y397; Biosource), anti-FAK (Upstate), and anti-β1 integrin (Millipore).
Mice
Conditional integrin β1 mice (β1fl/fl), kindlin-3−/−, integrin β1 hypomorphic (β1Hpm/fl) mice, and Mx1-Cre transgenic mice have been described previously.1,17-19 Bone marrow chimeras were generated by injecting 6 × 106 bone marrow cells into the tail vein of lethally irradiated 8-week-old C57BL/6 mice. Three weeks later, Cre expression in the hematopoietic system was induced by two intraperitoneal injections of 250 µg of polyI/C (Amersham Biosciences) with a time lag of 2 days.
Platelet preparation
Heparinized whole blood was centrifuged to isolate platelet-rich plasma. Platelets were washed in Tyrodes buffer, pH 6.5 (134 mM NaCl, 2.9 mM KCl, 12 mM NaHCO3, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) containing 5 mM glucose and 0.35% bovine serum albumin (BSA) and were kept at 37°C. For experiments ex vivo, platelets were resuspended in Tyrodes buffer, pH 7.4, containing 5 mM glucose, 0.35% BSA, 1 mM CaCl2, and 1 mM MgCl2.
Flow cytometry
Heparinized blood was diluted 1:50 in Tyrodes buffer, pH 7.4, containing 5 mM glucose, 0.35% BSA, 1 mM CaCl2, and 1 mM MgCl2. To quantify surface receptor expression level, blood samples were incubated with fluorophore-conjugated antibodies for 15 minutes at room temperature and analyzed on a FACScalibur flow cytometer (Becton Dickinson, Heidelberg, Germany). For integrin activation assays, washed platelets were stimulated for 15 minutes with the indicated agonists and incubated with fluorophore-conjugated conformation sensitive antibodies.
Platelet spreading
Coverslips were coated with 1 mg/mL fibrinogen (Sigma-Aldrich) or 25 μg/mL acid soluble collagen I (Sigma-Aldrich) overnight, washed and blocked with 1% BSA. Washed platelets (1 × 106) were suspended in 150 μL of Tyrodes buffer (pH 7.4; 1 mM CaCl2, 1 mM MgCl2), stimulated with 0.01U/mL thrombin, and seeded on coated coverslides in the presence or absence of 0.75 mM Mn2+. After 45 minutes at 37°C, differential interference contrast microscopy was performed with a Zeiss Axiovert 200M microscope with a Plan-NEOFLUAR, ×100, 1.45 oil objective (Zeiss, Jena, Germany). Pictures were acquired via the use of Metamorph software (Molecular Devices). Platelet spreading area was analyzed using ImageJ software (http://rsbweb.nih.gov/ij/).
Carotid ligation model
Experiments were performed as described by Massberg et al.20 In brief, carotid arteries of anesthetized mice were dissected and ligated by the use of a small suture for 5 minutes. To visualize platelet adhesion, fluorescently 2′,7′-dichlorofluorescein (Molecular Probes)-labeled platelets (200 × 106/250 μL) from control and mutant mice were infused, and the number of adherent platelets at the lesion site was quantified by video-fluorescence microscopy with a high-speed, wide-field fluorescent microscope with a long distance condenser and a 20× (NA 0.95) water immersion objective and a coupled camera (ORCA-ER; Hamamatsu Photonics). Images were acquired and analyzed using a Cell^R software (Olympus). The number of adherent platelets per mm2 is represented as percentage of adherent β1+/+ platelets at the 5-minute time point.
Analysis of bleeding time
Ten- to twelve-week-old mice were anesthetized by constant isoflurane gas-narcosis and subcutaneous application of weight-adapted fentanyl (0.05 mg/kg; CuraMed Pharma GmbH) and placed on a 37°C heating pad. An 8-mm segment of the tail tip was cut off, and the bleeding tail was immediately placed into 37°C warm physiological saline solution. Time until bleeding vanished was measured. All experiments were stopped latest after 15 minutes by cauterization to prevent excessive blood loss.
Aggregation
To determine platelet aggregation, light transmission was measured over 10 minutes in a two-channel aggregometer (CHRONO-LOG) and was expressed as arbitrary units. Washed platelets (2 × 108/mL) were stimulated with different agonists in Tyrodes buffer (pH 7.4, 1 mM CaCl2, 1 mM MgCl2) in the presence or absence of fibrinogen (100 μg/mL) or Mn2+ (0.75 mM), respectively.
Evaluation of adenosine triphosphate (ATP) release
A luciferin-luciferase detection kit (CHRONO-LOG) was used to quantify the release of ATP platelets. For these experiments, platelets were stimulated in a lumino-aggregometer (CHRONO-LOG) under stirring conditions, in the presence of luciferase and luciferin. Emitted luminescence was recorded as arbitrary units, and values were expressed as signal ratio of collagen and CVX-stimulated platelets.
Serotonin and PF-4 release assays
Platelets were stimulated in an aggregometer (CHRONO-LOG) under stirring conditions for 8 minutes. Platelets were pelleted by centrifugation for 30 seconds. The serotonin and PF-4 contents within the supernatants were determined using the Serotonin ELISA (LDN, Germany) and PF-4 ELISA (Abcam) Kits following the manufacturer’s instructions.
