Key Points
PKCα and PKCθ cooperate in T-cell alloresponses, which contribute to GVHD.
Pharmacologic inhibition of PKCα and PKCθ prevents GVHD and largely preserves GVL responses.
Abstract
Allogeneic hematopoietic cell transplantation (HCT) is the most effective therapy for hematopoietic malignancies through T-cell–mediated graft-vs-leukemia (GVL) effects but often leads to severe graft-vs-host disease (GVHD). Given that protein kinase Cθ (PKCθ), in cooperation with PKCα, is essential for T-cell signaling and function, we have evaluated PKCθ and PKCα as potential therapeutic targets in allogeneic HCT using genetic and pharmacologic approaches. We found that the ability of PKCα−/−/θ−/− donor T cells to induce GVHD was further reduced compared with PKCθ−/− T cells in relation with the relevance of both isoforms to allogeneic donor T-cell proliferation, cytokine production, and migration to GVHD target organs. Treatment with a specific inhibitor for both PKCθ and PKCα impaired donor T-cell proliferation, migration, and chemokine/cytokine production and significantly decreased GVHD in myeloablative preclinical murine models of allogeneic HCT. Moreover, pharmacologic inhibition of PKCθ and PKCα spared T-cell cytotoxic function and GVL effects. Our findings indicate that PKCα and θ contribute to T-cell activation with overlapping functions essential for GVHD induction while less critical to the GVL effect. Thus, targeting PKCα and PKCθ signaling with pharmacologic inhibitors presents a therapeutic option for GVHD prevention while largely preserving the GVL activity in patients receiving HCT.
Introduction
Protein kinase Cθ (PKCθ) is a viable target for intervention in detrimental donor T-cell alloreactivity to host antigens, as it maintains the immunologic synapse between T effector (Teff) cells and ligated antigen-presenting cells (APCs) and aids in signal propagation downstream from T-cell receptor (TCR) and CD28 ligation.1,2 Our group has shown that deletion of PKCθ is an effective target strategy for the prevention of graft-vs-host disease (GVHD) while preserving graft-vs-leukemia (GVL) effects in murine models of allogeneic hematopoietic cell transplantation (HCT).3 Blocking or deleting formerly proposed targets such as β2 and β7 integrins and CC chemokine receptors (CCRs) has yielded few practical results for the abolition of lethal GVHD.3-9 Likewise, the extent of GVHD prevention by the current potential care regimens of calcineurin inhibitors and rapamycin therapy is mild to moderate.10-12 Deletion of PKCθ partially blocks TCR signals leading to activation of the interleukin (IL) 2 promoter, nuclear factor of activated T cell (NFAT), activator protein-1, and nuclear factor κB (NF-κB) mediated cytokine storm activation through the caspase-associated recruitment membrane–associated protein (CARMA) complex of scaffolding proteins,13,14 which reduces the severity of GVHD. Importantly, recent work has characterized PKCα as a cooperative and surrogate T-cell activation signaling partner for PKCθ.15-17 Specifically, PKCα mimics or contributes to PKCθ signaling pathways by providing activation of IL-2 feedback, NFAT, activator protein-1, and NF-κB. PKCα can propagate PKCθ-redundant activation signals to NF-κB through a CARMA complex with B-cell leukemia/lymphoma 10, tumor necrosis factor receptor-associated factor 6, and I κβ kinase.18 Overall, little is known regarding the true extent of PKCα signaling contributions to T-cell activation, alloresponse, or PKCθ surrogacy.
With regard to GVHD-related donor T-cell pathogenicity, cooperation or overlapping functions of PKCθ and α in Teff cells could be important to consider as our previous work has shown that bone marrow transplant (BMT) recipients of PKCθ−/− T cells still retain some capacity for alloreactivity to host antigens3 and does not solidify PKCθ alone as an ideal target for GVHD prevention. Here, we have defined the effects of dual inhibition of PKCθ and PKCα on donor T-cell alloreactivity, GVHD pathology, and GVL responses with regard to specific alterations in donor T-cell proliferation, homing, and chemokine/cytokine production capacity. PKCα/θ abrogation inhibits GVHD while preserving functional GVL immune responses. Congruence between anti-GVHD outcomes resulting from genetic PKCα/θ deficiency in donor T cells and pharmacologic inhibition in multiple preclinical models of myeloablative allogeneic HCT verify the validity and realistic therapeutic potential of PKCα/θ small-molecule inhibition as a new potential clinical modality.
Materials and methods
Mice
C57BL/6 (B6;H-2b), BALB/c (H-2d) (NCI), C3.SW-H2b/SnJ (Jax), and PKCα−/−/θ−/− mice used for backcrossing (donated by Dr Amer Beg, at H. Lee Moffitt Cancer Center [Moffitt]) were housed in specific pathogen-free conditions in the American Association for Laboratory Animal Care–accredited Animal Resource Center at Moffitt. To ensure background equivalence, wild-type (WT), PKCα−/−, PKCθ−/−, and PKCα−/−/θ−/− mice were littermates bred at Moffitt and were offspring of PKCα/θ heterozygous breeding pairs resultant from >8 generations of backcrossing. B6 β-actin luciferase transgenic mice were originally provided by Dr Robert Negrin at Stanford. All work was approved by the Institutional Animal Care and Use Committee of University of South Florida.
Flow cytometry, intracellular cytokine staining, and serum cytokine detection
Mononuclear cell isolation from recipient spleen, liver, and lung was carried out as previously noted.5,19-21 Standard flow cytometric surface staining protocols were used. Intracellular cytokines were detected from APC-stimulated T cells or BMT recipient spleen, lung, and liver lymphocytes at specified times following in vitro phorbol-myristate-acetate/ionomycin stimulation and processed as previously described.3 Cells were analyzed using Diva software, LSR II (BD Biosciences), and FlowJo (TreeStar). Blood was collected from BMT recipients 14 days posttransplantation, and cytokine quantification from serum samples was performed using a cytometric bead assay,22 according to manufacturer’s instructions (BD Biosciences).
GVHD models
Magnetic bead negative depletion of non–T cells from whole spleen and lymph node was previously described.20 Purity of T cells ranged from 95% to 98%. T-cell–depleted bone marrow (TCD-BM) was prepared as previously described.20 Donor TCD-BM genotype was matched to that of the donor T-cell genotype. B6→BALB/c MHC-mismatched20 and C3.SW-H2b/SnJ→B6 minor histocompatibility antigen (MiHA)-mismatched23 recipient mice 8 to 10 weeks of age were conditioned with irradiation based on weight at 750 to 800 cGy (single dose) for BALB/c and 1200 cGy (split 3 hours apart) for B6. Twenty-four hours postconditioning, irradiated recipients received intravenous TCD-BM alone or with purified T cells (1 × 106 B6 total T cells or 3 × 106 CD4+/CD8+/CD44–/CD25– C3.SW T cells, respectively). In vivo pharmacologic experiments, a small molecular inhibitor to PKCθ/α (R524; Rigel Pharmaceuticals) was used. R524 was solubilized in 25 mM glacial acetic acid (pH 6) and administered by twice-daily oral gavage at 40 mg/kg body weight in a volume of 200 μL per dose per mouse. Vehicle and R524 dosing began on the day of BMT (day 0) and continued daily until experiment termination or 6 weeks. Mice losing 35% of their original body weight were deemed moribund and counted removed from study due to GVHD lethality. Representative samples of GVHD target organs were excised from recipients 14 days post-BMT and subjected to pathology scoring as previously described.5
Leukemia/lymphoma models
B6→BALB/c recipients received 2 × 103 luc/neo plasmid-transduced A20 B-cell lymphoma cells (A20-luc), whereas C3.SW-H2b/SnJ→B6 recipients received 5 × 104 C1498 luciferase-transduced atypical myeloid leukemia cells generated in B6 mice at the time of BMT.24 Tumor mortality and GVHD mortality were distinguished by bioluminescent imaging (BLI) for tumor load and weight loss indicative of GVHD. Ex vivo BLI was performed as previously described.25 BLI data were analyzed and quantified using Living Image Software (Xenogen) as previously described.9,26
Statistics
BMT recipient weight changes, absolute numbers, and percentages of cell types in GVHD target organs, T-cell cytokine production in GVHD target organs, and BLI, as well as in vivo and in vitro T-cell proliferation, were subject to 2-tailed Student t test. Pathology scores were compared using a nonparametric Mann-Whitney test. Log-rank test was used to compare survival curves in GVHD experiments.
