Key Points
Macrophage migration to lymph nodes during acute inflammation is quantitatively minor.
Macrophages are cleared from acute inflammation by local death.
Abstract
Chronic inflammatory diseases such as atherosclerosis are characterized by an accumulation of macrophages. To design therapies that would reduce macrophage burden during disease, understanding the cellular and molecular mechanisms that regulate macrophage removal from sites of resolving inflammation is critical. Although past studies have considered the local death of macrophages or the possibility that they emigrate out of inflammatory foci, methods to quantify death or emigration have never been employed. Here, we applied quantitative competition approaches and other methods to study resolution of thioglycollate-induced peritonitis, the model in which earlier work indicated that emigration to lymph nodes accounted for macrophage removal. We show that migration to lymph nodes occurred in a CC chemokine receptor 7–independent manner but, overall, had a quantitatively minor role in the removal of macrophages. Blocking migration did not significantly delay resolution. However, when macrophages resistant to death were competed against control macrophages, contraction of the macrophage pool was delayed in the apoptosis-resistant cells. These data refute the concept that macrophages are dominantly cleared through emigration and indicate that local death controls macrophage removal. This finding alters the emphasis on which cellular processes merit targeting in chronic diseases associated with accumulation of macrophages.
Introduction
Resolution of acute inflammation is a crucial step toward return to tissue homeostasis after initiation of tissue injury.1 In the case of infection, it is self-evident that clearance of the inciting microorganism or microorganisms would be necessary before resolution could be completed, but there are a growing list of health conditions not explicitly associated with infection in which low-grade chronic inflammation is observed, including metabolic diseases (obesity, atherosclerosis), neurodegenerative diseases, and cancers.2-4 A better understanding of the mechanisms that support resolution of inflammation might facilitate new strategies aimed to combat these diseases.5
Initiation of inflammation is typically characterized by early neutrophil recruitment, followed by an influx of monocytes that develop into inflammatory macrophages. The neutrophils undergo death within hours and are cleared by macrophages,6,7 with efferocytosis of neutrophils handled by neighboring macrophages. Efferocytosis triggers the production of mediators such as transforming growth factor β1, which in turn generate macrophages that facilitate resolution and tissue repair.8,9 Novel lipid mediators participate critically in control of these steps.7 Although there is consensus that the fate of neutrophils in acute inflammation is regulated through local death and efferocytosis,6,7 with a small number of neutrophils emigrating to draining lymph nodes (LNs),10 consensus is still lacking on the fate of monocyte-derived cells that become inflammatory macrophages. Two concepts are apparent in the literature: inflammatory macrophages die locally,11-14 or alternatively, these cells are primarily cleared by emigration to draining LNs.15 Although none of the studies is quantitative, the latter concept has received the most attention, cited as a classic in the field (www.nature.com/ni/focus/inflammation/classics/macrophages.html).
Accordingly, we earlier wondered whether the failure of resolution in atherosclerosis16 might be linked to the failure of macrophages to acquire the appropriate emigratory phenotype.17 However, although some evidence supports the link between atherosclerosis and emigration to LNs,18-20 emigration from atherosclerotic plaques is not required to promote clearance of macrophages from plaques.21 Instead, a combination of macrophage death and cessation of monocyte entry is sufficient for macrophage contraction during disease regression.21 These observations and the overall qualitative nature of the previous studies11-13,15 led us to reexamine the cellular mechanisms that underlie removal of macrophages from sites of resolving inflammation. We chose to use the model of thioglycollate-induced peritonitis that previously claimed a key role for emigration to LNs and added techniques to quantitatively evaluate emigration and death. We show that emigration to LNs plays only a minor quantitative role in the removal of macrophages relative to a dominant role for local death. Considering that the peritonitis model used in our study was the same as that used in the work claiming that emigration to LNs mediates macrophage removal, our findings indicate that the concept of emigration to LNs as a quantitatively significant driver of macrophage clearance no longer holds experimental support.
Methods
Animals and treatments
C57BL/6J mice carrying the Ly5.1 allele (CD45.2) were purchased from The Jackson Laboratories or the National Cancer Institute. CD45.1 C57BL/6 mice were from the National Cancer Institute. CD45.2 and CD45.1 mice were bred to obtain CD45.1 × CD45.2 F1 mice. CC chemokine receptor (CCR)7−/−, CCR2−/−, and Bim−/− mice were obtained from The Jackson Laboratories. Bone marrow cells from CD68-hBcl2 (Mϕ-hBcl2) mice22 were used to transplant lethally irradiated mice, as described.23
Resolving acute peritonitis was induced by intraperitoneal injection of 1 mL sterile thioglycollate (Sigma-Aldrich; 3% weight/volume). Pertussis toxin (PTX; Sigma-Aldrich) was injected intraperitoneally (2.5 µg/mouse). PKH26 Red Fluorescent Cell Linker Kit for Phagocytic Cell Labeling was from Sigma-Aldrich; 200 µL of a 5-μM solution was injected intraperitoneally. Mice were housed in a specific pathogen-free environment and used in accordance with protocols approved by the Animal Care and Utilization Committees at Mount Sinai School of Medicine or Washington University.
Antibodies
Anti-mouse CD115 (AFS98), F4/80 (BM8), CD45 (30-F11), MHC-II (IA-IE, M5/114.15.2), CD45.2 (104), CD45.1 (A20), and CD11c (N418) were from eBiosciences. Anti-mouse Gr-1 (Ly-6C/G, RB6-8C5) and CD36 (HM36) were from Biolegend. Anti-mouse F4/80 (CI:A3-1) was from Serotec. Apoptosis was determined using annexin V after cell surface labeling by following the manufacturer’s instructions (Miltenyi Biotec).