Granule protein release
Platelets were stimulated in an aggregometer in the absence of BSA as described previously. After 8 minutes, platelets were pelleted by centrifugation (20 000 × g for 30 seconds) and immediately lysed in Laemmli buffer (60 mM Tris-Cl, pH 6.8; 2% sodium dodecyl sulfate; 10% glycerol; 5% β-mercaptoethanol; 0.01% bromophenol blue). Lysates were subjected to immunoblotting and probed with the indicated antibodies.
Rac-1 activity assay
Rac-1 activity was analyzed with a Rac-1 Activation-Assay-Kit (Cell Biolabs). Platelets were stimulated with 5 μg/mL fibrillar collagen for 60 seconds in an aggregometer as described and then lysed by addition of 2× lysis buffer (125 mM HEPES, pH 7.5; 750 mM NaCl; 5% NP-40; 50 mM MgCl2; 5 mM EDTA; 10% glycerol) on ice. Cleared lysates were incubated with PAK-PBD agarose and processed according to the manufacturer’s protocol. Rac-1-GTP binding was determined by western blot analysis.
Measurement of filamentous actin content
Platelets were stimulated with 5 μg/mL fibrillar collagen in an aggregometer under stirring conditions in the presence of 0.5 mM RGDS (Sigma-Aldrich). At indicated time points, platelets were fixed in 1.8% parformaldehyde, permeabilized in 0.02% Triton X-100 phosphate-buffered saline, and stained with FITC-phalloidin (Invitrogen). F-actin content was quantified using flow cytometry.
Statistical analysis
All data are shown as mean ± SEM. To test significance level, an unpaired Student t test was performed, and a value of P < .05 was considered significant. If indicated, one-way analysis of variance followed by a Tukey multiple comparison test was performed.
Ethics statement
All animal experiments were performed with approval by the District Government of Bavaria (Munich, Germany).
Results
Genetic targeting of β1 integrin
To address the role of platelet β1 integrins for platelet activation, adhesion, and aggregation, we generated mouse strains with different β1 integrin expression levels and an activation-deficient β1 integrin mutant. We derived platelets from offspring (β1fl/fl Mx1-Cre; β1+/fl Mx1-Cre) of intercrosses between floxed β1 integrin mice and the inducible Mx1-Cre strain,18 from β1 integrin hypomorphic mice,17 and from Kindlin binding−deficient mice carrying the TT788/789AA substitutions in the β1 integrin cytoplasmic domain.21 Because homozygous hypomorphic β1 integrin (β1hpm/hpm) mice expressing very low levels of functional β1 integrin and homozygous β1TTAA/TTAA mice die at peri-implantation, we crossed β1+/hpm and β1+/TTAA mice, respectively, with floxed β1 integrin and Mx1-Cre mice to generate β1hpm/fl Mx1-Cre and β1TTAA/fl Mx1-Cre mice. Injection of poly-IC induced the expression of the Cre-recombinase, leading to the deletion of the floxed β1 integrin gene predominantly in hematopoietic cells resulting in blood cell-restricted β1-null (KO), heterozygous (HT), hypomorphic (Hpm), and β1TTAA (TTAA) mice. To prevent off-target effects of the Mx1-Cre−mediated gene deletion described in other tissues, we restricted the deletion to the hematopoietic system by using bone marrow chimeras for in vivo experiments.18
Differential β1 integrin expression in mutant mice
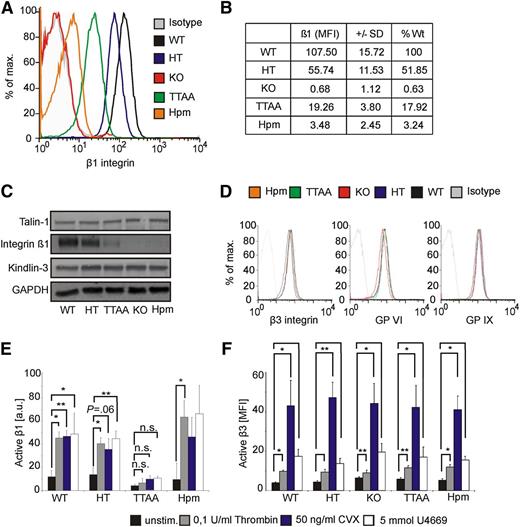
We first quantified the surface expression of platelet β1, α2, α5, and α6 integrins by using flow cytometry. Poly-IC treatment resulted in a loss of β1 integrin surface levels on platelets from β1fl/fl Mx1-Cre mice, reduced the levels by approximately 51% in β1+/fl Mx1-Cre mice, approximately 82% in β1TTAA/fl Mx1-Cre, and approximately 97% in β1hpm/fl Mx-Cre mice (Figure 1A-B). The differences in β1 integrin expression levels were confirmed by western blot analysis (Figure 1C). A similar reduction in the surface expression of the β1 integrin−associated α2, α5, and α6 integrin subunits was measured in the different platelet populations (supplemental Table 1A; see the Blood Web site). Furthermore, we analyzed the distribution of β1 integrin expression on hypomorphic platelets, which showed a mean expression of 3% compared with wild-type platelets. Fluorescence-activated cell sorting analysis revealed that 80% of the platelets from hypomorphic mice express <8.5% of β1 integrins, whereas only 5% express more than 25% of β1 integrins compared with controls (supplemental Table 1B). It has been previously shown that the reduced expression of β1TTAA integrin was caused by increased degradation.21 Expression of talin-1 (for quantification, see supplemental Figure 1) and kindlin-3 was similar in platelets of all genotypes (Figure 1C). Furthermore, surface levels of β3 integrin, GPVI, and GPIX also were unaffected by the different β1 levels (Figure 1D).