Results
Combined PKCθ and PKCα deficiency further impairs T-cell function and ameliorates GVHD severity beyond PKCθ deficiency alone
PKCα and PKCθ combine forces to contribute to T-cell alloresponses.15 In the current study, we directly compared the abilities of PKCα−/−, PKCθ−/−, and PKCα−/−/θ−/− T cells to induce GVHD. Gruber et al have previously reported normal CD4 and CD8 T-cell ratios in PKCα−/−/θ−/− mice.15 We also observed that the T-cell percentages and ratios are comparable across different PKC genotypes (data not shown). CD4 and CD8 T cells purified from WT and PKC−/− littermates on a B6 background (H2b) were transplanted with TCD-BM into lethally irradiated MHC-mismatched BALB/c recipients (H2d). Given that more regulatory T cells (Tregs) are present in PKCα−/−/θ−/− mice,26 CD25+ cells were depleted from all grafts prior to BMT to prevent any immune skew that may affect GVHD development. All PKCα −/− T-cell recipients and 82% of WT T-cell recipients succumbed to GVHD within 60 days post-BMT. In contrast, PKCθ−/− and PKCα−/−/θ−/− T-cell recipients developed little to mild GVHD, and all survived more than 100 days (Figure 1A-B). These results indicate that PKCθ−/− and PKCα−/−/θ−/− T cells have similar impaired abilities to induce GVHD. Both WT and PKCα−/− T-cell recipients displayed severe injuries to GVHD target organs, whereas PKCθ−/− and PKCα−/−/θ−/− recipient tissues appeared normal (Figure 1C). In contrast, histologic evidence in PKCθ−/− and PKCα−/−/θ−/− T-cell recipients showed maintenance of normal cellular architecture in GVHD target organs and a lack of characteristic severe lymphocyte infiltration (Figure 1C). The recipients of PKCα−/−/θ−/− T cells had the fewest pathological injuries compared with those of WT T cells (P < .01 in liver, lung, small intestine, and colon) (Figure 1D). PKCα−/−/θ−/− recipients displayed significantly improved GVHD pathological scores compared with PKCθ−/− recipients (Figure 1D). BALB/c mice receiving PKCα−/−/θ−/− T cells displayed the greatest splenic reconstitution of CD4+ and CD8+ T cells and B220+ B cells 120 days posttransplantation (Figure 1E-F), numbers significantly greater than those of PKCθ−/− recipients. As few WT recipients remained, it was only conclusive that a positive trend existed among the recipients transplanted with PKCθ−/− and PKCα−/−/θ−/− T cells. Likewise, the response of reconstituted B cells to lipopolysaccharide (LPS) mitogen in the recipients of PKCα−/−/θ−/− T cells was comparable to that in the recipients of TCD-BM alone, and significantly better than those from recipients of WT or PKCθ−/− T cells (Figure 1G). Because B- and T-cell reconstitution is impaired by GVHD, these results reflect that the recipients of PKCα−/−/θ−/− T cells suffered only mild GVHD. Anti-CD3–induced proliferation of reconstituted PKCα−/−/θ−/− T cells was impaired significantly more so than PKCθ−/− T cells (Figure 1G), which presumably was due to lack of endogenous PKCα/θ per se rather than GVHD-induced immunosuppression. These data indicate the additive roles of PKCα and PKCθ to the impairment of T-cell reconstitution and function during the development of acute GVHD.
Absence of PKCα and θ in donor T cells more comprehensively abrogates GVHD lethality and pathology than deficiency of PKCθ alone. Recipient BALB/c mice were lethally irradiated (800 cGy) and transplanted with 5 × 106 genotype-matched TCD-BM cells alone (n = 2 for each genotype) or in addition to 1 × 106 T cells (CD4+, CD8+, and CD25–) from littermate WT C57BL/6 or PKCα−/−, PKCθ−/−, or PKCα−/−/θ−/− mice (n = 11, 10, 15, and 15 recipients, respectively). Recipient mice were monitored throughout the experimental period for survival (A) and weight change (B), and pooled data from 3 separate experiments are represented. In separate experiments, recipients (n = 4 recipients per group per experiment) were euthanized 2 weeks posttransplant, and samples of skin, liver, lung, small intestine, and large intestine were collected in formalin for routine hematoxylin and eosin and scored for microscopic GVHD severity by a pathologist blinded to the treatment groups. Photomicrographs depicting the average disease score morphology from 1 representative experiment out of 3 separate experiments (C) and average scores for GVHD target organs across 3 separate experiments (D) are depicted. Recipients surviving to 120 days post-BMT in experiments depicted in panels A-B were euthanized, and their spleens were subjected to total splenocyte count (E) and flow cytometric staining and analysis for H2kb, CD4, CD8, and B220 surface expression (F) and plated with 1 ug/mL anti-CD3 or 5 ug/mL LPS for 72 hours followed by overnight tritium thymidine incorporation to observe activation of T cells or B cells, respectively (G). T- and B-cell activities are depicted as per cell activity normalized to the original spleen count. *P < .05; **P < .01; ***P < .001 (compared with WT [brackets indicate statistical significance for comparisons between PKCα−/−/θ−/− and PKCθ−/− treatment groups]). All error bars indicate standard error of the mean (SEM).
Absence of PKCα and θ in donor T cells more comprehensively abrogates GVHD lethality and pathology than deficiency of PKCθ alone. Recipient BALB/c mice were lethally irradiated (800 cGy) and transplanted with 5 × 106 genotype-matched TCD-BM cells alone (n = 2 for each genotype) or in addition to 1 × 106 T cells (CD4+, CD8+, and CD25–) from littermate WT C57BL/6 or PKCα−/−, PKCθ−/−, or PKCα−/−/θ−/− mice (n = 11, 10, 15, and 15 recipients, respectively). Recipient mice were monitored throughout the experimental period for survival (A) and weight change (B), and pooled data from 3 separate experiments are represented. In separate experiments, recipients (n = 4 recipients per group per experiment) were euthanized 2 weeks posttransplant, and samples of skin, liver, lung, small intestine, and large intestine were collected in formalin for routine hematoxylin and eosin and scored for microscopic GVHD severity by a pathologist blinded to the treatment groups. Photomicrographs depicting the average disease score morphology from 1 representative experiment out of 3 separate experiments (C) and average scores for GVHD target organs across 3 separate experiments (D) are depicted. Recipients surviving to 120 days post-BMT in experiments depicted in panels A-B were euthanized, and their spleens were subjected to total splenocyte count (E) and flow cytometric staining and analysis for H2kb, CD4, CD8, and B220 surface expression (F) and plated with 1 ug/mL anti-CD3 or 5 ug/mL LPS for 72 hours followed by overnight tritium thymidine incorporation to observe activation of T cells or B cells, respectively (G). T- and B-cell activities are depicted as per cell activity normalized to the original spleen count. *P < .05; **P < .01; ***P < .001 (compared with WT [brackets indicate statistical significance for comparisons between PKCα−/−/θ−/− and PKCθ−/− treatment groups]). All error bars indicate standard error of the mean (SEM).
Dual PKCα/θ deficiency is superior to PKCθ deficiency in suppressing inflammatory cytokine production
To determine the mechanisms by which PKCα/θ regulate T-cell–mediated GVHD, we measured T-cell activation, effector phenotypes, expansion, and migration. At an early time point (4 days post-BMT), the proliferative and effector responses of PKCθ−/− and PKCα−/−/θ−/− donor T cells were examined in allogeneic and syngeneic mouse models of BMT. We found that PKCθ−/− donor T cells proliferated at lower levels than WT T cells, and PKC α−/−/θ−/− T cells proliferated even less than PKCθ−/− T cells in allogeneic recipients (supplemental Figure 1A,D; see the Blood Web site). However, PKCθ−/− or PKCα−/−/θ−/− T cells exhibited preserved homeostatic proliferation in syngeneic recipients (supplemental Figure 1B,E,F). Increased apoptosis of PKCθ−/− and PKCα−/−/θ−/− donor CD8 T cells in syngeneic recipients suggests that these donor cells underwent a higher rate of apoptosis while proliferating at an elevated level (supplemental Figure 1C). The levels of interferon γ (IFN-γ) production by donor PKCθ−/− CD4 and CD8 T cells were significantly reduced compared with those by WT counterparts, and PKCα−/−/θ−/− T cells produced even lower levels of IFN-γ than PKCθ−/− T cells (supplemental Figure 2). Among the IFN-γ+ population, the majority of these cells were CD25+CD62Llow Teff cells, whereas a substantial proportion of T cells were CD25–CD62Llow, nonactivated cells among the IFN-γ– population (supplemental Figure 2). Interestingly, percentages of CD25+CD62Lhigh T cells were increased among PKCθ−/− T cells and even more so among PKCα−/−/θ−/− T cells, suggesting that higher percentages of Tregs were generated from resting T cells in the absence of PKCθ or PKCα/θ within those IFN-γ– populations (supplemental Figure 2).
At 2 weeks post-BMT, absolute numbers of total splenocytes were significantly increased in the recipients of PKCθ−/− and PKCα−/−/θ−/− donor T cells compared with those of WT or PKCα−/− (Figure 2A, left). Donor CD4+ and CD8+ T-cell numbers were increased in spleens (Figure 2A, left) but decreased in livers (Figure 2B, left) of recipients transplanted with PKCθ−/− or PKCα−/−/θ−/− T cells compared with WT, indicating that PKCθ−/− or PKCα−/−/θ−/− donor T cells have impaired abilities to migrate into the liver. We also observed significantly increased Treg populations in the spleens and livers of recipients of PKCα−/−/θ−/− T cells (supplemental Figure 3). As T helper (Th) 1 responses including IFN-γ and tumor necrosis factor (TNF) α are known to contribute primarily to GVHD progression,27 we examined donor CD4+ and CD8+ intracellular expression of these cytokines in recipient spleens and livers (Figure 2B; supplemental Figure 4). Only PKCα−/−/θ−/− CD4+ donor T cells displayed significantly reduced production of IFN-γ and TNF-α in recipient spleens compared with WT (P < .05; P < .001, respectively). Likewise, PKCα−/−/θ−/− CD8+ splenic donor T cells produced significantly less IFN-γ and TNF-α compared with WT donor cells (P < .001; P < .05, respectively), whereas PKCθ−/− CD8+ donor T cells only significantly downregulated production of IFN-γ (P < .001). In recipient livers, PKCα−/−/θ−/− T cells were significantly more impaired than PKCθ−/− T cells in the production of Th1 cytokines (Figure 2B, middle and right; supplemental Figure 4). Decreases in intracellular cytokines were parallel in recipient sera, indicating that production of IFN-γ, TNF-α, and IL-6 was significantly decreased in the recipients of PKCα−/−/θ−/− T cells as compared with WT T cells, and IFN-γ and IL-6 significantly decreased compared with PKCθ−/− T cells (Figure 2C). These data demonstrate that PKCα−/−/θ−/− donor T cells vs WT or PKCθ−/− T cells had decreased abilities to produce proinflammatory Th1 cytokines and to migrate to GVHD target organs, thus preventing substantial allogeneic responses.
Donor T cells deficient for PKCα and θ produce reduced inflammatory cytokines in vivo. Lethally irradiated (800 cGy) BALB/c mice were transplanted with 5 × 106 genotype-matched TCD-BM cells alone (n = 2 for each genotype) or in addition to 1 × 106 T cells (CD4+, CD8+, and CD25–) from littermate WT C57BL/6 or PKCα−/−, PKCθ−/−, or PKCα−/−/θ−/− mice (n = 4 recipients per group per experiment). Two weeks posttransplant, recipient spleens (A) and livers (B) were harvested, and organ cell counts determined and stained for H2Kb, CD4, CD8, IFNγ, TNFα, and IL-4/5 analysis by flow cytometry. Absolute cell numbers depicted were calculated from whole spleen and liver counts at necropsy with flow cytometric percentages. Serum collected at necropsy was subjected to cytokine bead analysis (C) to quantify serum cytokine concentrations of IFN, TNF, IL-2, IL-6, IL-10, and IL-17. Cytokines that are not graphically represented were undetectable in serum. Averages for all values are represented with error bars indicating SEM from 1 representative experiment out of 3 separate experiments. *P < .05; **P < .01; ***P < .001 (compared with WT [brackets indicate statistical significance for comparisons between PKCα−/−/θ−/− and PKCθ−/− treatment groups]).
Donor T cells deficient for PKCα and θ produce reduced inflammatory cytokines in vivo. Lethally irradiated (800 cGy) BALB/c mice were transplanted with 5 × 106 genotype-matched TCD-BM cells alone (n = 2 for each genotype) or in addition to 1 × 106 T cells (CD4+, CD8+, and CD25–) from littermate WT C57BL/6 or PKCα−/−, PKCθ−/−, or PKCα−/−/θ−/− mice (n = 4 recipients per group per experiment). Two weeks posttransplant, recipient spleens (A) and livers (B) were harvested, and organ cell counts determined and stained for H2Kb, CD4, CD8, IFNγ, TNFα, and IL-4/5 analysis by flow cytometry. Absolute cell numbers depicted were calculated from whole spleen and liver counts at necropsy with flow cytometric percentages. Serum collected at necropsy was subjected to cytokine bead analysis (C) to quantify serum cytokine concentrations of IFN, TNF, IL-2, IL-6, IL-10, and IL-17. Cytokines that are not graphically represented were undetectable in serum. Averages for all values are represented with error bars indicating SEM from 1 representative experiment out of 3 separate experiments. *P < .05; **P < .01; ***P < .001 (compared with WT [brackets indicate statistical significance for comparisons between PKCα−/−/θ−/− and PKCθ−/− treatment groups]).
T cells deficient for both PKCα and θ are more impaired in their GVL activity than those deficient for PKCθ
To further ask how PKCα/θ contributes to donor T-cell–mediated GVL effects, the GVL activity of WT, PKCθ−/−, and PKCα−/−/θ−/− T cells was directly compared by using various numbers of donor T cells. In the range of cell doses tested, we observed that the GVL activity was reduced in PKCθ−/− T cells and further impaired in PKCα−/−/θ−/− T cells as compared with WT counterparts. However, the recipients with either PKCθ−/− or PKCα−/−/θ−/− T cells displayed significantly prolonged tumor relapse compared with those of BM alone (supplemental Figure 5), indicating that these deficient T cells had residual GVL activity.
Pharmacologic PKCα/θ inhibition by R524 hinders T-cell proliferation in vivo and in vitro
Genetic evidence that PKCα−/−/θ−/− impairs allogeneic Teff function to a greater extent than PKCθ−/− indicates likelihood of greater therapeutic potential by cotargeting PKCα/θ when considering pharmacologics for clinical application. As such, we tested the ability of a novel PKCα/θ small-molecule inhibitor (R524) to alter the proliferative kinetics and Th1 cytokines in allogeneic major histocompatibility complex (MHC)–mismatched T-cell responses in vitro and in vivo. R524 is specific for PKCθ (50% inhibition concentration = 1.6 nM) and PKCα (50% inhibition concentration = 8.5 nM) with lower activity against other PKC isoforms. With regard to off-target kinases, in a panel of 123 kinase targets, only 3 kinases (fms-like tyrosine kinase 3, glycogen synthase kinase 3, and vascular endothelial growth factor receptor 2) were inhibited more than 50% by 250 nM R524 (supplemental Table 1).