Microarray analysis
Male C57BL/6 mice at 6 weeks of age were used for gene expression analysis in macrophages. Inflammatory peritoneal macrophages were identified as CD115+ F4/80int B220− MHC-II+ or MHC-II− and sorted using a BD FACSAria II cell sorter. Resident peritoneal macrophages, including CD115+ F4/80hi MHC-II− B220− and CD115+ F4/80lo MHC-II+ B220− populations, were sorted at steady state and after thioglycollate injection. Microarrays were carried out as part of the Immunological Genome Project. Flow plots of sorted cells can be found on the Immunological Genome Project website (www.immgen.org). RNA was prepared from sorted populations from C57BL/6J mice after double sorting directly into TRIzol reagent (www.immgen.org/Protocols/Total RNA Extraction with Trizol.pdf). Then it was amplified and hybridized on the Affymetrix Mouse Gene 1.0 ST array. For data analysis, Immunological Genome Project data sets were used. Raw data were normalized using the RMA algorithm. Extensive quality control documents are available on the Immunological Genome Project website (www.immgen.org/Protocols/ImmGen QC Documentation_ALL-DataGeneration_0612.pdf). All data sets have been deposited at the National Center for Biotechnology Information/Gene Expression Omnibus under accession number GSE15907 (www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE15907). Data were analyzed with the GenePattern genomic platform (www.broadinstitute.org/cancer/software/genepattern/). A common threshold for positive expression at 95% confidence across the data set was determined to be 120 (www.immgen.org/Protocols/ImmGen QC Documentation_ALL-DataGeneration_0612.pdf). Differences in gene expression were identified and visualized with the Multiplot module of GenePattern. Clustering analysis considered the 15% most variable genes. Genes were clustered (centered on the mean) with the Hierarchical Clustering module of GenePattern, employing Pearson's correlation as a metric, and data were visualized with the Hierarchical Clustering Viewer heat-map module. Enrichment for gene ontology terms was done using List2Networks software.24
Tissue sample preparation for flow cytometry
Peritoneal exudates were collected in 4 mL cold Hanks’ balanced saline solution supplemented with EDTA (0.3 µM final) and bovine serum albumin (0.06% final) into the peritoneal cavity. LN and omentum cell suspensions were obtained after digestion of the teased organ in collagenase D (Roche) for 30 minutes. Cell suspensions were then stained with appropriate antibodies for 30 minutes on ice. Data were acquired on a BD FACS Canto II or BD Fortessa Flow Cytometer (BD Biosciences) and analyzed with FlowJo software (Treestar).
Adoptive transfer and competition assay
Peritoneal macrophages were retrieved by lavage from donors injected with thioglycollate 5 days earlier. These macrophages were derived from CD45.1 wild-type (WT) mice, CD45.2 CCR7−/− mice, CD68-hBcl2 mice (human Bcl2 cDNA expression driven by the mouse CD68 promoter),22 or CD45.2 Bim−/− mice. Mixtures composed of 1:1 CD45.1 WT macrophage and CD45.2 macrophages from one of the respective CD45.2 strains were made. These mixtures were injected in CD45.1 × CD45.2 F1 recipients that also received thioglycollate 5 days earlier, with a total of 2 to 5 million macrophages from the 1:1 mixture injected into each recipient. Ratios at the time of injection were assessed by flow cytometry on the mixture prepared for injection. At day 8, the peritoneal lavage or LN was analyzed for the presence and ratio of the transferred cells.
Efferocytosis assay
Freshly isolated thymocytes were labeled with carboxyfluorescein diacetate succinimidyl ester (10 µM) and then treated with UV light for 10 minutes, followed by a 3-hour incubation at 37°C. Apoptotic thymocytes (2.5 × 107 per mouse, 40% annexin V+) were then injected intraperitoneally in mice that had received thioglycollate 5 days earlier. Peritoneal lavage was performed 30 minutes after carboxyfluorescein diacetate succinimidyl ester–labeled apoptotic thymocyte injection, and uptake was determined by flow cytometry.
Statistical analysis
Data are expressed as mean ± SEM. Statistical differences were assessed using a 2-tailed t test or analysis of variance (with Tukey’s posttest analysis), using GraphPad Prism software. A P value of less than .05 was considered statistically significant.
Results
Resident and inflammatory macrophages during onset and resolution of acute inflammation
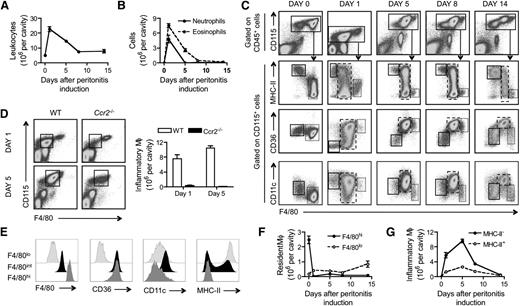
On induction of peritonitis after thioglycollate administration intraperitoneally, a prominent recruitment of leukocytes into the peritoneal cavity occurred within 24 hours, as expected,15 followed by gradual clearance to reach steady-state numbers 8 days later (Figure 1A). Peak leukocyte accumulation at 24 hours was associated with a massive influx of neutrophils (Gr-1+ CD115lo F4/80−) and eosinophils (Gr-1int CD115lo F4/80+) (Figure 1B). Neutrophils were cleared completely within 5 days, whereas eosinophils remained longer (Figure 1B). Macrophages prominently expressed CD115 (Csf-1 receptor). We noted, as previously described,25 that 2 resident macrophage populations were present in the steady-state peritoneum: CD115+ F4/80lo CD11clo CD36− MHC-II+ macrophages (5%-10% of all CD115+ macrophages) and CD115+ F4/80hi CD11c− MHC-II− (90%-95% of all CD115+ macrophages; Figure 1C, day 0). These populations are macrophages, as evidenced from gene expression profiling.26
Phenotype and dynamics of resident and inflammatory macrophages during acute peritonitis. Quantification of total leukocytes (A) or granulocytes (neutrophils and eosinophils) (B) in the peritoneal cavity at the steady state and during a 14-day period after i.p. administration of thioglycollate. (C) Fluorescence-activated cell sorter (FACS) plots illustrating the gating strategy used for identification of macrophages at the steady state and during a 14-day period after i.p. administration of thioglycollate. Macrophages were found in CD115hi gates (first row) at all times; CD115 expression on these cells was reduced at day 1. Three macrophage populations were discerned on the basis of F4/80 intensity, and these populations are depicted in the second through the fourth rows of the dot plots. F4/80low macrophages (solid-line gates) and F4/80hi macrophages (finely dotted gates) were resident macrophages, and inflammatory peritoneal macrophages (wide-dashed gates) appeared only in response to thioglycollate. (D) FACS plots illustrating the gating of inflammatory macrophages and corresponding cell counts in the peritoneum of CCR2-deficient mice and controls 1 and 5 days after initiation of peritonitis. (E) Comparison of F4/80, CD36, CD11c, and MHC-II cell surface expression levels between resident (F4/80low and F4/80hi) and inflammatory (F4/80int) macrophages 5 days after initiation of peritonitis. (F) Quantification of resident macrophages and (G) inflammatory macrophages in the peritoneal cavity during the steady state and a 14-day period after i.p. administration of thioglycollate. Data are derived from at least 2 experiments performed with 5 replicates per experimental condition.
Phenotype and dynamics of resident and inflammatory macrophages during acute peritonitis. Quantification of total leukocytes (A) or granulocytes (neutrophils and eosinophils) (B) in the peritoneal cavity at the steady state and during a 14-day period after i.p. administration of thioglycollate. (C) Fluorescence-activated cell sorter (FACS) plots illustrating the gating strategy used for identification of macrophages at the steady state and during a 14-day period after i.p. administration of thioglycollate. Macrophages were found in CD115hi gates (first row) at all times; CD115 expression on these cells was reduced at day 1. Three macrophage populations were discerned on the basis of F4/80 intensity, and these populations are depicted in the second through the fourth rows of the dot plots. F4/80low macrophages (solid-line gates) and F4/80hi macrophages (finely dotted gates) were resident macrophages, and inflammatory peritoneal macrophages (wide-dashed gates) appeared only in response to thioglycollate. (D) FACS plots illustrating the gating of inflammatory macrophages and corresponding cell counts in the peritoneum of CCR2-deficient mice and controls 1 and 5 days after initiation of peritonitis. (E) Comparison of F4/80, CD36, CD11c, and MHC-II cell surface expression levels between resident (F4/80low and F4/80hi) and inflammatory (F4/80int) macrophages 5 days after initiation of peritonitis. (F) Quantification of resident macrophages and (G) inflammatory macrophages in the peritoneal cavity during the steady state and a 14-day period after i.p. administration of thioglycollate. Data are derived from at least 2 experiments performed with 5 replicates per experimental condition.
Inflammatory macrophages appeared in the peritoneal cavity after induction of inflammation (Figure 1C; day 1, 5, 8, and 14) and were distinguished from resident macrophage populations by intermediate cell surface levels of F4/80 (Figure 1C). In addition, these inflammatory macrophages (CD115+ F4/80int) expressed CD11c and CD36. Approximately 20% to 30% of them displayed cell surface expression of MHC-II (Figure 1C). The gene expression profile of these inflammatory cells classified them as macrophages,26 despite expression of CD11c and MHC-II that are also associated with dendritic cells (DCs) and despite their similar secretome to in vitro cultured granulocyte macrophage–CSF–derived DCs.27 Inflammatory F4/80int macrophages likely arose from circulating Ly-6Chi monocytes, as their numbers were approximately 70-fold decreased 5 days after injection of thioglycollate in CCR2−/− mice with reduced circulating levels of Ly-6Chi monocytes28 and deficiency in monocyte trafficking into the inflamed peritoneum29 (Figure 1D). Cell surface expression of CD36 and CD11c distinguished inflammatory macrophages (F4/80int) from the 2 populations of resident macrophages (F4/80lo and F4/80hi) (Figure 1E). Thus, F4/80hi and F4/80lo cells represent tissue resident macrophage populations; whereas F4/80int cells are Ly-6Chi monocyte–derived inflammatory macrophages recruited after induction of peritonitis. As expected,30 the dominant CD115+ F4/80hi resident macrophages almost disappeared after initiation of inflammation (Figure 1F). Their numbers remained low at all times studied after thioglycollate was administered, including as long as days 8 to 14, when total leukocyte counts had returned to baseline (Figure 1F). In contrast, CD115+ F4/80low resident macrophages were overall unchanged over time (Figure 1F). Both MHC-II+ and MHC-II− inflammatory macrophages peaked in magnitude 5 days after injection of thioglycollate. Then they were cleared rapidly between day 5 and 8 (Figure 1G), as expected.15,31 Thus, we chose the window of time between day 5 and 8 as the interval to study events associated with the clearance of inflammatory macrophages.
Gene expression signature of inflammatory macrophages
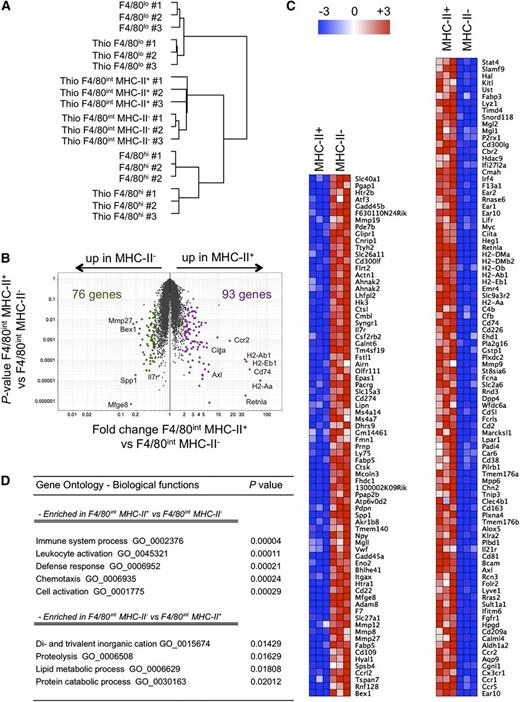
CD115+ F4/80int MHC-II− and CD115+ F4/80int MHC-II+ cells were separately sorted from mice injected 5 days earlier with thioglycollate and further analyzed using microarray. In addition, gene expression profiles were carried out on resident CD115+ F4/80lo MHC-II+ and CD115+ F4/80hi MHC-II− macrophages during the steady state and 5 days after thioglycollate injection. Clustering analysis of these populations revealed that CD115+ F4/80lo macrophages clustered closely together with or without thioglycollate treatment (Figure 2A), and CD115+ F4/80hi macrophages also clustered together with or without thioglycollate (Figure 2A). Furthermore, both MHC-II+ and MHC-II− monocyte-derived inflammatory macrophages were more similar to each other than to the resident macrophages (Figure 2A). These clustering patterns confirm that cell surface levels of F4/80 for the different macrophage populations remain stable, and therefore are useful as means to track given populations after induction of inflammation with thioglycollate.
Gene expression analysis of inflammatory macrophage populations. (A) Hierarchical clustering of steady-state and inflammatory macrophage populations (3 replicates for each populations) based on the 15% of genes with the greatest variability. (B) Volcano plot (P value vs fold change, with each dot representing 1 probe set), highlighting the 76 genes upregulated in MHC-II− inflammatory macrophages (green) and the 93 genes upregulated in MHC-II+ inflammatory macrophages (purple). Some differentially expressed probe sets were tagged with the gene name they correspond to. (C) Heat map depicting genes differentially expressed (P < .05; fold > 2) between MHC-II− and MHC-II+ inflammatory macrophages. (D) Pathways associated with differentially expressed genes between MHC-II− and MHC-II+ inflammatory macrophages.
Gene expression analysis of inflammatory macrophage populations. (A) Hierarchical clustering of steady-state and inflammatory macrophage populations (3 replicates for each populations) based on the 15% of genes with the greatest variability. (B) Volcano plot (P value vs fold change, with each dot representing 1 probe set), highlighting the 76 genes upregulated in MHC-II− inflammatory macrophages (green) and the 93 genes upregulated in MHC-II+ inflammatory macrophages (purple). Some differentially expressed probe sets were tagged with the gene name they correspond to. (C) Heat map depicting genes differentially expressed (P < .05; fold > 2) between MHC-II− and MHC-II+ inflammatory macrophages. (D) Pathways associated with differentially expressed genes between MHC-II− and MHC-II+ inflammatory macrophages.
Focusing on the recruited inflammatory macrophages, the MHC-II+ and MHC-II− populations were overall highly similar. Fewer than 200 genes were significantly different when the 2 populations were compared (≥twofold; P < .05; Figure 2B). The differentially regulated genes between the 2 populations are shown in Figure 2C, and included Stat4, Mgl1 and Mgl2, Irf4, Il21r, and Lyve1 upregulated in the MHC-II+ macrophages (Figure 2C), whereas proteases were especially prominent in MHC-II− macrophages, including genes encoding cathepsins L and K and several metalloproteases (Figure 2C). Pathway analysis of the differentially expressed genes revealed pathways associated with proteolysis and lipid catabolic processes in F4/80int MHC-II− macrophages (Figure 2D), whereas pathways associated with T-cell activation and migratory behavior were enriched in F4/80int MHC-II+ macrophages (Figure 2D). These data raised the possibility that MHC-II+ inflammatory macrophages were poised to emigrate.
Emigration of macrophages to the LN peaks before the phase of clearance
We used flow cytometry to assess arrival of inflammatory macrophages in the mediastinal LN that drains the peritoneal cavity15,32 after intraperitoneal thioglycollate administration. F4/80+ CD11c+ CD36+ MHC-II+ macrophages resembling the inflammatory MHC-II+ macrophages from the peritoneum could be recovered from the inflamed, but not steady state, mediastinal LN (Figure 3A). The population was also absent from any other LNs tested (inguinal, mesenteric, and brachial) 5 days after induction of peritonitis. These cells fell into a typical DC gate with regard to their expression of CD11c and MHC-II (Figure 3B), but they expressed higher levels of F4/80 and CD36 compared with classical DCs. To confirm that these cells were from the inflamed peritoneal cavity, we injected the phagocytic tracer PKH26 into the thioglycollate-treated peritoneum (Figure 3C). Nearly all PHK26+ cells in the mediastinal LN were F4/80+ CD36+ (and also CD11c+ and MHC-II+) (Figure 3D). Conversely, when we first gated on F4/80+ CD36+ cells in the LN, they were almost all PKH26+ (Figure 3D). Transfer of peritoneal cells from thioglycollate-inflamed CD45.1 congenic mice into the peritoneum of thioglycollate-inflamed CD45.2 recipients also demonstrated that inflammatory CD45.1 macrophages migrated from the peritoneum to the mediastinal LN (Figure 3E), and all F4/80+CD45.1+ cells bore the CD36+ phenotype (Figure 3E). Thus, F4/80+ CD36+ cells appearing in the mediastinal LN after thioglycollate injection in the peritoneum indeed originated from the peritoneum, and this phenotype included the majority of phagocytic cells that emigrated from the peritoneum.
Kinetics of inflammatory macrophage migration to LNs. (A) FACS plots depicting the appearance of inflammatory macrophages (F4/80+CD36+ or F4/80+ MHC-II+) in the mediastinal LN over the course of 8 days after i.p. administration of thioglycollate. (B) Total LN cells (Left) or total cells with F4/80+ CD36+ cells gated out (Right) based on cell surface expression of CD11c and MHC-II. Bottom plot overlays F4/80+ CD36+ gated cells on total LN cells, plotted to show CD11c vs MHC-II. (C) Labeling of peritoneal inflammatory macrophages 24 hours after i.p. injection of the phagocytic tracer dye PKH26 in mice inflamed 2 days earlier by thioglycollate injection. (D) PKH26+ cells were identified in the mediastinal LN 3 days after injection of PKH26 in the peritoneal cavity (5 days after inflammation was induced by thioglycollate) and analyzed for F4/80 and CD36 cell surface expression. Far-right plot shows PKH26 levels after gating on all F4/80+ CD36+ LN cells. (E) Inflammatory macrophages were retrieved from CD45.1 mice that had been injected 1 day earlier with thioglycollate. These macrophages were adoptively transferred into CD45.2 mice at the same stage of inflammation. CD45.1+ inflammatory macrophages were gated in the mediastinal LN cell suspension (left FACS plot) 4 days later and analyzed for CD36 expression. (F-H) Inflammatory macrophages in the peritoneum, draining LN and omentum at different times after induction of peritonitis by thioglycollate (n = 4-6 mice per group per time point). Data are representative of at least 2 independent experiments.
Kinetics of inflammatory macrophage migration to LNs. (A) FACS plots depicting the appearance of inflammatory macrophages (F4/80+CD36+ or F4/80+ MHC-II+) in the mediastinal LN over the course of 8 days after i.p. administration of thioglycollate. (B) Total LN cells (Left) or total cells with F4/80+ CD36+ cells gated out (Right) based on cell surface expression of CD11c and MHC-II. Bottom plot overlays F4/80+ CD36+ gated cells on total LN cells, plotted to show CD11c vs MHC-II. (C) Labeling of peritoneal inflammatory macrophages 24 hours after i.p. injection of the phagocytic tracer dye PKH26 in mice inflamed 2 days earlier by thioglycollate injection. (D) PKH26+ cells were identified in the mediastinal LN 3 days after injection of PKH26 in the peritoneal cavity (5 days after inflammation was induced by thioglycollate) and analyzed for F4/80 and CD36 cell surface expression. Far-right plot shows PKH26 levels after gating on all F4/80+ CD36+ LN cells. (E) Inflammatory macrophages were retrieved from CD45.1 mice that had been injected 1 day earlier with thioglycollate. These macrophages were adoptively transferred into CD45.2 mice at the same stage of inflammation. CD45.1+ inflammatory macrophages were gated in the mediastinal LN cell suspension (left FACS plot) 4 days later and analyzed for CD36 expression. (F-H) Inflammatory macrophages in the peritoneum, draining LN and omentum at different times after induction of peritonitis by thioglycollate (n = 4-6 mice per group per time point). Data are representative of at least 2 independent experiments.
When we used this strategy to identify and quantify macrophages in the mediastinal LN, the magnitude of inflammatory macrophage accumulation in the LN did not increase during the 5- to 8-day window when macrophages were clearing from the peritoneum, as would be expected if the LN were the major depot for the clearing cells. Instead, the accumulation of inflammatory macrophages in the LN roughly paralleled the kinetics of accumulated macrophages in the peritoneum, with a peak accumulation in the LN at day 5 before macrophage clearance from the peritoneum began (Figure 3F). A day-by-day analysis of inflammatory macrophages in the peritoneum and the LN from day 5 to day 8 further revealed a similar rate of decrease at both sites (Figure 3G). There was also no increase in macrophage numbers in the omentum during days 5 to 8, when macrophage numbers contracted (Figure 3H). Thus, macrophages emigrate to mediastinal LNs from the inflamed peritoneum, but the kinetics suggest that the LN is simply sampling a portion of the cells in the peritoneum, rather than serving as the target for removal of macrophages. If the latter were true, a transient increase in macrophages in the LN (or omentum) during the window from day 5 to day 8, when macrophages are lost from the peritoneum, would be expected.
Blocking CCR7-independent, Gαi-sensitive macrophage migration to the LN has a quantitatively minimal effect on macrophage contraction
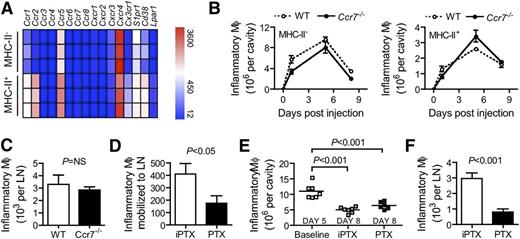
LN-migrating macrophages were uniformly MHC-II+. In the peritoneum, we observed a significant enrichment in genes involved in migratory behavior such as chemokine receptors (Ccr2, Ccr5, Ccr1, and Cx3cr1), the cyclic adenosine 5′-diphosphate-ribose hydrolase (Cd38), and sphingosine-1-phosphate receptor (S1pr1), selectively, in F4/80int MHC-II+ macrophages (Figures 2C and 4A). Ccr7 mRNA was not detected in inflammatory macrophages in the peritoneum (Figure 4A). However, Ccr7 is, with little exception, universally associated with the migration of leukocytes to LNs through lymphatic vessels,33,34 including the emigration of monocyte-derived cells from skin to draining LNs.35 Discussion has argued that it would be essential for resolution of inflammation.20 Thus, we tested whether CCR7 was needed for inflammatory macrophage migration to the mediastinal LN and resolution.
Blocking inflammatory macrophages migration marginally impaired their clearance during resolution. (A) Heat map showing the expression of chemotactic receptors in MHC-II− and MHC-II+ inflammatory macrophages 5 days after intraperitoneal administration of thioglycollate (3 replicates were generated for each population). (B) Kinetics of MHC-II− and MHC-II+ inflammatory macrophages during the course of thioglycollate-induced peritonitis in CCR7-deficient mice and controls (n = 5 mice per group per time). (C) Accumulation of inflammatory macrophage in the mediastinal LN of CCR7-deficient mice and controls 5 days after intraperitoneal administration of thioglycollate (n = 5 mice per group). (D) Mobilization to the mediastinal LN of adoptively transferred F4/80+ CD36+ CD45.1+ inflammatory macrophages in CD45.2 mice injected with thioglycollate in the presence of PTX or iPTX (n = 5-7 per group). To maximize the recovery, cells and PTX or iPTX (single injection) were injected in the peritoneum at day 2, and LNs were analyzed at day 5. (E) Baseline number of inflammatory macrophage in the peritoneum at day 5 and numbers of macrophages recovered at day 8 after treatment with either active (PTX) or inactive (iPTX) pertussis toxin at day 5. Each symbol represents one mouse. (F) The number of F4/80+CD36+ macrophages recovered in the mediastinal LN at day 8 from the experiment in panel E, illustrating that active pertussis toxin prevented emigration to the LN.
Blocking inflammatory macrophages migration marginally impaired their clearance during resolution. (A) Heat map showing the expression of chemotactic receptors in MHC-II− and MHC-II+ inflammatory macrophages 5 days after intraperitoneal administration of thioglycollate (3 replicates were generated for each population). (B) Kinetics of MHC-II− and MHC-II+ inflammatory macrophages during the course of thioglycollate-induced peritonitis in CCR7-deficient mice and controls (n = 5 mice per group per time). (C) Accumulation of inflammatory macrophage in the mediastinal LN of CCR7-deficient mice and controls 5 days after intraperitoneal administration of thioglycollate (n = 5 mice per group). (D) Mobilization to the mediastinal LN of adoptively transferred F4/80+ CD36+ CD45.1+ inflammatory macrophages in CD45.2 mice injected with thioglycollate in the presence of PTX or iPTX (n = 5-7 per group). To maximize the recovery, cells and PTX or iPTX (single injection) were injected in the peritoneum at day 2, and LNs were analyzed at day 5. (E) Baseline number of inflammatory macrophage in the peritoneum at day 5 and numbers of macrophages recovered at day 8 after treatment with either active (PTX) or inactive (iPTX) pertussis toxin at day 5. Each symbol represents one mouse. (F) The number of F4/80+CD36+ macrophages recovered in the mediastinal LN at day 8 from the experiment in panel E, illustrating that active pertussis toxin prevented emigration to the LN.
We injected CCR7-deficient animals with thioglycollate and followed the accumulation and clearance of inflammatory macrophages in the peritoneum. Other than a minor shift toward a slightly increased proportion of MHC-II+ macrophages (Figure 4B), there was no difference in their accumulation or clearance during the resolution phase compared with WT controls (Figure 4B). The numbers of inflammatory macrophages that migrated to the LN at day 5 (peak macrophage accumulation) were similar between Ccr7−/− mice and WT controls (Figure 4C), illustrating that CCR7 is unnecessary for LN homing of macrophages. Chemokine receptors selectively expressed by MHC-II+ inflammatory macrophages (Figure 4A) are all coupled to Gαi proteins, and are thus subject to inhibition by PTX. Thus, we adoptively transferred CD45.1+ inflammatory macrophages in CD45.2+ recipients and locally injected PTX or PTX inactivated by boiling (iPTX) simultaneously. PTX treatment substantially reduced migration of inflammatory macrophages to the mediastinal LN compared with treatment with iPTX (Figure 4D), revealing that emigration to the draining LN was dependent on Gαi-mediated signals. However, cohorts of mice injected with PTX (compared with iPTX) at the onset of resolution at day 5 had only a nonsignificant trend toward an increased number of inflammatory macrophages (+25%) that were retained in the peritoneum at day 8 (Figure 4E), even though we recovered few inflammatory macrophages from the LNs of the same PTX-treated mice (Figure 4F). PTX did not affect inflammatory macrophage survival, but it increased their proliferation (supplemental Figure 1A-B, available on the Blood Web site), although the percentage of proliferating cells was very low, which might explain the nonsignificant 25% increase in the number of inflammatory macrophages observed at day 8 in the PTX-treated group (Figure 4E). Thus, macrophage migration to LNs fails to account quantitatively for removal of macrophages during the phase of intense macrophage contraction observed during resolution.
Apoptosis of inflammatory macrophages promotes macrophage clearance during resolution
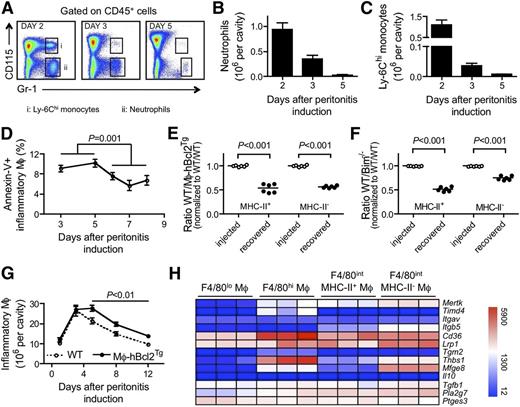
A key step necessary to allow for macrophage clearance is the shutdown in the recruitment of their precursors, circulating Ly-6Chi monocytes.31 The recruitment of neutrophils (gate ii, defined as Gr-1+ CD115-) and Ly-6Chi monocytes (gate i, Gr-1+ CD115+) gradually and dramatically decreased before the onset of resolution (Figure 5B-C). However, cessation in recruitment alone could not account for a net reduction in macrophage numbers. Thus, we wondered whether concomitant local death would promote macrophage contraction between days 5 and 8 after intraperitoneal thioglycollate injection. F4/80int inflammatory macrophages recovered from the peritoneum showed annexin V+–positive cells, even as early as day 3 (Figure 5D). Annexin V–reactive macrophages were relatively more abundant, approaching 8% at early stages of resolution, and were present at all times studied (Figure 5D). To obtain a quantitative sense of the importance of apoptosis in macrophage clearance, we induced peritonitis using thioglycollate in 3 cohorts of mice, including CD45.1 WT mice, CD45.1 × CD45.2 F1 hybrid WT mice, and CD45.2 transgenic mice containing a transgene in which the CD68 promoter was used to drive expression of the antiapoptotic factor Bcl2.22 In all cohorts of mice, we injected thioglycollate and maintained the mice until day 5. Macrophages from CD45.1 WT and CD45.2 CD68-Bcl2 transgenic mice were mixed 1:1 and then immediately transferred to the site of peritonitis in the CD45.1 × CD45.2 F1 WT mice. We then recovered cell suspensions of the peritoneal lavage at day 8. CD68-Bcl2 transgenic (Tg) macrophages cleared less efficiently from the peritoneum than nontransgenic WT cells, such that in the 3-day period of analysis, the Bcl2-overexpressing macrophages represented two-thirds of the transferred macrophages (Figure 5E). As expected, overexpression of Bcl2 reduced, although it did not eliminate, death by apoptosis (supplemental Figure 2A); it did not affect proliferation (supplemental Figure 2B).
Inflammatory macrophages contraction during resolution is controlled by apoptotic cell death. (A) FACS plot illustrating the gating strategy used for neutrophils (ii, CD115− Gr-1+) and Ly-6Chi monocytes (i, CD115+ Gr-1+) in the inflamed peritoneal cavity. Quantification of neutrophils (B) as well as Ly-6Chi monocytes (C) up to day 5 in mice injected with thioglycollate (n = 5 per time). (D) Quantification of annexin V staining in inflammatory macrophages from day 3 to 8 after intraperitoneal administration of thioglycollate (n = 5 per time). (E-F) Ratios of F4/80int inflammatory macrophages competed after injection into thioglycollate-inflamed peritoneum at day 5 (injected) and recovered at day 8 (recovered). Two competitions are shown: between macrophages derived from WT and CD68-Bcl2 (Mφ-hBcl2Tg) transgenic mice and between macrophages derived from WT and Bim−/− mice. Each symbol represents data from 1 mouse. (G) Quantification of F4/80int inflammatory macrophages in the peritoneal of irradiated recipient mice transplanted with bone marrow from CD68-Bcl2 (Mφ-hBcl2Tg) or WT mice during a 12-day period after i.p. administration of thioglycollate. (H) Heat maps depict gene expression patterns of mRNA transcripts that mediate or are induced in response to efferocytosis. Three replicates are shown for different macrophage populations.
Inflammatory macrophages contraction during resolution is controlled by apoptotic cell death. (A) FACS plot illustrating the gating strategy used for neutrophils (ii, CD115− Gr-1+) and Ly-6Chi monocytes (i, CD115+ Gr-1+) in the inflamed peritoneal cavity. Quantification of neutrophils (B) as well as Ly-6Chi monocytes (C) up to day 5 in mice injected with thioglycollate (n = 5 per time). (D) Quantification of annexin V staining in inflammatory macrophages from day 3 to 8 after intraperitoneal administration of thioglycollate (n = 5 per time). (E-F) Ratios of F4/80int inflammatory macrophages competed after injection into thioglycollate-inflamed peritoneum at day 5 (injected) and recovered at day 8 (recovered). Two competitions are shown: between macrophages derived from WT and CD68-Bcl2 (Mφ-hBcl2Tg) transgenic mice and between macrophages derived from WT and Bim−/− mice. Each symbol represents data from 1 mouse. (G) Quantification of F4/80int inflammatory macrophages in the peritoneal of irradiated recipient mice transplanted with bone marrow from CD68-Bcl2 (Mφ-hBcl2Tg) or WT mice during a 12-day period after i.p. administration of thioglycollate. (H) Heat maps depict gene expression patterns of mRNA transcripts that mediate or are induced in response to efferocytosis. Three replicates are shown for different macrophage populations.
When we repeated this experiment, substituting CD68-Bcl2 transgenic macrophages with macrophages from Bim−/− mice, the same outcome was observed, in which Bim−/− macrophages that are partially resistant to death36 came to dominate over WT macrophages locally in the peritoneum during resolution of thioglycollate-induced peritonitis (Figure 5F).
Next we performed a detailed kinetic analysis of F4/80int inflammatory macrophages in irradiated recipients mice repopulated with bone marrow from CD68-Bcl2 transgenic or WT mice during the course of thioglycollate-induced peritonitis. The appearance of inflammatory macrophages in the peritoneal cavity was comparable between both genotypes until day 3 (Figure 5G). However, macrophages from CD68-Bcl2 transgenic mice showed no contraction between days 3 and 5, whereas WT macrophages contracted (Figure 5G). Accumulation in draining LNs was comparable in both groups (supplemental Figure 2C). Together, these data indicate that local death accounts for macrophage contraction during inflammatory resolution and further suggest that efficient efferocytosis would be crucial to promote resolution. Using our microarray data, we probed the expression of key molecules involved in apoptotic cell clearance, as well as the response to efferocytosis (Tgfb1, Pla2g7, Ptges3) (Figure 5H). These data revealed that F4/80hi resident and F4/80int inflammatory macrophages seemed overall better-equipped than F4/80lo resident macrophage for efferocytic function, as they expressed higher levels of Mertk, Cd36, and Lrp1, for example. This was confirmed experimentally after injecting exogenous apoptotic cells in vivo into the peritoneum of mice injected 5 days earlier with thioglycollate (supplemental Figure 3). Finally, all macrophages expressed Tgfb1, but not Il10, as well as the genes encoding the enzymes producing the lipid mediators PAF (Pla2g7) and PGE2 (Ptges3), all of which are induced in response to efferocytosis.37
Discussion
Resolution of inflammation is a key homeostatic response to injury critical to maintain tissue integrity. However, the fundamental mechanisms underlying the removal of infiltrated inflammatory macrophages are poorly understood. For decades, different studies have argued variably for local apoptosis,11-13 akin to the mechanism removing neutrophils,6,7 vs emigration to LNs15 as the fundamental process governing macrophage removal. Although in fact only a single study, that of Bellingan and colleages,15 concluded that emigration to LNs dominated, the conclusions of this single study have taken hold in the field. Others argued that CD3638 and CD11b39-41 could modulate macrophage migration and thereby delay resolution, but they used a model of “resolution” that is triggered by lipopolysaccharide (LPS),39 even though LPS promotes, rather than resolves, inflammation. The current study used the model of thioglycollate-induced peritoneal inflammation similarly to Bellingan and colleagues, although seemingly minor differences existed, including volume of injection and supplier. Local apoptosis can be difficult to appreciate because of the rapid and seemingly “invisible” clearance of apoptotic cells at the histological level. We got around this problem using a competitive approach that would allow us to quantity whether the ability to die (or emigrate) conferred a disadvantage to resolution. Although a low level of emigration of macrophages to LNs occurred during resolution of thioglycollate-induced inflammation, the concept that emigration is either required for or serves as the major mechanism in removing macrophages from resolving inflammatory lesions failed to stand. Instead, the fundamental mechanism most relevant to removal of macrophages is none other than local apoptosis. This revised concept will now need to be tested, using other models of inflammation in the peritoneum as well as in other tissues, but resolution of inflammation in the heart already appears to follow the paradigm we observe here.42
We argue that the straightforward body of work presented here is of fundamental importance with respect to how data in the literature are interpreted. Indeed, the Bellingan study has strongly influenced the interpretation and design of many studies. For example, a few years ago, we discussed how impaired macrophage emigration out of atherosclerotic vessels could negatively affect the course of atherosclerosis if this process were required for resolution.17,43,44 However, when we observed that macrophage contraction from plaques did not depend on emigration out of the plaque environment,21 we were prompted to return to studies of acute inflammation and reconsider how macrophages are cleared therein. We then recognized that the study of Bellingan and colleagues was generally qualitative and needed further investigation. Other studies have discussed their own findings with interpretations that reflect an assumption that the conclusions of Bellingan and colleagues are correct.10,20 Indeed, lipid mediators that promote resolution, including resolvins and protectins, increase migration of macrophages to LNs,10 but it remains unclear whether this is their dominant means for controlling macrophage burden. It would now be valuable to evaluate the role of resolvins and protectins, along with other mediators associated with macrophage removal, such as CD3638 and netrin,20 in monocyte recruitment and macrophage apoptosis. Although the implications of our work include the idea that macrophage apoptosis is beneficial to the resolution of inflammation, we recognize that in a context such as atherosclerosis, the failure to clear apoptotic bodies through efferocytosis can significantly diminish the benefits of macrophage death and even contribute to disease pathology.45 Along these lines, developing mouse models that would allow for inducible and macrophage-specific alterations of efferocytic pathways would be useful to probe the requirement of this process in favoring silent inflammatory macrophage removal during resolution.
Even if the majority of macrophages are not cleared via emigration to LNs through lymphatic vessels, lymphatic transport is still a vital route for resolution of inflammation. For example, the decoy chemokine receptor D6 that sequesters and removes pro-inflammatory chemokines46 is primarily expressed on lymphatic vessels.47 Furthermore, removal of soluble mediators through the lymph is critical for resolution.47 Indeed, in the context of atherosclerosis, our recent work revealed that cholesterol loaded onto high-density lipoproteins is cleared from tissues, including the artery wall, through the lymphatic vasculature, suggesting that intact lymphatic drainage would be important in the resolution of atherosclerosis.48 Furthermore, although our data suggest that macrophage emigration to LNs during resolution of inflammation does not quantitatively prevail in the removal of macrophages and that the emigration is not otherwise essential for resolution (or else pertussis toxin would have delayed resolution), there is likely some relevance to the migration that does occur. For instance, emigratory macrophages may contribute to antigen presentation in the LN that, for pathogens, may be essential for mounting a response needed to ultimately eradicate the cause of the inflammatory response in the first place.31 All emigratory macrophages in the LNs were MHC-II+, and their MHC-II+ counterparts in the peritoneum expressed many genes relevant to antigen presentation. Although they bore many features of dendritic cells, these cells could not be classified as dendritic cells by gene expression profiling26,49 ; instead, their expression profile is that of a macrophage. Genes associated with migration to LNs strongly cluster with dendritic cells, including CCR7, and did not appear to be induced in inflammatory macrophages. CCR7 also mediates the emigration of T cells34 and neutrophils50 to LNs from the skin, so we suspected it would govern the emigration of inflammatory macrophages from the peritoneum to the mediastinal LN. However, surprisingly, we found that CCR7 was not required for this step. This instance is one of only few documented cases in which emigration to LNs is CCR7-independent. Another example is found in chronic skin inflammation.34 Thus, if CCR7 is a mediator of resolution, as recently proposed,19,20 its role in resolution may not directly relate to a role in macrophage migration from the inflammatory environment.
In closing, this study revises the conclusions of an earlier influential body of work and, in so doing, alters the fundamental cellular pathways that merit strong consideration for therapeutics that seek to recapitulate the events that promote resolution of inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Gerald Morris for help with mice irradiation. The authors thank the Immunological Genome Project for microarray and bioinformatics support associated with this work.
This work was funded by National Institutes of Health grants R01 AI049653 and R01 AI061741 (to G.J.R.). E.G. was supported in part by a postdoctoral fellowship from the American Heart Association, Heritage Affiliate (10POST4160140). The Immunological Genome Project is funded by R24 AI072073 to Christophe Benoist (Harvard Medical School). Earlier studies included in this body of work were carried out at the Mount Sinai School of Medicine before the laboratory relocated to the Washington University School of Medicine.
Authorship
Contribution: E.L.G. designed and conducted experiments and wrote the manuscript; S.I. conducted experiments; P.L. provided key reagents; and G.J.R. designed and supervised experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gwendalyn J. Randolph, Department of Pathology & Immunology, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8118, St. Louis, MO 63110; email: grandolph@path.wustl.edu.