Characterization of platelets from β1 integrin mouse mutants. Platelet β1 integrin surface expression of wild-type (WT; β1+/+), HT (β1+/−), KO (β1−/−), TTAA (β1TTAA), and Hpm (β1Hpm) β1 integrin mice were analyzed by flow cytometry. (B) Geometric mean values of β1 integrin expression shown in panel A were corrected for isotype control and expressed as % of WT β1-integrin surface levels (n = 6 per group). (C) Platelet lysates were subjected to immunoblotting for β1-integrin, talin-1, kindlin-3, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (D) Surface expression of β3 integrin, GPVI, and GPIX on platelets of indicated mouse strains. (E) Platelet β1 integrin activation was determined with the conformation-specific 9EG7 antibody via the use of flow cytometry upon stimulation with 50 ng/mL CVX, 0.1 U/mL thrombin, or 5 mM U46619. Acquired mean fluorescence intensity values were normalized to total β1 integrin expression levels and are shown as arbitrary unit (n = 3 for TTAA and Hpm, n = 4 for all other groups; bars represent mean values ± SEM; significance levels are indicated; *P < .05; **P < .01; n.s., not significant). (F) Active β3 integrins were determined with JON/A antibody after platelet stimulation with indicated stimuli. No significant difference between all tested groups was determined (n = 5 for HT and WT, n = 4 for all other groups; bars represent geometric mean values ± SEM).
Characterization of platelets from β1 integrin mouse mutants. Platelet β1 integrin surface expression of wild-type (WT; β1+/+), HT (β1+/−), KO (β1−/−), TTAA (β1TTAA), and Hpm (β1Hpm) β1 integrin mice were analyzed by flow cytometry. (B) Geometric mean values of β1 integrin expression shown in panel A were corrected for isotype control and expressed as % of WT β1-integrin surface levels (n = 6 per group). (C) Platelet lysates were subjected to immunoblotting for β1-integrin, talin-1, kindlin-3, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (D) Surface expression of β3 integrin, GPVI, and GPIX on platelets of indicated mouse strains. (E) Platelet β1 integrin activation was determined with the conformation-specific 9EG7 antibody via the use of flow cytometry upon stimulation with 50 ng/mL CVX, 0.1 U/mL thrombin, or 5 mM U46619. Acquired mean fluorescence intensity values were normalized to total β1 integrin expression levels and are shown as arbitrary unit (n = 3 for TTAA and Hpm, n = 4 for all other groups; bars represent mean values ± SEM; significance levels are indicated; *P < .05; **P < .01; n.s., not significant). (F) Active β3 integrins were determined with JON/A antibody after platelet stimulation with indicated stimuli. No significant difference between all tested groups was determined (n = 5 for HT and WT, n = 4 for all other groups; bars represent geometric mean values ± SEM).
β1 integrin inside-out signaling depends on an intact kindlin-binding site
We then analyzed the β1 integrin activation state on platelets from the different mice. Active β1 integrins were quantified by flow cytometry by use of the conformation-specific 9EG7 antibody that recognizes the active conformation of β1 integrins and the measured values were then corrected for the different β1 integrin expression. Treatment with CVX, the thromboxane A analog U46619, and thrombin led to a similar β1 integrin activation on platelets from wild-type, heterozygous, and hypomorphic mice. In sharp contrast, all three agonists failed to activate the kindlin binding−deficient β1TTAA integrin (Figure 1E). Importantly, the activation of αIIbβ3 integrins was not affected by β1 integrin surface levels or the kindlin binding mutation (Figure 1F). These data show that an intact kindlin-3 binding site in β1 integrins is required for their activation in vivo and that an intact binding site for talin-1 alone is not sufficient to activate β1 integrins.
β1 integrin−mediated outside-in signaling and spreading is independent of kindlin-integrin interaction
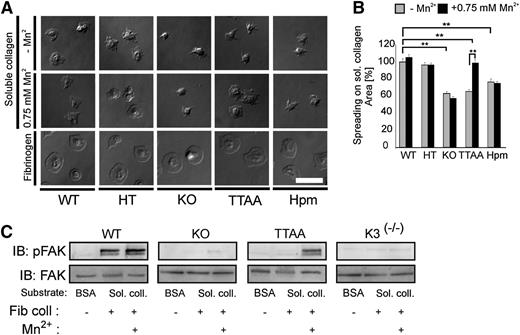
Next, we analyzed platelet adhesion and spreading on acidic soluble collagen. Acidic soluble collagen I is bound by α2β1 integrin via a repetitive GFOGER motif. This motif is not recognized by GPVI, which binds GPOn exposed on fibrillar collagen.5,22 In contrast to human platelets that can bind soluble collagen and generate intrinsic signaling without previous activation, mouse platelets require a weak intrinsic activation to spread on soluble collagen.23 Taking this into account, we stimulated washed mouse platelets with 0.01 U/mL thrombin in the presence or absence of 0.75 mM Mn2+. As depicted in Figure 2A, β1Hpm and β1-null platelets showed a severe spreading defect on soluble collagen I both in the presence or absence of Mn2+. Furthermore, they formed elongated filopodia and only occasionally small lamellipodia. Activation of β1TTAA platelets also failed to form lamellipodia and showed a significantly reduced spreading (Figure 2A-B). Notably, spreading of β1Hpm platelets was not improved by increasing the coating concentration of collagen (supplemental Figure 2A). Interestingly, however, bypassing integrin inside-out signaling by Mn2+-treatment rescued the spreading defect in β1TTAA platelets (Figure 2A-B). In light of our previous observation showing that Mn2+-induced platelet spreading is abolished in kindlin-3−deficient platelets,1 we conclude that a direct interaction between kindlin-3 and β1 integrin is not required for integrin outside-in signaling.
β1 integrin−mediated spreading and outside-in signaling is independent of kindlin-integrin interaction. (A) Spreading of thrombin-stimulated platelets on soluble collagen in the absence or presence of 0.75 mM Mn2+ and on fibrinogen. Representative pictures are shown 45 minutes after platelet seeding (scale bar represents 5 μm). (B) Spreading area of mutant platelets on soluble collagen is shown relative to wild-type (WT) platelets at 45 minutes (bars represent mean values ± SEM; significance level are indicated; *P < .05; **P < .01). (C) Integrin outside-in signaling was evaluated by FAK autophosphorylation at Tyr379. Washed platelets from wild-type (WT), β1 knockout (KO), and TTAA mice as well as from kindlin-3−deficient mice (K3(−/−)) were stimulated with 5 μg/mL fibrillar collagen and seeded on soluble collagen-coated surfaces in the absence or presence of Mn2+. To analyze basal FAK phosphorylation, unstimulated platelets were seeded on BSA. Cells were lysed after 30 minutes and subjected to immunoblotting for FAK Y397 phosphorylation and total FAK.
β1 integrin−mediated spreading and outside-in signaling is independent of kindlin-integrin interaction. (A) Spreading of thrombin-stimulated platelets on soluble collagen in the absence or presence of 0.75 mM Mn2+ and on fibrinogen. Representative pictures are shown 45 minutes after platelet seeding (scale bar represents 5 μm). (B) Spreading area of mutant platelets on soluble collagen is shown relative to wild-type (WT) platelets at 45 minutes (bars represent mean values ± SEM; significance level are indicated; *P < .05; **P < .01). (C) Integrin outside-in signaling was evaluated by FAK autophosphorylation at Tyr379. Washed platelets from wild-type (WT), β1 knockout (KO), and TTAA mice as well as from kindlin-3−deficient mice (K3(−/−)) were stimulated with 5 μg/mL fibrillar collagen and seeded on soluble collagen-coated surfaces in the absence or presence of Mn2+. To analyze basal FAK phosphorylation, unstimulated platelets were seeded on BSA. Cells were lysed after 30 minutes and subjected to immunoblotting for FAK Y397 phosphorylation and total FAK.
To further corroborate this finding we analyzed autophosphorylation of focal adhesion kinase (FAK) at position Tyr397 as an immediate effect of collagen-bound β1 integrin signaling. Indeed, β1TTAA platelets showed robust FAK phosphorylation in the presence of Mn2+, which was neither seen in β1-null nor Kindlin-3−null platelets (Figure 2C). Of note, platelet adhesion and spreading on the α6β1 ligand laminin was similarly affected (supplemental Figure 2B). In contrast, adhesion and spreading on the αIIbβ3 ligands fibrinogen (Figure 2A) and fibronectin or on the GPVI substrate collagen-related peptide (supplemental Figure 2B) was not altered in any of the genotypes examined here. Because Mn2+ treatment of β1TTAA platelets show normal spreading, these findings indicate that a direct interaction between the integrin cytoplasmic domain and kindlin-3 is required for inside-out signaling, but this interaction is dispensable for integrin-mediated, outside-in signaling and cytoskeletal reorganization during platelet spreading.
β1 integrins are important for platelet adhesion and hemostasis
We next investigated whether the in vitro defects have consequences in vivo. The reduced β1 integrin expression in β1hpm mice and the expression of β1TTAA integrins had no impact on thrombopoiesis because platelet counts were similar to control animals (data not shown). For studying platelet adhesion to vascular lesions in vivo, we used the in vivo carotid ligation model, in which platelets accumulate in a collagen-dependent manner20 and which allows monitoring single-platelet adhesion to a defined subendothelial vessel wound by intravital microscopy over time. Consistent with an important role of collagen for platelet adhesion, we observed that β1 integrin−deficient platelets as well as hypomorphic and β1TTAA mutant platelets, but not platelets from heterozygous β1 integrin knockout mice, showed a strong decrease in platelet adhesion to the wounded vessel wall 5 to 60 minutes after vascular injury (Figure 3A). These findings indicate that β1 integrins significantly contribute to primary adhesion to subendothelial lesions and that 50% but not the 3% of wild-type β1 integrins (present on β1 hypomorphic platelets) are sufficient to mediate this task. The reduced adhesion of β1TTAA platelets is likely attributable to the activation defect of the β1 integrins, although we cannot fully rule out that the expression level of approximately 18% compared with wild-type platelets also contributes to the observed adhesion defect.
Reduced platelet adhesion and aggregate formation onto injured vessel walls of β1-null, β1TTAA, and β1hpmmice, whereas tail bleeding times of β1hpmmice are normal. (A) Number of adherent fluorescently labeled platelets to the injured vessel wall was quantified after 5, 10, 15, and 60 minutes. Values are expressed as percentage of adherent wild-type (WT) platelets (n/mm2) at 5 minutes (WT, n = 9; HT, n = 6; knockout [KO], n = 6; TTAA, n = 4; Hpm, n = 8; bars represent mean values ± SEM in percent, significance level are indicated; *P < .05; **P < .01 by one-way analysis of variance followed by a Tukey multiple comparison test). (B) Tail-bleeding times of chimeric mice carrying the bone marrow of the indicated genotype (WT, n = 19; fl/fl, n = 6; HT, n = 10; KO, n = 9; TTAA, n = 15; TTAA/fl, n = 6; Hpm, n = 13; Hpm/fl, n = 6; Kindlin-3−/−, n = 4; cross line represents mean bleeding time and bars represent SEM, significance level are indicated; **P < .01).
Reduced platelet adhesion and aggregate formation onto injured vessel walls of β1-null, β1TTAA, and β1hpmmice, whereas tail bleeding times of β1hpmmice are normal. (A) Number of adherent fluorescently labeled platelets to the injured vessel wall was quantified after 5, 10, 15, and 60 minutes. Values are expressed as percentage of adherent wild-type (WT) platelets (n/mm2) at 5 minutes (WT, n = 9; HT, n = 6; knockout [KO], n = 6; TTAA, n = 4; Hpm, n = 8; bars represent mean values ± SEM in percent, significance level are indicated; *P < .05; **P < .01 by one-way analysis of variance followed by a Tukey multiple comparison test). (B) Tail-bleeding times of chimeric mice carrying the bone marrow of the indicated genotype (WT, n = 19; fl/fl, n = 6; HT, n = 10; KO, n = 9; TTAA, n = 15; TTAA/fl, n = 6; Hpm, n = 13; Hpm/fl, n = 6; Kindlin-3−/−, n = 4; cross line represents mean bleeding time and bars represent SEM, significance level are indicated; **P < .01).
Next, we tested whether β1 integrins also play a role for occlusive thrombus formation and hemostasis using the tail-bleeding assay. Although wild-type, heterozygous, and animals carrying a floxed β1 integrin allele but no Mx1-Cre allele (β1fl/fl, β1TTAA/fl, β1Hpm/fl) showed similar bleeding times of 2 to 3 minutes, β1 integrin knockout and β1TTAA mice showed significantly prolonged tail bleeding times of 8 to 9 minutes (Figure 3B), and kindlin-3− deficient mice exhibited the most severe bleeding defect as the result of impaired β1 and αIIbβ3 integrin activation.1 Surprisingly, hypomorphic mice showed normal bleeding times. Taken together, these data indicate that β1 integrins play a crucial role for stopping bleeding and as little as 3% of them are sufficient enabling a normal hemostasis.
Defective aggregation and granule secretion in β1TTAA and β1-null mice
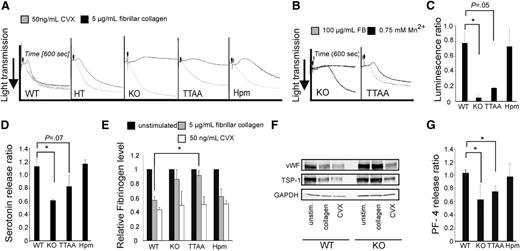
Occlusive thrombus formation requires a second wave of platelet recruitment, activation, and subsequent aggregation, which is triggered by the release of paracrine factors stored in platelet α and dense granules. To find an explanation for the normal tail bleeding times of β1 hypomorphic mice, we investigated platelet aggregation in more detail. In brief, washed platelets were activated with either 5 µg/mL fibrillar collagen or with 50 ng/mL CVX, which activates GPVI by inducing GPVI receptor dimerization and downstream signaling.24 CVX induced aggregation of platelets of all genotypes (Figure 4A; supplemental Figure 3A). In contrast, β1-deficient and β1TTAA platelets failed to aggregate after stimulation with fibrillar collagen, whereas β1hpm platelets showed an intermediate response (Figure 4A). Exogenous activation of β1TTAA platelets by Mn2+ resulted in normal aggregation, but this did not occur in β1-deficient platelets (Figure 4B). This latter finding further supports the concept that the double threonine motif within the β1 integrin cytoplasmic domain is required for integrin inside-out but not for integrin outside-in signaling.
Collagen-induced platelet aggregation and granule secretion requires β1 integrins. (A) Washed platelets were stimulated with 5 μg/mL fibrillar collagen (black curves) or 50 ng/mL CVX (gray curves) and aggregation was recorded for 600 seconds. (B) Aggregation of β1-null (KO) and β1TTAA (TTAA) platelets in the presence of 100 μg/mL fibrinogen (gray curves) or 0.75 mM Mn2+ (black curves). (C) ATP release from platelet dense granules after stimulation of platelets with collagen. ATP was measured by luminescence after addition of luciferase luciferin reagent using the Lumi-aggregometer (Chronolog, Havertown, PA). Platelets were stimulated with either 5 μg/mL fibrillar collagen or 50 ng/mL CVX for 10 minutes. The relative luminescence after 8 minutes (ratio of collagen to CVX stimulated cells) is shown (bars represent mean values ± SEM of n = 3 independent experiments). (D) Serotonin release after stimulation with collagen. Platelets were stimulated with either 5 μg/mL fibrillar collagen or 50 ng/mL CVX for 8 minutes. The relative serotonin level (ratio of collagen to CVX stimulation) is shown (bars represent mean values ± SEM of n ≥ 3 independent experiments). (E) Quantification of the cellular fibrinogen content in resting platelets or 8 minutes after stimulation with either fibrillar collagen (5 μg/mL) or CVX (50 ng/mL; bars represent mean values ± SEM; significance levels are indicated; *P < .05; wild-type (WT), n = 3; knockout (KO), n = 3; TTAA, n = 3; hypomorphic (Hpm), n = 3; representative blots are shown in supplemental Figure 4B). (F) Platelet content of the α-granule cargo proteins vWF and thrombospondin-1 (TSP-1) in wild-type (WT) and β1-null (KO) platelets upon collagen and stimulation with CVX. (G) Ratio of PF-4 release from platelets stimulated with either 5 μg/mL fibrillar collagen or 50 ng/mL CVX for 8 minutes (bars represent mean values ± SEM of n ≥ 3 independent experiments).
Collagen-induced platelet aggregation and granule secretion requires β1 integrins. (A) Washed platelets were stimulated with 5 μg/mL fibrillar collagen (black curves) or 50 ng/mL CVX (gray curves) and aggregation was recorded for 600 seconds. (B) Aggregation of β1-null (KO) and β1TTAA (TTAA) platelets in the presence of 100 μg/mL fibrinogen (gray curves) or 0.75 mM Mn2+ (black curves). (C) ATP release from platelet dense granules after stimulation of platelets with collagen. ATP was measured by luminescence after addition of luciferase luciferin reagent using the Lumi-aggregometer (Chronolog, Havertown, PA). Platelets were stimulated with either 5 μg/mL fibrillar collagen or 50 ng/mL CVX for 10 minutes. The relative luminescence after 8 minutes (ratio of collagen to CVX stimulated cells) is shown (bars represent mean values ± SEM of n = 3 independent experiments). (D) Serotonin release after stimulation with collagen. Platelets were stimulated with either 5 μg/mL fibrillar collagen or 50 ng/mL CVX for 8 minutes. The relative serotonin level (ratio of collagen to CVX stimulation) is shown (bars represent mean values ± SEM of n ≥ 3 independent experiments). (E) Quantification of the cellular fibrinogen content in resting platelets or 8 minutes after stimulation with either fibrillar collagen (5 μg/mL) or CVX (50 ng/mL; bars represent mean values ± SEM; significance levels are indicated; *P < .05; wild-type (WT), n = 3; knockout (KO), n = 3; TTAA, n = 3; hypomorphic (Hpm), n = 3; representative blots are shown in supplemental Figure 4B). (F) Platelet content of the α-granule cargo proteins vWF and thrombospondin-1 (TSP-1) in wild-type (WT) and β1-null (KO) platelets upon collagen and stimulation with CVX. (G) Ratio of PF-4 release from platelets stimulated with either 5 μg/mL fibrillar collagen or 50 ng/mL CVX for 8 minutes (bars represent mean values ± SEM of n ≥ 3 independent experiments).
Unexpectedly, the addition of exogenous fibrinogen to washed platelets rescued the aggregation defect of β1TTAA and β1-null platelets (Figure 4B). Platelets contain substantial amounts of fibrinogen within their granules, which they release upon stimulation (eg, by collagen). Hence, the finding that exogenous fibrinogen is able to rescue defective aggregation of β1TTAA and β1-null platelets suggests a novel role of β1 integrin in the regulation of fibrinogen release from α granules in platelets. We therefore tested whether β1 integrin-mediated signals are important for granule secretion in general. Dense granule secretion was quantified by measuring ATP release after addition of luciferin/luciferase reagent in an aggregometer. Platelets were stimulated either with 50 ng/mL CVX or 5 μg/mL fibrillar collagen, and values were calculated as a ratio between fibrillar collagen- and CVX-induced luminescence.
Although β1-null and β1TTAA platelets showed a severe defect in dense granule secretion after fibrillar collagen stimulation, β1hpm platelets showed an almost-normal exocytosis of dense granules upon collagen stimulation (Figure 4C; supplemental Figure 3B). Furthermore, the defect in dense granule secretion of β1-null and β1TTAA platelets was corroborated by reduced secretion of serotonin (Figure 4D). To analyze platelet α-granule secretion, we determined the cellular level of the α-granule cargo fibrinogen (Figure 4E; supplemental Figure 3C) before and 8 minutes after agonist treatment (5 μg/mL fibrillar collagen or 50 ng/mL CVX) by immunoblotting. Our data show that stimulation with fibrillar collagen led to a decrease in cellular fibrinogen in wild-type and hypomorphic platelets but not in β1-null and β1TTAA platelets. Importantly, the total fibrinogen content was similar in unstimulated platelets of all groups (supplemental Figure 3D), and exocytosis of other α-granule cargo proteins such as thrombospondin, vWF or PF-4 were also β1 integrin–dependent, suggesting that β1 integrin−mediated signals control platelet granule release (Figure 4F-G; supplemental Figure 3E).
β1 integrins control platelet actin polymerization and Rac-1 activation upon collagen stimulation
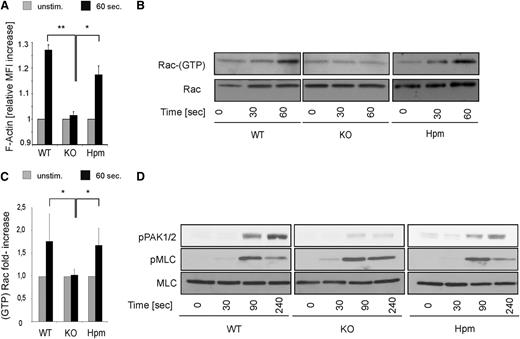
How does β1 integrin control platelet granule secretion? Platelet granule fusion with the outer platelet membrane requires the reorganization of the actin cytoskeleton.25,26 Therefore, we analyzed F-actin dynamics after collagen stimulation by flow cytometry. Indeed β1-null platelets failed to increase their F-actin content after 60 seconds, whereas ∼3% of β1 integrin were sufficient to rescue F-actin polymerization in hypomorphic platelets (Figure 5A).
Normal actin polymerization, Rac activation, and PAK phosphorylation in β1 hypomorphic platelets. (A) Platelets from wild-type (WT), β1-null (KO), and hypomorphic (Hpm) mice were stimulated with 5 µg/mL fibrillar collagen for 60 seconds in the presence of 0.5 mM RGDS peptide, and the relative F-actin content was measured by flow cytometry. Curves represent mean ± SEM increase in FITC phalloidin MFI (n = 3, significance level are indicated). (B) Same platelet populations were stimulated with 5 µg/mL fibrillar collagen for 30 and 60 seconds lysed and subjected to Rac-1 GTP pulldown experiments. Total Rac-1 loading is shown below. (C) Quantification of GTP-bound active Rac-1 pulldown experiments at 60 seconds (bars represent mean values ± SEM; significance level are indicated; *P < .05; **P < .01; wild-type (WT), n = 4; β1-null (KO), n = 3; hypomorphic (Hpm), n = 4). (D) Platelets from WT, KO, and Hpm mice were stimulated with 5 µg/mL fibrillar collagen for the indicated time points, lysed and subjected to immunoblotting for p-Thr432/402 PAK1/2 and p-Ser19 myosin light chain (pMLC). Total MLC is shown as loading control.
Normal actin polymerization, Rac activation, and PAK phosphorylation in β1 hypomorphic platelets. (A) Platelets from wild-type (WT), β1-null (KO), and hypomorphic (Hpm) mice were stimulated with 5 µg/mL fibrillar collagen for 60 seconds in the presence of 0.5 mM RGDS peptide, and the relative F-actin content was measured by flow cytometry. Curves represent mean ± SEM increase in FITC phalloidin MFI (n = 3, significance level are indicated). (B) Same platelet populations were stimulated with 5 µg/mL fibrillar collagen for 30 and 60 seconds lysed and subjected to Rac-1 GTP pulldown experiments. Total Rac-1 loading is shown below. (C) Quantification of GTP-bound active Rac-1 pulldown experiments at 60 seconds (bars represent mean values ± SEM; significance level are indicated; *P < .05; **P < .01; wild-type (WT), n = 4; β1-null (KO), n = 3; hypomorphic (Hpm), n = 4). (D) Platelets from WT, KO, and Hpm mice were stimulated with 5 µg/mL fibrillar collagen for the indicated time points, lysed and subjected to immunoblotting for p-Thr432/402 PAK1/2 and p-Ser19 myosin light chain (pMLC). Total MLC is shown as loading control.
The small GTPase Rac-1 is a central regulator of actin polymerization and granule secretion in platelets and is activated rapidly after platelet stimulation.27 Previous studies showed that genetic and pharmacologic inhibition of Rac-1 efficiently blocks collagen-induced platelet granule secretion and reduces the stability of platelet aggregates under flow.28 Moreover, it has also been shown that α2β1 integrins regulate actin dynamics and lamellipodia formation in platelets spread on soluble collagen in a Rac-1−dependent manner.29 Hence, we next examined the formation of active, GTP-bound Rac-1 within 60 seconds after collagen exposure. Hypomorphic and wild-type platelets showed robust Rac-1 activation within 60 seconds, which was not observed in β1 integrin−null platelets (Figure 5B-C). In line with the reduced Rac-1 activity, phosphorylation of the Rac-1 target proteins PAK1/2 was strongly reduced in β1-null platelets 90 and 240 seconds after collagen treatment. Importantly, also hypomorphic platelets showed significant PAK phosphorylation. In contrast, phosphorylation of MLC in β1 integrin–null platelets was not different (Figure 5D). These results show that 3% of β1 integrin expression is sufficient for rapid Rac-1 activation required to remodel the platelet actin cytoskeleton and granule secretion in response to fibrous collagen.
Discussion
Previous authors have investigated platelet-collagen interactions by using various knockout mouse mutants, pharmacologic inhibitors, and disease models in arterial thrombosis and hemostasis. Despite conflicting results, the current model suggests that both GPVI and integrin α2β1 play significant roles as adhesion receptors; however, whether α2β1 plays an additional crucial role in platelet intracellular signaling controlling platelet aggregation and thrombus formation has not been elaborated. To address the molecular role of β1 integrins for these processes, we generated mice expressing either very low levels of functional (wild-type) β1 integrin or an activation-deficient β1 integrin, which harbors a mutation in the binding site for the essential integrin activating protein kindlin. Our studies reveal critical and distinct roles for β1 integrins in thrombosis and hemostasis dependent on their expression levels: (1) high β1 integrin levels critically contribute to platelet adhesion at sites of vascular injury; (2) β1 integrins assemble signaling platforms required to initiate full-fledged platelet activation and platelet granule secretion in response to fibrillar collagen; and (3) the level of β1 integrins can decrease to as little as 3% for sufficient activation of Rac-1 and its downstream effector molecules to finally promote F-actin formation and initiate platelet granule secretion.
Despite robust Rac-1 activation, hypomorphic platelets fail to spread on plates coated with high concentrations of soluble collagen I, suggesting that a certain number of β1 integrins (>3%) are required for platelet adhesion during spreading. Furthermore, the efficient release of dense granule cargo such as ATP or serotonin and α-granule cargo proteins such as fibrinogen is controlled by β1 integrin outside-in signaling and cannot be compensated by GPVI in the absence of β1 integrins. More potent, although rather nonphysiologic, stimuli such as CVX may mask the contribution of β1 integrin signaling and thereby underestimate its role during thrombotic processes. However, our experimental conditions might not account for complex in vivo situations, in which additional platelet-collagen interactions through vWF and β3 integrin as well as mechanical forces or soluble stimuli might contribute to platelet activation in the presence of plasma proteins such as fibrinogen.
In addition, our data on platelets expressing an activation-deficient β1 integrin are in line with the proposed model that α2β1 integrins directly participate in collagen signaling in an activation-dependent manner.10 Inside-out activation by α2β1 integrin is probably triggered by signals generated by GPVI. These signals can be overcome by external integrin activation by manganese, which rescues the defects in platelet adhesion, spreading, and aggregation of β1TTAA platelets. Thus, upon GPVI-mediated α2β1 activation, collagen-bound α2β1 integrin generates GPVI independent outside-in signals that are sufficient to trigger the release of secondary agonists to fully activate platelets.
Our findings also revealed that β1 integrins on platelets require an intact kindlin binding motif for their activation. This important finding further corroborates previous studies by showing that a direct interaction between kindlin-3 and the β1 integrin cytoplasmic domain is required for platelet β1 integrin activation in vivo.21 However, we also observed that the direct interaction of kindlin-3 with the β1 tail is not required for integrin outside-in signaling and for achieving platelet spreading, FAK phosphorylation, and platelet aggregation. A similar observation has been reported recently for β2 integrins, which form a trimeric complex together with kindlin-3 and Rack-1 in T cells, and that kindlin-3 binding to β2 integrin tails can even occur to a kindlin binding-deficient β2 integrin tail.30 Thus, kindlins need to directly bind the integrin tails for inducing inside-out signaling but a direct contact is either not required or does not occur for triggering outside-in signals. In such a scenario the dissociation of kindlin-3 from the integrin tail after activation has occurred will enable binding of other cytoplasmic proteins to the integrin tail.
Because high levels of β1 integrins are required for primary platelet adhesion to exposed matrix at sites of vascular disease but very low levels are already sufficient to trigger platelet signals required for normal hemostasis, the use of novel therapeutic agents targeting β1 integrins might be a worthwhile approach to reduce arterial thrombosis without increasing the risk for bleeding.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Reinhard Fässler for continuous support, discussions, and help in writing the paper. They also thank Roy Zent and Ambra Pozzi for critically reading the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 914 TP A01 and B02) and the Max Planck Society.
Authorship
Contribution: T.P. designed and performed most part of the study, analyzed data, and assisted in writing the paper; R.R. performed research and analyzed data; D.P. performed research and analyzed data; V.B. performed research and analyzed data; H.M. contributed vital reagents; M.L. performed research and analyzed data; L.Z. performed research and analyzed data; W.S. designed research and contributed to the discussion; S.M. designed research, analyzed data, and wrote the paper; and M.M. designed and performed research, analyzed data, and wrote the paper
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus Moser, Max-Planck-Institute of Biochemistry, Am Klopferspitz 18, D-82152 Martinsried, Germany; e-mail: moser@biochem.mpg.de.
References
Author notes
R.R. and D.P. contributed equally to this study.

![Figure 3. Reduced platelet adhesion and aggregate formation onto injured vessel walls of β1-null, β1TTAA, and β1hpm mice, whereas tail bleeding times of β1hpm mice are normal. (A) Number of adherent fluorescently labeled platelets to the injured vessel wall was quantified after 5, 10, 15, and 60 minutes. Values are expressed as percentage of adherent wild-type (WT) platelets (n/mm2) at 5 minutes (WT, n = 9; HT, n = 6; knockout [KO], n = 6; TTAA, n = 4; Hpm, n = 8; bars represent mean values ± SEM in percent, significance level are indicated; *P < .05; **P < .01 by one-way analysis of variance followed by a Tukey multiple comparison test). (B) Tail-bleeding times of chimeric mice carrying the bone marrow of the indicated genotype (WT, n = 19; fl/fl, n = 6; HT, n = 10; KO, n = 9; TTAA, n = 15; TTAA/fl, n = 6; Hpm, n = 13; Hpm/fl, n = 6; Kindlin-3−/−, n = 4; cross line represents mean bleeding time and bars represent SEM, significance level are indicated; **P < .01).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/15/10.1182_blood-2013-06-508721/4/m_2723f3.jpeg?Expires=1765895521&Signature=TM~~V-sErj-1m76ZhOv3CUWeA5L8BApjAAyQp8LApMFNli60rg5WNHkvh0YI5nLaRNLNcaCGk~7GpZLlJpPWAqJYtOFJya0fufilFCsW9KULqv03-Xf26MHp1s~qEQfp6KtcQ8gYPTdMP~ZGBEsR~VAnHOQMk3RwXtjDKla-t6IVOwIqYfy~fMrNfozHCY4EF7UbNEkbX1Bb6y-oCBrVNCueAVTjb1ZEfo0AYklAXuaX8p7RgWYLOG524pbFNfgVdsgk9~fNWoAZqyCOuOFZ0adP9zQkkKy30tyHki~meR5LJXbzpUADWfS9fyddU1GvWi-Aq71YiN-QgGGlU4zu~w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal