In this issue of Blood, Vockel and Vestweber identify the molecular signaling pathway leading from T-lymphocyte adhesion to dissociation of the transmembrane phosphatase vascular endothelial–specific receptor protein tyrosine phosphatase (VE-PTP) from VE-cadherin, a key step in allowing leukocyte transmigration.1
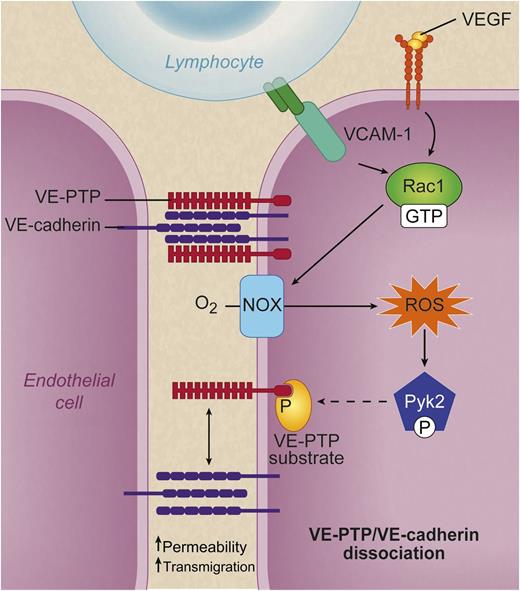
When lymphocyte α4β1 integrin (dark green dimer) binds to endothelial VCAM-1 (turquoise) or VEGF (yellow spheres) binds VEGF receptor 2 (top right, orange), a signaling cascade is set in motion that leads to activation of Rac1 (GTP bound). Rac1 in turn activates NOX, which converts molecular oxygen to ROS. These ROS activate the kinase Pyk2, which phosphorylates an unidentified substrate (yellow) for VE-PTP and causes VE-PTP (red) to dissociate from VE cadherin (purple). This dissociation is associated with increased leukocyte transmigration and increased macromolecular permeability. See Figure 7D in the paper by Vockel and Vestweber that begins on page 2512. Professional illustration by Debra T. Dartez
When lymphocyte α4β1 integrin (dark green dimer) binds to endothelial VCAM-1 (turquoise) or VEGF (yellow spheres) binds VEGF receptor 2 (top right, orange), a signaling cascade is set in motion that leads to activation of Rac1 (GTP bound). Rac1 in turn activates NOX, which converts molecular oxygen to ROS. These ROS activate the kinase Pyk2, which phosphorylates an unidentified substrate (yellow) for VE-PTP and causes VE-PTP (red) to dissociate from VE cadherin (purple). This dissociation is associated with increased leukocyte transmigration and increased macromolecular permeability. See Figure 7D in the paper by Vockel and Vestweber that begins on page 2512. Professional illustration by Debra T. Dartez
An excellent summary of their findings is contained in their figure 7D1 : when a lymphocyte’s α4β1 integrin binds endothelial vascular adhesion molecule (VCAM)-1, this causes activation of the small GTPase Rac1. Rac1 in turn activates the endothelial nicotinamide adenine dinucleotide phosphate oxidase (NOX complex), which produces superoxide and hydrogen peroxide, also known as reactive oxygen species (ROS). ROS activate Pyk2, a kinase related to the integrin-organizing focal adhesion kinase.2 The Pyk2 kinase phosphorylates an unknown substrate for VE-PTP,3 and voila, VE-PTP dissociates from VE-cadherin.
VE-cadherin is a member of the large cadherin family of cell-cell adhesion proteins and the only cadherin that is exclusively expressed in endothelial cells. Endothelial cells separate the flowing blood from the extravascular (interstitial) space. They serve critical functions in vascular biology by expressing anticoagulants to keep the blood flowing, by regulating vasoconstriction and vasodilation, by controlling macromolecular permeability, and by denying most leukocytes access to the extravascular space. However, under conditions of inflammation and immune responses, endothelial cells allow transmigration of certain leukocytes including neutrophils and monocytes and, in the case of immune-driven or allergic inflammation, effector T lymphocytes. Exquisite regulation of leukocyte access to extravascular tissues is needed to prevent unwanted inflammation yet allow leukocytes to infiltrate tissues upon infection or trauma.
VE-cadherin dissociation from VE-PTP is a key regulator of leukocyte migration through the endothelial lining of microvessels. Most leukocytes including lymphocytes transmigrate out of the vascular system using a paracellular pathway, as opposed to the alternative transcellular pathway that accounts for ∼10% of transendothelial migration under most conditions. Paracellular transmigration requires that molecules in the endothelial junction move out of the way to allow passage of the leukocyte. Amazingly, this “moving-out-of-the-way” happens without blowing holes in the VE lining. Such holes would allow plasma proteins to leak. Once plasma proteins leak, fluid follows, because the natural difference in oncotic (colloid osmotic) pressure between intra- and extravascular space is destroyed. During inflammation, some plasma proteins do escape, which leads to tissue edema, a common consequence of inflammation.4
So how does selective access for leukocytes and limited access for macromolecules work? Probably by the formation of small holes or slits between endothelial cells that persist just long enough to let a cell squeeze through, but not long enough to allow large amounts of plasma proteins to escape. In experimental systems, it is possible to induce leukocyte transmigration without leakage of plasma proteins5 or plasma protein leakage without significant leukocyte transmigration.6 Although this has been known for >25 years, it was not known how the endothelium differentially regulates cellular and protein traffic. This was clarified by Vockel and Vestweber’s recent work on VE-cadherin, culminating in the present paper.1
VE-cadherin reversibly binds to catenins, which bridge to the cytoskeleton.7 This interaction is regulated by mediators that influence VE permeability such as histamine or VE growth factor (VEGF), but also by leukocyte adhesion and transmigration. In a knockin mouse in which VE-cadherin is fused to the C terminus of α catenin, VEGF or histamine cannot induce vascular permeability.8 In this mouse, leukocyte recruitment induced by interleukin-1β in cremaster muscle venules or by bacterial lipopolysaccharide in the lung is also blocked. Similarly, T-cell recruitment in response to the hapten 1-fluoro-2,4-dinitrobenzene is reduced. By contrast, naïve lymphocyte homing to peripheral lymph nodes or inflammatory cell recruitment to the peritoneal cavity in response to thioglycollate-induced sterile inflammation is not affected. Similar results were found in a mouse in which VE-cadherin association with VE-PTP was forced by introducing inducible dimerization elements.9 These findings show that VE-cadherin attachment to the cytoskeleton regulates microvascular permeability and leukocyte migration in many, but not all, tissues.
VE-cadherin has 9 tyrosines that can be phosphorylated. In vitro assays identified 2 of these as likely phosphorylation sites that regulate VE-cadherin interaction with other molecules. It is possible that differential phosphorylation might provide a molecular explanation for the differential regulation of leukocyte extravasation and macromolecular permeability.
The present paper1 defines the signaling pathway from leukocyte adhesion to endothelial VCAM-1 to dissociation of VE-PTP from VE-cadherin. Although many other molecules are found in endothelial junctions, including junctional adhesion molecules, platelet endothelial cell adhesion molecule-1, and CD99,10 it appears that VE-cadherin plays the role of master regulator of endothelial permeability and leukocyte transendothelial migration in many vascular beds. The data assembled by Vestweber’s group over the last 10 years conclusively established this in mice. Most likely, the regulation of vascular permeability and leukocyte extravasation is similar in humans. Amazingly, the VE-cadherin mechanisms are not universal throughout the organism: the mechanisms regulating leukocyte migration in high endothelial venules of lymphatic tissues and in the lining of the peritoneal cavity remain to be discovered. The present paper1 shows how VCAM-1 binding can destabilize endothelial junctions in postcapillary venules of skin and muscle. However, some leukocytes like neutrophils express no or very limited amounts of α4β1, the integrin binding VCAM-1. Therefore, other pathways regulating leukocyte migration must exist even in these microvessels. Whether or not these other mechanisms also converge on VE-cadherin remains to be seen.
Conflict-of-interest disclosure: The author declares no competing financial interests.