To mimic MHC-mismatched alloresponses in vitro, T cells from C57BL/6 mice were mixed with irradiated BALB/c APCs or LPS-activated bone marrow–derived dendritic cells in the presence or absence of R524. R524 was able to inhibit IL-2 and IFN-γ production and proliferation in response to alloantigen in vitro in a dose-dependent manner (supplemental Figure 6A-B). Annexin-V staining for viability revealed that R524 did not increase apoptosis at doses ranging from 0.125 to 5 μM (data not shown). Additionally, R524 yielded similar IL-2 and T-cell activation inhibitory effects on primary human cells in vitro (supplemental Table 1).
We next evaluated the effect of R524 on T-cell alloresponses and GVHD development in vivo. R524 was first titrated and tested for toxicity in irradiated TCD-BM recipients. Pharmacokinetics indicated that R524 completely clears the blood at 24 hours, dictating that twice-daily doses would be required (data not shown). Thirty days of continual R524 dosing revealed that twice-daily 40 mg/kg R524 dosed by gavage, the highest dose tested, was safe for irradiated recipients (supplemental Figure 7A-B). Importantly, twice-daily gavages with 40 mg/kg R524 did not adversely affect engraftment of donor lymphocytes (supplemental Figure 7C). Observation of the recovery of donor nonlymphocyte H2kb+ hematopoietic cells from recipients treated with 40 mg/kg twice-daily R524 gavage indicated no significant effects of R524 on hematopoietic cell recovery (data not shown).
To test R524 effects on allogeneic T-cell proliferation in vivo, carboxyfluorescein diacetate succinimidyl ester–labeled WT or PKCα−/−/θ−/− B6 splenocytes were transplanted into MHC-mismatched, lethally irradiated BALB/c recipients. Following 5 days of vehicle or 40 mg/kg R524 twice-daily gavage, R524 significantly reduced WT donor T-cell proliferation when compared with vehicle controls (P < .001 for all comparisons) (supplemental Figure 8A-B). The magnitude of R524 inhibition of T-cell proliferation above and beyond that of PKCα−/−/θ−/− T-cell proliferation was substantially reduced as compared with that on WT T-cell proliferation, suggesting both PKCα and θ target-specific and nontarget-specific effects. R524 treatment significantly suppressed the production of IFN-γ and TNF-α by WT but not by PKCα−/−/θ−/− T cells in vivo (supplemental Figure 8C-D). Collectively, these data indicate that pharmacologic inhibition of PKCα and PKCθ with a single pharmacologic agent effectively inhibits allogeneic T-cell cytokine and proliferative responses.
R524 inhibition of PKCα and PKCθ abrogates GVHD
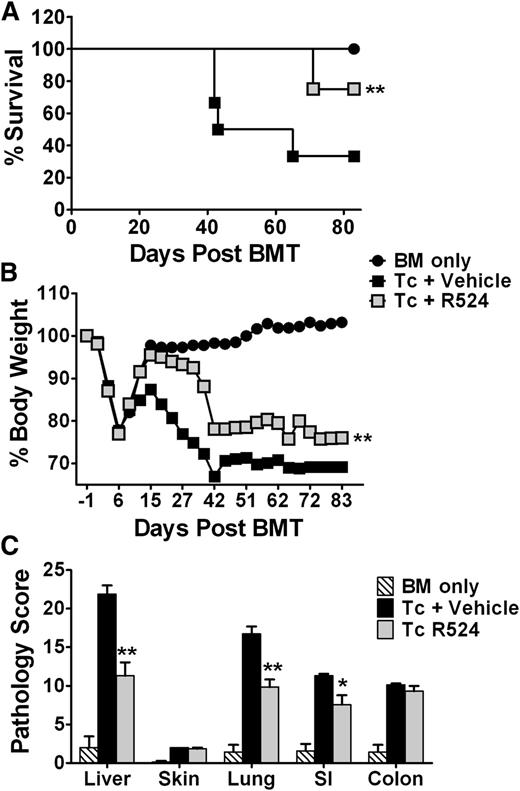
We next tested R524 efficacy in the prevention of GVHD using a MHC-mismatched murine model of myeloablative HCT (B6 → BALB/c), in which recipient mice received vehicle or R524 twice daily beginning on the day of transplantation and continually thereafter for 6 weeks. Eighty days posttransplant, R524-treated mice exhibited significantly reduced symptoms of GVHD including reduced weight loss (P < .01) and 75% survival (P < .01) compared with vehicle-treated controls (Figure 3A-B). Histologic analysis 14 days post-BMT revealed that R524 prophylaxis resulted in reduced GVHD pathology scores in recipient livers, lungs, and small intestines (P < .01; P < .01; P < .05, respectively) when compared with vehicle-treated controls (Figure 3C). These data demonstrate that a safe and effective regimen of R524 was identified in the recipients after allogeneic BMT. Thus, pharmacologic inhibition of PKCα/θ achieves significant GVHD amelioration and organ sparing in the context of MHC-mismatched myeloablative BMT.
Inhibition of PKCα and θ significantly ameliorates acute GVHD after myeloablative allogeneic BMT. Recipient BALB/c mice were lethally irradiated (800 cGy) and transplanted with 5 × 106 C57BL/6 TCD-BM cells alone (n = 6) or in addition to 1 × 106 total T cells from WT C57BL/6 donors and treated twice daily by gavage with vehicle (n = 15) or 40 mg/kg R524 (n = 12) beginning on day 0 (the day of BMT) and continuing daily for 6 weeks. Recipient mice were monitored throughout the experimental period for survival (A) and weight change (B), and pooled data from 3 separate experiments are represented. In separate experiments, recipients (n = 4 recipients per group per experiment) were euthanized 2 weeks posttransplant, and samples of skin, liver, lung, small intestine, and large intestine were collected in formalin for routine hematoxylin and eosin and scored for microscopic GVHD severity by a pathologist blinded to the treatment groups. Average scores for GVHD target organs across 3 separate experiments (C) are depicted. *P < .05; **P < .01; ***P < .001 (compared with vehicle-treated recipients). All error bars indicate SEM.
Inhibition of PKCα and θ significantly ameliorates acute GVHD after myeloablative allogeneic BMT. Recipient BALB/c mice were lethally irradiated (800 cGy) and transplanted with 5 × 106 C57BL/6 TCD-BM cells alone (n = 6) or in addition to 1 × 106 total T cells from WT C57BL/6 donors and treated twice daily by gavage with vehicle (n = 15) or 40 mg/kg R524 (n = 12) beginning on day 0 (the day of BMT) and continuing daily for 6 weeks. Recipient mice were monitored throughout the experimental period for survival (A) and weight change (B), and pooled data from 3 separate experiments are represented. In separate experiments, recipients (n = 4 recipients per group per experiment) were euthanized 2 weeks posttransplant, and samples of skin, liver, lung, small intestine, and large intestine were collected in formalin for routine hematoxylin and eosin and scored for microscopic GVHD severity by a pathologist blinded to the treatment groups. Average scores for GVHD target organs across 3 separate experiments (C) are depicted. *P < .05; **P < .01; ***P < .001 (compared with vehicle-treated recipients). All error bars indicate SEM.
R524 decreases T-cell proliferation and increases splenic retention in BMT recipients
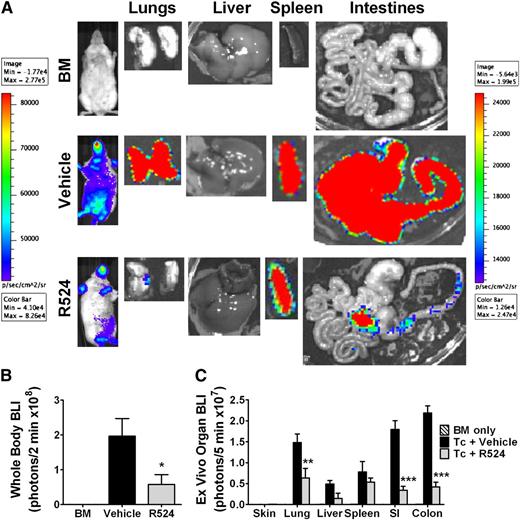
To delineate allogeneic donor T-cell expansion and migration capacity under R524 prophylaxis, we used B6 donor T cells transgenic for luciferase under β-actin promoter control. Two weeks posttransplant, BALB/c recipients were subjected to BLI and euthanized, and GVHD target organs imaged by ex vivo BLI for determination of donor T-cell expansion and migration. Recipients treated with R524 had limited T-cell expansion and reduced T-cell migration to the lungs and gut while retaining these cells in the spleen (Figure 4A). Quantification of BLI intensity from whole body, lung, small intestine, and colon from the individual organs of R524-treated recipients indicated significant reductions from vehicle-treated controls (P < .05; P < .01; P < .001; P < .001, respectively) (Figure 4B-C).
Inhibition of PKCα and θ restricts alloreactive donor T-cell expansion and migration to GVHD target organs. BALB/c mice were irradiated at 800 cGy and transplanted with 5 × 106 WT C57CL/6 TCD-BM cells alone (n = 2 per experiment) or in addition to 1 × 106 total T cells from β-actin luciferase transduced C57BL/6 mice and treated twice daily by gavage with vehicle or 40 mg/kg R524 (n = 4 per group per experiment) beginning on day 0 and continuing daily for 2 weeks. Fourteen days posttransplant, recipients were subjected to intraperitoneal luciferin injection and whole body BLI followed by a second luciferin injection, euthanization, organ removal, and BLI of GVHD target organs (A). Bioluminescence was quantified using whole body (B) or whole organ (C) region of interest gating and signal normalization with Living Image software. Average representative whole body images, ex vivo organ images, and average bioluminescence intensities ± SEM depicted are from 1 representative experiment of 2 separate experiments. *P < .05; **P < .01; ***P < .001 (compared with vehicle-treated controls).
Inhibition of PKCα and θ restricts alloreactive donor T-cell expansion and migration to GVHD target organs. BALB/c mice were irradiated at 800 cGy and transplanted with 5 × 106 WT C57CL/6 TCD-BM cells alone (n = 2 per experiment) or in addition to 1 × 106 total T cells from β-actin luciferase transduced C57BL/6 mice and treated twice daily by gavage with vehicle or 40 mg/kg R524 (n = 4 per group per experiment) beginning on day 0 and continuing daily for 2 weeks. Fourteen days posttransplant, recipients were subjected to intraperitoneal luciferin injection and whole body BLI followed by a second luciferin injection, euthanization, organ removal, and BLI of GVHD target organs (A). Bioluminescence was quantified using whole body (B) or whole organ (C) region of interest gating and signal normalization with Living Image software. Average representative whole body images, ex vivo organ images, and average bioluminescence intensities ± SEM depicted are from 1 representative experiment of 2 separate experiments. *P < .05; **P < .01; ***P < .001 (compared with vehicle-treated controls).
R524 prophylaxis significantly reduces expression of chemokine receptors and cytokine production
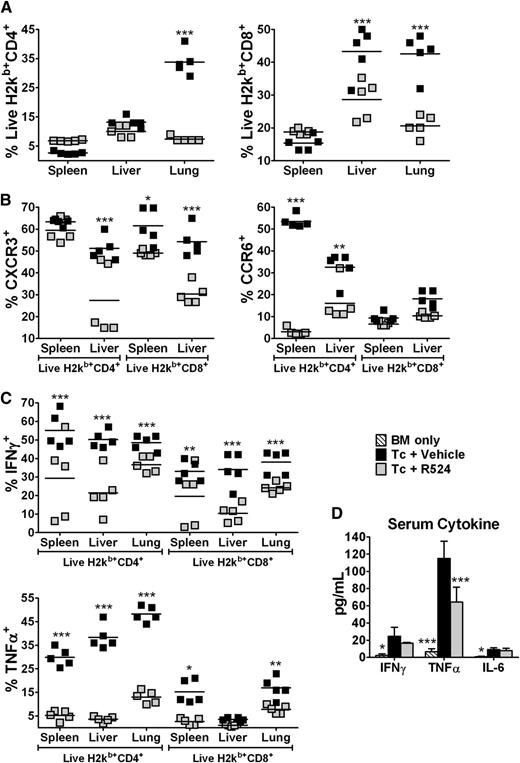
Together with inflammatory cytokine signals, the chemokine receptors CXCR3 and CCR6 are known to support Th1 T-cell migration to the liver, gut, and skin, as well as Th17 T-cell migration to the lungs and skin, respectively.8,28-30 Supportive of BLI results, flow cytometric analysis of recipient lymphocytes isolated from spleens, livers, and lungs of R524-treated mice indicate a similar retention of donor T cells in spleens, but significant reductions of donor T cells in the lungs and livers of these mice (CD4+ lung and CD8+ livers/lungs, P < .001) (Figure 5A). The trend of effects on spleen cellularity observed with R524 treatment or PKCα/θ deficiency is similar but of different magnitude (Figures 2 and 5), which was likely because pharmacologic inhibition was not as potent or target specific as genetic deletion. Mononuclear cells were isolated from recipient spleen and liver and stained for the Th1 effector chemotactic factor CXCR3 and the Th1 and Th17 chemotactic factor CCR6. Th1 responses are primarily involved in the development of GVHD, but Th17 cells can contribute to GVHD pathogenesis.20,25 R524 prophylaxis significantly reduced CXCR3 donor CD8+ T-cell expression from spleens and CD4+ and CD8+ CXCR3 in livers compared with vehicle controls (P < .05 for spleen T cells; P < .01 for liver T cells). Only CD4+ spleen and liver T cells from R524-treated recipients displayed reduced expression of the Th17 and Th1 chemokine receptor CCR6 (P < .001; P < .01, respectively) (Figure 5B; supplemental Figure 9). CCR6 was not detected in significant amounts in recipient lungs (data not shown). Also, intracellular IL-17 was not detected in significant amounts in spleen, liver, or lung samples at any time point tested across multiple experiments, regardless of the presence or absence of PKC genetic or pharmacologic inhibition (data not shown). In addition, R524 prophylaxis resulted in significant (P < .01) reductions in IFN-γ production by CD4+ and CD8+ donor T cells isolated from recipient spleens, livers, and lungs compared with vehicle treatment. Significant decreases were seen in TNF-α production by donor T cells from R524-treated recipients (Figure 5C; supplemental Figure 9). Serum cytokine analysis also revealed that R524-treated recipients had significantly (P < .001) reduced concentrations of TNF-α and a trend of reduced IFN-γ (Figure 5D). Together, these results indicate that R524 inhibition of PKCα/θ in vivo significantly reduced Th1 cytokine production, T-cell migration, and expression of Th1 and Th17 chemotactic CXCR3 and CCR6, respectively. This could explain reduced Teff cell migration and GVHD pathology in GVHD target organs.
Inhibition of PKCα and θ reduces alloreactive chemokine receptor expression and inflammatory cytokine production in vivo. Total-body irradiation (800 cGy) was dosed to BALB/c mice, which were then transplanted with 5 × 106 WT C57BL/6 TCD-BM cells alone (n = 2 per experiment) or in addition to 1 × 106 total T cells and treated twice daily by gavage with vehicle or 40 mg/kg R524 (n = 4 per group per experiment) beginning on day 0 and continuing daily for 2 weeks. Fourteen days posttransplant, recipient spleens, livers, and lungs were harvested; organ cell counts determined; and samples stained for H2Kb, CD4, CD8, CXCR3, CCR6, IFN-γ, and TNF-α. Average percentages ± SEM are based on live H2Kb+ (donor) cell gate (A), T-cell chemokine receptor expression (B), and intracellular T-cell cytokine expression (C). Serum collected at necropsy was subjected to cytokine bead analysis (D) to quantify serum cytokine concentrations of IFN, TNF, IL-2, IL-6, IL-10, and IL-17. Cytokines that are not graphically represented were undetectable in serum. Data are representative of 1 experiment out of 3 separate repeat experiments. *P < .05; **P < .01; ***P < .001 (compared with vehicle-treated controls).
Inhibition of PKCα and θ reduces alloreactive chemokine receptor expression and inflammatory cytokine production in vivo. Total-body irradiation (800 cGy) was dosed to BALB/c mice, which were then transplanted with 5 × 106 WT C57BL/6 TCD-BM cells alone (n = 2 per experiment) or in addition to 1 × 106 total T cells and treated twice daily by gavage with vehicle or 40 mg/kg R524 (n = 4 per group per experiment) beginning on day 0 and continuing daily for 2 weeks. Fourteen days posttransplant, recipient spleens, livers, and lungs were harvested; organ cell counts determined; and samples stained for H2Kb, CD4, CD8, CXCR3, CCR6, IFN-γ, and TNF-α. Average percentages ± SEM are based on live H2Kb+ (donor) cell gate (A), T-cell chemokine receptor expression (B), and intracellular T-cell cytokine expression (C). Serum collected at necropsy was subjected to cytokine bead analysis (D) to quantify serum cytokine concentrations of IFN, TNF, IL-2, IL-6, IL-10, and IL-17. Cytokines that are not graphically represented were undetectable in serum. Data are representative of 1 experiment out of 3 separate repeat experiments. *P < .05; **P < .01; ***P < .001 (compared with vehicle-treated controls).
R524 allows for maintained GVL effects despite significant T-cell impairment
We next tested for GVL maintenance under the treatment of R524 utilizing the B6→BALB/c allogeneic BMT model supplemented with A20 murine B-cell lymphoma cells transduced with luciferase/neo plasmid (A20-luc) for tumor tracking by BLI. Under twice-daily vehicle or R524 gavage for 6 weeks, GVHD vs tumor mortality was separated by BLI signal (indicative of tumor load) and weight loss (indicative of GVHD onset). Recipients were euthanized and checked for tumor at necropsy when moribund. As expected, all recipients of A20-luc and TCD-BM succumbed to tumor within 3 weeks. Vehicle-treated recipients of allogeneic T cells all succumbed to GVHD by day 60 posttransplant as evidenced by lack of tumor signal, lack of visible tumor when moribund, and notable weight loss. In contrast, R524-treated recipients maintained original body weights, indicative of abrogated GVHD, and only 20% succumbed to tumor. Weight maintenance and survival were significantly improved (P < .001; P < .05, respectively) in R524-treated recipients above vehicle controls (Figure 6A-C). Given that GVL effects are primarily mediated by CD8 T cells, we measured the cytolytic function of T cells from BMT recipients against P815 (H2Kd) tumor target cells and found that cytolytic function was largely preserved in T cells from R524-treated recipients (Figure 6D).
GVL is preserved in myeloablated allogeneic BMT recipients treated with R524. BALB/c mice were lethally irradiated (800 cGy) and transplanted with 5 × 106 C57BL/6 TCD-BM cells alone (n = 4) or in addition to 1 × 106 total T cells from WT C57BL/6 and treated twice daily by gavage with vehicle (n = 10) or 40 mg/kg R524 (n = 10) beginning on day 0 (the day of BMT) and continuing daily for 6 weeks. Additionally, recipients were intravenously injected with 2 × 103 A20 luciferase-transduced lymphoma cells at the time as BMT. Recipients were monitored throughout the experimental period for survival (A), weight change (B), and tumor expansion by luciferin intraperitoneal injection and whole body BLI (C). Average survival and weight changes across 2 separate repeat experiments and recipient BLI images from 1 of the 2 replicated experiments are shown. *P < .05; **P < .01; ***P < .001 (compared with vehicle-treated recipients). In a separate experiment in which recipient mice were treated with vehicle or R524 twice daily for 14 days post-BMT, splenocytes were assayed for preserved cytotoxic T lymphocyte (CTL) activity against control EL4 or mismatched P815 tumor target cells ex vivo (D). CTL assay was run in triplicate and normalized to % CD8+ cells in recipient spleens.
GVL is preserved in myeloablated allogeneic BMT recipients treated with R524. BALB/c mice were lethally irradiated (800 cGy) and transplanted with 5 × 106 C57BL/6 TCD-BM cells alone (n = 4) or in addition to 1 × 106 total T cells from WT C57BL/6 and treated twice daily by gavage with vehicle (n = 10) or 40 mg/kg R524 (n = 10) beginning on day 0 (the day of BMT) and continuing daily for 6 weeks. Additionally, recipients were intravenously injected with 2 × 103 A20 luciferase-transduced lymphoma cells at the time as BMT. Recipients were monitored throughout the experimental period for survival (A), weight change (B), and tumor expansion by luciferin intraperitoneal injection and whole body BLI (C). Average survival and weight changes across 2 separate repeat experiments and recipient BLI images from 1 of the 2 replicated experiments are shown. *P < .05; **P < .01; ***P < .001 (compared with vehicle-treated recipients). In a separate experiment in which recipient mice were treated with vehicle or R524 twice daily for 14 days post-BMT, splenocytes were assayed for preserved cytotoxic T lymphocyte (CTL) activity against control EL4 or mismatched P815 tumor target cells ex vivo (D). CTL assay was run in triplicate and normalized to % CD8+ cells in recipient spleens.
It is possible that the treatment with R524 inhibits tumor growth independent of T-cell–mediated GVL effect. To test this alternative hypothesis, we cultured A20 cells with R524 at a wide range of concentrations in vitro for 2 to 3 days and found that the growth or death of A20 cells was unaffected by R524 up to 10 µM (data not shown). Furthermore, we observed that R524 at the dose and schedule effectively preventing GVHD had no effect on A20 growth in the recipients with TCD-BM alone (supplemental Figure 10). Taken together, these experiments make it clear that R524 impairs donor T-cell responses, which contribute to GVHD while sparing GVL effects mediated by cytotoxic lymphocytes.
R524 abrogates GVHD in an MiHA-antigen mismatched model while preserving GVL effects
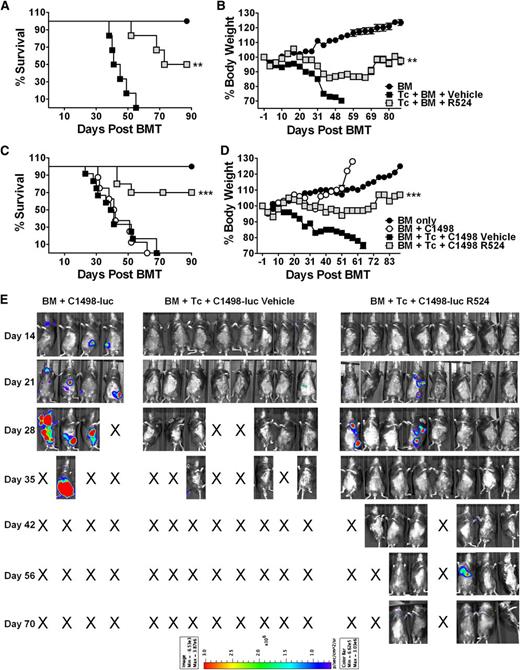
MiHA-antigen mismatches play an important role in clinical GVHD development in HLA-identical BMT conditions.31 Accordingly, R524 was examined in a C3.SW→B6 MiHA-mismatched myeloablative preclinical model of BMT including twice-daily vehicle or R524 gavage for 6 weeks. GVHD lethality of vehicle-treated recipients was 100%. R524 was again able to significantly reduce GVHD-mediated weight loss (P < .01) and maintain 50% survival (P < .01) (Figure 7A-B).
GVHD induction is impaired, whereas GVL effects are preserved in R524-treated recipients in an MiHA-mismatch BMT model. C57BL/6 recipients received total-body irradiation (1200 cGy) and were transplanted with donor C3SW.H TCD-BM alone (n = 6) or in combination with 3 × 106 T cells (CD4+, CD8+, CD25–, and CD44–) and treated with vehicle (n = 6) or 40 mg/kg R524 (n = 6) twice daily for 6 weeks beginning on day 0. Additional recipients were intravenously injected with 5 × 104 C1498 luciferase-transduced leukemia cells at the time as BMT (n = 10 vehicle treated and n = 10 R524 treated). Recipients without tumor (A-B) and with tumor (C-E) were monitored throughout the experimental period for survival (A,C) and weight change (B,D). In leukemia recipients, tumor expansion was tracked by luciferin intraperitoneal injection and whole body BLI (E). Leukemia growth was confirmed in recipients at necropsy. Average survival and weight changes across 3 separate repeat experiments and recipient BLI images from 1 of 2 repeating experiments are shown. *P < .05; **P < .01; ***P < .001 (compared with vehicle-treated recipients).
GVHD induction is impaired, whereas GVL effects are preserved in R524-treated recipients in an MiHA-mismatch BMT model. C57BL/6 recipients received total-body irradiation (1200 cGy) and were transplanted with donor C3SW.H TCD-BM alone (n = 6) or in combination with 3 × 106 T cells (CD4+, CD8+, CD25–, and CD44–) and treated with vehicle (n = 6) or 40 mg/kg R524 (n = 6) twice daily for 6 weeks beginning on day 0. Additional recipients were intravenously injected with 5 × 104 C1498 luciferase-transduced leukemia cells at the time as BMT (n = 10 vehicle treated and n = 10 R524 treated). Recipients without tumor (A-B) and with tumor (C-E) were monitored throughout the experimental period for survival (A,C) and weight change (B,D). In leukemia recipients, tumor expansion was tracked by luciferin intraperitoneal injection and whole body BLI (E). Leukemia growth was confirmed in recipients at necropsy. Average survival and weight changes across 3 separate repeat experiments and recipient BLI images from 1 of 2 repeating experiments are shown. *P < .05; **P < .01; ***P < .001 (compared with vehicle-treated recipients).
To observe GVL effects, murine C1498 leukemia cells transduced with luciferase allowed BLI imaging of tumor expansion (Figure 7C-E). All the recipients with TCD-BM plus tumor died with extensive tumor load (Figure 7E), and all vehicle-treated recipients with T cells plus tumor died without relapse following significant weight loss and associated GVHD clinical features. In contrast, 70% of R524-treated recipients survived (P < .001 compared with vehicle) without weight loss (P < .001 compared with vehicle) (Figure 7C-D). The 30% of R524-treated recipients that did not survive had no detectable tumor by BLI signal or necropsy but incurred weight loss indicative of GVHD. During in vitro culture, the growth or death of C1498 cells was not affected by R524 up to 10 µM (data not shown). In conclusion, as in the MHC-mismatched model, R524 significantly ameliorates GVHD while leaving GVL mechanisms intact in an MiHA-mismatched BMT model.
Discussion
Here, we show that an optimal T-cell alloresponse requires both PKCθ and α in the development of acute GVHD after allogeneic BMT. The novel dual PKCα/θ inhibitor, R524, was able to support anti-GVHD efficacy pharmacologically with specificity and safety in recipients while largely preserving GVL effects in MHC-mismatched and MiHA-mismatched BMT models, although relatively less potency and some off-target effects were observed compared with genetic deletion of PKCα and θ. Together, the 2 complementary approaches, genetic and pharmacologic, underscore the critical role of PKCα and PKCθ in GVHD-related alloresponses.
To date, no form of clinical intervention proposed has effectively cleared the way for safe and efficacious application of BMT as a hematopoietic therapy. Evidence presented here clearly illustrates the superior suppression of donor T-cell TCR-induced activation, proliferation, Th1 cytokine production, and subsequent GVHD pathology in the lungs, livers, and colons of PKCα−/−/θ−/− recipients compared with PKCθ−/− recipients. The current study also provided compelling evidence that accumulation of PKCθ−/− or PKCα−/−/θ−/− donor T cells in recipient spleens at later time points likely resulted from intact or even elevated homeostatic proliferation and impairment of migration into GVHD target organs. We confirm that PKCα is employed in complementary and redundant functions with PKCθ in T cells. Lack of both PKCα and θ would block the primary TCR→PKCθ-mediated→CARMA1→NF-κB signals as well as the potential alternative PKCα→CARMA3 route and thus lower the ability of deficient T cells to activate and exert effector functions vital to optimal Th1 immune responses.32,33 Likewise, only dispensing of both PKCα and θ would abrogate both NFAT and NF-κB–mediated IL-2 production in T cells.13,15
Our data depict significantly reduced percentages of CD4+ donor T cells in livers of PKCα−/−/θ−/− but not PKCθ−/− recipients, supporting the redundancy of PKCα in these mechanisms. We examined R524 in vivo for anti-GVHD efficacy as PKCα and PKCθ work collaboratively to promote production of inflammatory mediators known to contribute significantly to T-cell alloreactivity in GVHD and are themselves frequent therapeutic targets for T-cell alloreactivity.34-38 Supportive of PKCα therapeutic redundancy in T cells for PKCθ activation of IL-2 production and NF-κB, initial in vitro tests using R524 revealed relevant and dose-dependent biological activity against IL-2 production, IFN-γ production, and TCR-activation induced proliferation in allogeneic T cells in a dose-dependent manner. Related to known PKCα and θ participation in T-cell migration and invasion signaling,39,40 we have shown here that R524 treatment is associated with reduced expression of Th1 and Th17 chemokine receptors for GVHD target organ homing. This effect is likely linked to blocking NF-κB activation of gene transcription for Th1 cytokines through PKCθ inhibition and inhibition of redundant PKCα signaling as well. Further, R524 likely blocks NFAT signaling through PKCα and θ inhibition.13,15,16,18,41 More work will also be required to establish the precise toxicity profile of PKC inhibitors, such as R524, but this type of inhibitor has the potential to be well tolerated as suggested by our genetic and pharmacologic in vivo data. Although our work indicates greater potential for Treg conversion in recipients of PKCα−/−/θ−/− compared with PKCθ−/− or donor T cells, we found no positive or negative impact of R524 on Treg conversion or function. This result seems to be different from the observation by Zanin-Zhorov et al.21 We suspect that different inhibitors (irreversible PKCθ vs reversible PKCα/θ inhibitor) and cell types (human vs mouse) used in these studies might contribute to the discrepancy. Additionally, although PKCθ is rather exclusively expressed on T cells, PKCα is shown to be present in professional and nonprofessional APCs,42 and we thus cannot exclude the possibility that observed outcomes with R524 treatment were partially due to its effect on other types of cells besides T cells.
Of clinical importance, we show here that R524 yields significant anti-GVHD efficacy and preserves GVL responses in 2 preclinical BMT models. Unlike inhibition of PKCα/θ, we observed that the GVL activity of PKCθ−/− T cells was significantly reduced and that of PKCα−/−/θ−/− T cells was further impaired. However, the median survival of recipients with BM + PKCα−/−/θ−/− T cells was significantly longer than that in the recipients of TCD-BM alone (supplemental Figure 5), indicating that the GVL activity of PKCα−/−/θ−/− T cells was not absent. Taken together, we interpret that inhibition but not absence of PKCα/θ permits the preservation of GVL effects.
Interestingly, we observed in the BMT models tested that discontinuation of R524 treatment lead to moderate GVHD recurrence in the absence of tumor, whereas in the presence of A20 or C1498 tumor cells, no such GVHD development occurred following withdraw of R524 after 6 weeks of treatment. This could indicate that tumor cells rendered donor T cells more susceptible to PKC inhibition through either cell contact with APCs or cytokine mediation. The precise mechanisms of this phenomenon remain to be further investigated. Because donor CTL machinery is critically important for GVL responses, we demonstrated that cytolytic activity was largely intact for T cells in R524-treated recipients. Our data suggest that PKCα and PKCθ signaling is not required for all the GVL responses. Precise mechanisms accounting for the difference remain for further study.
In conclusion, our work provides novel evidence of the requirement of both α and θ isoforms for severe GVHD induction and maintenance, but not for GVL immunologic effects. Given that similar PKC inhibitors, such as AEB071, have proved efficacious in clinical trials in patients suffering with plaque psoriasis as well as in renal-transplant recipients,43,44 the current study provides a strong rationale to evaluate the efficacy of PKCα/θ inhibitors for the prevention of GVHD while preserving GVL activity after clinical allogeneic HCT.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Esteban Celis (H. Lee Moffitt Cancer Center and Research Institute) for his consultation and advice. The authors are grateful to Matthew Duncton, lead PKC program chemist at Rigel, and Gary Park, lead drug metabolism and pharmacokinetics scientist at Rigel, for their kind support of these studies. Further, the authors recognize the technical assistance provided by Jodi Kroeger and Kate Shapland of the Moffitt Flow Cytometry Core Facility, as well as Epi Ruiz and Jennifer Morse of the Moffitt Imaging Core and Vivarium Facilities.
This work was supported in part by grants from the National Institutes of Health, National Cancer Institute (R01s CA11816, CA143812, and AI 082685) (X.-Z.Y.) (R01 CA72669) (B.R.B.).
A materials transfer agreement was established between Rigel Pharmaceuticals and H. Lee Moffitt Cancer Center and Research Institute for the use of the compound R524.
Authorship
Contribution: K.M.K.H. participated in experimental design, performed research, collected, analyzed, and interpreted data, performed statistical analysis, and drafted and revised the manuscript; J.L., J.H., D.W., C.C.B., K.K., and S.H. performed research, collected and analyzed data, and edited the manuscript; C.L. performed pathological analysis; K.T. designed and discovered the compound R524; A.M.O., E.M., B.R.B., C.A., and A.A.B. participated in experimental design, interpreted data, and revised the manuscript; and X.-Z.Y. designed research, collected, analyzed, and interpreted data, and revised the manuscript.
Conflict-of-interest disclosure: A.M.O., S.H., E.M., and K.T. are employed by Rigel Pharmaceuticals and own stock and/or stock options in the company. The remaining authors declare no competing financial interests.
Correspondence: Dr Xue-Zhong Yu, Department of Microbiology & Immunology, Medical University of South Carolina, MSC 955, 86 Jonathan Luca St, Charleston, SC 29425; e-mail: yux@musc.edu.

![Figure 1. Absence of PKCα and θ in donor T cells more comprehensively abrogates GVHD lethality and pathology than deficiency of PKCθ alone. Recipient BALB/c mice were lethally irradiated (800 cGy) and transplanted with 5 × 106 genotype-matched TCD-BM cells alone (n = 2 for each genotype) or in addition to 1 × 106 T cells (CD4+, CD8+, and CD25–) from littermate WT C57BL/6 or PKCα−/−, PKCθ−/−, or PKCα−/−/θ−/− mice (n = 11, 10, 15, and 15 recipients, respectively). Recipient mice were monitored throughout the experimental period for survival (A) and weight change (B), and pooled data from 3 separate experiments are represented. In separate experiments, recipients (n = 4 recipients per group per experiment) were euthanized 2 weeks posttransplant, and samples of skin, liver, lung, small intestine, and large intestine were collected in formalin for routine hematoxylin and eosin and scored for microscopic GVHD severity by a pathologist blinded to the treatment groups. Photomicrographs depicting the average disease score morphology from 1 representative experiment out of 3 separate experiments (C) and average scores for GVHD target organs across 3 separate experiments (D) are depicted. Recipients surviving to 120 days post-BMT in experiments depicted in panels A-B were euthanized, and their spleens were subjected to total splenocyte count (E) and flow cytometric staining and analysis for H2kb, CD4, CD8, and B220 surface expression (F) and plated with 1 ug/mL anti-CD3 or 5 ug/mL LPS for 72 hours followed by overnight tritium thymidine incorporation to observe activation of T cells or B cells, respectively (G). T- and B-cell activities are depicted as per cell activity normalized to the original spleen count. *P < .05; **P < .01; ***P < .001 (compared with WT [brackets indicate statistical significance for comparisons between PKCα−/−/θ−/− and PKCθ−/− treatment groups]). All error bars indicate standard error of the mean (SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/14/10.1182_blood-2012-12-471938/4/m_2500f1.jpeg?Expires=1769124522&Signature=hVrcre8~W~LhP7XfexvNinnlzJQyXGhZtzZlTIbPSCottwmIZ8LVzh9xzVjCjKfREZoNqsj7Q9~YaUX7lENGVWOC7TXshlVV3mrjXHFmltkg5FBnXWOEKVdy8sReoYTIOgfx0owI4LN3deU7iaqiLvlnb2CGnNMpUUaDzm-FPq-izDj2os7FQ6~nR7uqvmBxGr5-Ybl8PfDjZA~mgy3TyATg1cDL90yWl3Kze0OcIkguPjnTAVaDVlwCPDgPtM5mpwNmuUxxqG5b3I~wX6BhA1-zkhW4L0x~0ASx6KpwfwcC1bie07rm3XE7vPWbeH-gEwOjTGwTixuQw2z6Atdrlg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Donor T cells deficient for PKCα and θ produce reduced inflammatory cytokines in vivo. Lethally irradiated (800 cGy) BALB/c mice were transplanted with 5 × 106 genotype-matched TCD-BM cells alone (n = 2 for each genotype) or in addition to 1 × 106 T cells (CD4+, CD8+, and CD25–) from littermate WT C57BL/6 or PKCα−/−, PKCθ−/−, or PKCα−/−/θ−/− mice (n = 4 recipients per group per experiment). Two weeks posttransplant, recipient spleens (A) and livers (B) were harvested, and organ cell counts determined and stained for H2Kb, CD4, CD8, IFNγ, TNFα, and IL-4/5 analysis by flow cytometry. Absolute cell numbers depicted were calculated from whole spleen and liver counts at necropsy with flow cytometric percentages. Serum collected at necropsy was subjected to cytokine bead analysis (C) to quantify serum cytokine concentrations of IFN, TNF, IL-2, IL-6, IL-10, and IL-17. Cytokines that are not graphically represented were undetectable in serum. Averages for all values are represented with error bars indicating SEM from 1 representative experiment out of 3 separate experiments. *P < .05; **P < .01; ***P < .001 (compared with WT [brackets indicate statistical significance for comparisons between PKCα−/−/θ−/− and PKCθ−/− treatment groups]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/14/10.1182_blood-2012-12-471938/4/m_2500f2.jpeg?Expires=1769124522&Signature=LhMa9stfLIhoAHKg6r00SYnXB4NfGTHOd3faKa86S7-bfY8muHTjJBoeUSNNCMJeyFcqNh2TQwQQB1UDDxh52veMyf9RVb1wCP435DupcBXwGvkpLBBXGMtqJme7eiXGNh4bzyxDubMSVGZ0tn-zYl1XLniA4n1G8dchh2t1T1P23b9rPnWph2bo9kst0n5qXoSAhS9U1-ok0nxjRphFD8pVRV8fLWVwOQGG9gpMfUiyXHdNsdFKfxzZ-34lNatEiJc0JeLBwIJsGNjKHO0r9BcZ0RHTmF7OgmzdFct-BX8BnhDlm3IVi30myoeIZoHLaI~oTXinz~-1Bi512qQLjw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal