Key Points
GVHD after HLA-DPB1–mismatched CD4+ DLI after TCD-alloSCT is mediated by allo-reactive HLA-DPB1–directed CD4+ T cells.
Viral infections after TCD-alloSCT can induce HLA class II on nonhematopoietic tissues, making them targets for CD4+ T cells in GVHD.
Abstract
CD8+ T cell–depleted (TCD) donor lymphocyte infusion (DLI) after TCD allogeneic hematopoietic stem cell transplantation (alloSCT) has been associated with a reduced risk of graft-versus-host disease (GVHD) while preserving conversion to donor hematopoiesis and antitumor immunity, providing a rationale for exploring CD4+ T cell–based immunotherapy for hematologic malignancies. Here, we analyzed the clinical course and specificity of T cell immune responses in 2 patients with acute myeloid leukemia (AML) who converted to full-donor chimerism but developed severe acute GVHD after prophylactic CD4+ DLI after 10/10-HLA–matched, but HLA-DPB1–mismatched TCD-alloSCT. Clonal analysis of activated T cells isolated during GVHD demonstrated allo-reactivity exerted by CD4+ T cells directed against patient-mismatched HLA-DPB1 molecules on hematopoietic cells and skin-derived fibroblasts only when cultured under inflammatory conditions. At the time of CD4+ DLI, both patients contained residual patient-derived T cells, including cytomegalovirus (CMV)-specific T cells as a result of CMV reactivations. Once activated by CMV antigens, these CMV-specific T cells could stimulate HLA-DPB1–specific CD4+ T cells, which in turn could target nonhematopoietic tissues in GVHD. In conclusion, our data demonstrate that GVHD after HLA-DPB1–mismatched CD4+ DLI can be mediated by allo-reactive HLA-DPB1–directed CD4+ T cells and that ongoing viral infections inducing HLA class II expression on nonhematopoietic cells may increase the likelihood of GVHD development. This trial is registered at http://www.controlled-trials.com/ISRCTN51398568/LUMC as #51398568.
Introduction
In allogeneic hematopoietic stem cell transplantation (alloSCT), T-cell depletion of the graft efficiently prevents the occurrence of severe acute graft-versus-host disease [GVHD])1,2 but also adversely affects posttransplant antitumor and antipathogen immunity.1-3 Early intervention with donor lymphocyte infusion (DLI) after T cell–depleted (TCD)-alloSCT may prevent relapse of the malignancy and improve immune reconstitution against pathogens but is frequently associated with reintroduction of GVHD.4,5 Therefore, exploration of treatment strategies to improve the balance between graft-versus-leukemia (GVL) reactivity and antipathogen immunity and GVHD is warranted.
To minimize the risk of GVHD, patients are preferably transplanted with stem cell grafts from HLA-matched sibling or unrelated donors (URD).6 HLA matching is generally performed for HLA-A, -B, -C, -DRB1, and -DQB1 alleles (10/10 match) but not for HLA-DPB1. Therefore, 70% to 80% of URD alloSCT are HLA-DPB1–mismatched.6,7 In contrast to HLA class I, constitutive expression of HLA class II molecules is mainly confined to normal and malignant hematopoietic cells,8-11 and HLA class II disparity in alloSCT may therefore induce GVL reactivity without GVHD. In accordance with this, we previously demonstrated induction of selective GVL reactivity by allo-reactive HLA-DPB1–specific CD4+ T cells after delayed infusion of DLI after HLA-DPB1–mismatched TCD-alloSCT.12 Other clinical studies also reported that HLA-DPB1 disparity in TCD-alloSCT is associated with GVL reactivity without increased GVHD incidence.13 In contrast, mismatching for HLA class II in non–TCD-alloSCT has been associated with severe GVHD.14-17 This may be explained by upregulated expression of HLA class II molecules on nonhematopoietic tissues in an inflammatory environment,18 which may be particularly relevant in the early posttransplantation period as a result of tissue damage from the transplant conditioning procedure and lymphopenia-induced homeostatic proliferation of donor T cells.19 As a consequence, recipient nonhematopoietic cells can become targets for allo-reactive CD4+ T cells. Therefore, the clinical effect of CD4+ T cell–mediated immune responses is likely to be strongly influenced by clinical circumstances.
It is well established that CD8+ T cells are potent effector cells in antitumor and antipathogen immunity,20 and that they play an essential role in GVHD.21 Although CD4+ T cells are generally regarded as helper cells in the induction and maintenance of CD8+ T-cell immunity,22 they can also exert direct cytolysis in antitumor and antipathogen immunity.12,23 Clinical application of CD8+ TCD DLI induced conversion to donor hematopoiesis and disease remission in patients with relapsed malignancies and was associated with a lower incidence of opportunistic infections in the absence of severe GVHD.24-26 These clinical findings provide a rationale for exploration of CD4+ T cell–based immunotherapy as a treatment modality for selective stimulation of GVL reactivity and protective immunity against pathogens after TCD-alloSCT.
We recently started a clinical study of treatment of patients with hematologic malignancies transplanted with stem cells from HLA-matched URD with prophylactic CD4+ DLI at 3 months after TCD-alloSCT. Two patients developed severe acute colonic GVHD after CD4+ DLI after 10/10-HLA–matched, but HLA-DPB1–mismatched TCD-alloSCT, and in these patients we analyzed the clinical course and specificity of T cell immune responses. Our results show induction of profound polyclonal CD4+ T cell immune responses directed against mismatched HLA-DPB1 alleles in both patients. Allo-reactive HLA-DPB1–specific CD4+ T cells recognized HLA class II–expressing patient hematopoietic cells and skin-derived fibroblasts, but only when they were cultured under proinflammatory conditions. As a consequence of cytomegalovirus (CMV) reactivation early after alloSCT, both patients contained high frequencies of residual patient-derived CMV-specific T cells at the time of CD4+ DLI. Once activated by CMV antigens, these CMV-specific T cells could stimulate and be targeted by HLA-DPB1–specific CD4+ T cells. Because of the release of proinflammatory factors by CMV-specific T cells in vivo, nonhematopoietic colonic cells of the patient are likely to have upregulated HLA class II expression, thereby becoming targets for HLA-DPB1–specific CD4+ T cells. Our data indicate that allo-reactive HLA-DPB1–specific CD4+ T cells can act as direct mediators of acute GVHD after HLA-DPB1–mismatched CD4+ DLI, and they suggest that ongoing viral infections resulting in upregulated HLA class II expression on nonhematopoietic tissues increase the likelihood of GVHD development.
Patients and methods
Case reports
Two patients with acute myeloid leukemia (AML) in first complete remission were transplanted with TCD-mobilized peripheral blood (PB) stem cells from a 10/10-HLA–matched, but HLA-DPB1–mismatched, URD (Table 1; Figure 1). After alloSCT, hematopoietic recovery was observed in the absence of acute GVHD and no posttransplant immune suppressive therapy was administered. Patients gave informed consent in accordance with the Declaration of Helsinki to participate in a phase II open-label, single-center, randomized clinical study approved by the Leiden University Medical Center (LUMC) Institutional Review Board and national authorities, which evaluated immunologic effects of prophylactic DLI of purified donor CD4+ T cells early after TCD-alloSCT for hematologic malignancies. CD4+ DLI cell products were manufactured within a good manufacturing practice–compliant process on the CliniMACS System (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Enrichment of CD4+ cells was performed according to the manufacturer’s instructions using CliniMACS CD4 Reagents. Patients received a single infusion of 0.5*106 purified CD4+ T cells/kg body weight at 3.5 (patient 1) and 3.0 (patient 2) months after alloSCT, at which time bone marrow (BM) mononuclear cell fraction showed mixed chimerism (Figure 1). After CD4+ DLI, both patients showed a decrease in patient chimerism, which coincided with initially limited acute GVHD of the skin requiring only topical corticosteroid treatment. Three to 8 weeks later, both patients developed grade 3/4 acute colonic GVHD that was successfully treated with systemic immune suppression. Early after alloSCT and after CD4+ DLI, both patients experienced CMV reactivations requiring antiviral treatment (Figure 1). At 1 year after alloSCT, both patients were in complete remission.
Patient and donor characteristics
| . | Patient 1 . | Patient 2 . |
|---|---|---|
| Diagnosis | AML-M1 | AML-M0 |
| Age, y | 66 | 58 |
| Gender of patient/donor | M/M | M/F |
| CMV serostatus of patient/donor | +/+ | +/+ |
| EBV serostatus of patient/donor | +/– | +/+ |
| HLA-DP typing* | ||
| Patient | B1*0101/B1*0301 A1*0103/A1*0201 | B1*0301/B1*0401 A1*0103 |
| Donor | B1*0201/B1*0401 A1*0103 | B1*0201/B1*0401 A1*0103 |
| T-cell composition of CD4+ DLI | ||
| CD3+CD4+ T cells, % | 98.29 | 98.22 |
| CD3+CD8+ T cells, % | 1.23 | 1.46 |
| CD3+CD4+CD8+ T cells, % | 0.11 | 0.22 |
| CD3+CD4-CD8- T cells, % | 0.36 | 0.22 |
| . | Patient 1 . | Patient 2 . |
|---|---|---|
| Diagnosis | AML-M1 | AML-M0 |
| Age, y | 66 | 58 |
| Gender of patient/donor | M/M | M/F |
| CMV serostatus of patient/donor | +/+ | +/+ |
| EBV serostatus of patient/donor | +/– | +/+ |
| HLA-DP typing* | ||
| Patient | B1*0101/B1*0301 A1*0103/A1*0201 | B1*0301/B1*0401 A1*0103 |
| Donor | B1*0201/B1*0401 A1*0103 | B1*0201/B1*0401 A1*0103 |
| T-cell composition of CD4+ DLI | ||
| CD3+CD4+ T cells, % | 98.29 | 98.22 |
| CD3+CD8+ T cells, % | 1.23 | 1.46 |
| CD3+CD4+CD8+ T cells, % | 0.11 | 0.22 |
| CD3+CD4-CD8- T cells, % | 0.36 | 0.22 |
CMV and EBV serostatus was determined by standard serology methods: +, positive; –, negative.
Mismatched patient HLA-DPB1 and HLA-DPA1 alleles are underlined.
HLA class I and HLA class II typing was performed by DNA analysis using sequence-specific primers. Patients and their URD were fully matched at HLA-A, -B, -C, -DRB, and -DQB1 alleles (not shown).
Clinical immune responses in patients treated with prophylactic CD4+ DLI after HLA-DPB1–mismatched URD alloSCT. After a nonmyeloablative conditioning regimen consisting of fludarabine, busulfan, alemtuzumab, and rabbit-derived antithymocyte globulin, patients received a stem cell transplant graft depleted of T cells by in vitro incubation with alemtuzumab. Percentage of donor chimerism (-▪-) in BM (left, Y-axis), absolute numbers of CD3+CD4+ (-●-), and CD3+CD8+ (-○-) T cells (first right, Y-axis [black]) in PB and CMV-DNA ( ) (log copies/mL) (second right, Y-axis [gray]) after alloSCT for patient 1 (A) and patient 2 (B) are shown (supplemental Methods). Arrows indicate CD4+ DLI infusion. Asterisks and stars indicate the time of diagnosis of acute skin GVHD (21 and 16 days after CD4+ DLI for patients 1 and 2, respectively) and colonic GVHD (35 and 58 days after CD4+ DLI for patients 1 and 2, respectively). § indicates time of detection of CMV infection in colonic biopsy of patient 1 (133 days after CD4+ DLI). Both patients developed CMV reactivation within the first month after alloSCT, which was treated with antiviral drugs (valganciclovir). Both patients cleared CMV reactivation at 2 months after alloSCT but again experienced several episodes of CMV reactivations requiring antiviral treatment after CD4+ DLI. Longitudinal bars indicate duration of antiviral therapy. Patient 2 experienced 1 episode of Epstein-Barr virus reactivation (black bar), which was treated with Rituximab (anti-CD20 monoclonal antibody). The intensity and duration of systemic immunosuppressive treatment is indicated by longitudinal bars. CsA, cyclosporine; MMF, mycofenolate mofetil; PNL, prednisolone.
) (log copies/mL) (second right, Y-axis [gray]) after alloSCT for patient 1 (A) and patient 2 (B) are shown (supplemental Methods). Arrows indicate CD4+ DLI infusion. Asterisks and stars indicate the time of diagnosis of acute skin GVHD (21 and 16 days after CD4+ DLI for patients 1 and 2, respectively) and colonic GVHD (35 and 58 days after CD4+ DLI for patients 1 and 2, respectively). § indicates time of detection of CMV infection in colonic biopsy of patient 1 (133 days after CD4+ DLI). Both patients developed CMV reactivation within the first month after alloSCT, which was treated with antiviral drugs (valganciclovir). Both patients cleared CMV reactivation at 2 months after alloSCT but again experienced several episodes of CMV reactivations requiring antiviral treatment after CD4+ DLI. Longitudinal bars indicate duration of antiviral therapy. Patient 2 experienced 1 episode of Epstein-Barr virus reactivation (black bar), which was treated with Rituximab (anti-CD20 monoclonal antibody). The intensity and duration of systemic immunosuppressive treatment is indicated by longitudinal bars. CsA, cyclosporine; MMF, mycofenolate mofetil; PNL, prednisolone.
Clinical immune responses in patients treated with prophylactic CD4+ DLI after HLA-DPB1–mismatched URD alloSCT. After a nonmyeloablative conditioning regimen consisting of fludarabine, busulfan, alemtuzumab, and rabbit-derived antithymocyte globulin, patients received a stem cell transplant graft depleted of T cells by in vitro incubation with alemtuzumab. Percentage of donor chimerism (-▪-) in BM (left, Y-axis), absolute numbers of CD3+CD4+ (-●-), and CD3+CD8+ (-○-) T cells (first right, Y-axis [black]) in PB and CMV-DNA ( ) (log copies/mL) (second right, Y-axis [gray]) after alloSCT for patient 1 (A) and patient 2 (B) are shown (supplemental Methods). Arrows indicate CD4+ DLI infusion. Asterisks and stars indicate the time of diagnosis of acute skin GVHD (21 and 16 days after CD4+ DLI for patients 1 and 2, respectively) and colonic GVHD (35 and 58 days after CD4+ DLI for patients 1 and 2, respectively). § indicates time of detection of CMV infection in colonic biopsy of patient 1 (133 days after CD4+ DLI). Both patients developed CMV reactivation within the first month after alloSCT, which was treated with antiviral drugs (valganciclovir). Both patients cleared CMV reactivation at 2 months after alloSCT but again experienced several episodes of CMV reactivations requiring antiviral treatment after CD4+ DLI. Longitudinal bars indicate duration of antiviral therapy. Patient 2 experienced 1 episode of Epstein-Barr virus reactivation (black bar), which was treated with Rituximab (anti-CD20 monoclonal antibody). The intensity and duration of systemic immunosuppressive treatment is indicated by longitudinal bars. CsA, cyclosporine; MMF, mycofenolate mofetil; PNL, prednisolone.
) (log copies/mL) (second right, Y-axis [gray]) after alloSCT for patient 1 (A) and patient 2 (B) are shown (supplemental Methods). Arrows indicate CD4+ DLI infusion. Asterisks and stars indicate the time of diagnosis of acute skin GVHD (21 and 16 days after CD4+ DLI for patients 1 and 2, respectively) and colonic GVHD (35 and 58 days after CD4+ DLI for patients 1 and 2, respectively). § indicates time of detection of CMV infection in colonic biopsy of patient 1 (133 days after CD4+ DLI). Both patients developed CMV reactivation within the first month after alloSCT, which was treated with antiviral drugs (valganciclovir). Both patients cleared CMV reactivation at 2 months after alloSCT but again experienced several episodes of CMV reactivations requiring antiviral treatment after CD4+ DLI. Longitudinal bars indicate duration of antiviral therapy. Patient 2 experienced 1 episode of Epstein-Barr virus reactivation (black bar), which was treated with Rituximab (anti-CD20 monoclonal antibody). The intensity and duration of systemic immunosuppressive treatment is indicated by longitudinal bars. CsA, cyclosporine; MMF, mycofenolate mofetil; PNL, prednisolone.
Sample and tissue collection
BM and PB samples and colonic biopsies were obtained from the patients, and PB samples were obtained from the URD and healthy individuals, after approval by the LUMC Institutional Review Board and receiving informed patient consent according to the Declaration of Helsinki. BM and PB mononuclear cells (PBMCs) were isolated by Ficoll-Isopaque separation and cryopreserved.
Phenotypic analysis of patient samples
Phenotypic analysis of T cells in patient PB samples and CMV-specific T cells was performed by staining with PE-labeled HLA-DP (Meridian Life Science, Memphis, TN), antigen-presenting cell (APC)-labeled HLA-DR (Becton Dickinson, San Jose, CA), and APC-H7–labeled CD3 (Pharmingen, San Diego, CA) moAb. Tetramer analysis was performed with viral-peptide/MHC complexes (supplemental Table 2, available on the Blood Web site).
Isolation and culture of T-cell clones
Activated CD4+ and CD8+ T cells were isolated from PBMCs obtained from the patients after CD4+ DLI, as previously described.27 PBMCs were stained with fluorescein isothiocyanate-labeled CD4 or CD8 (Becton Dickinson) and PE-labeled HLA-DR–specific moAb, and HLA-DR+CD4+ or HLA-DR+CD8+cells were single cell–sorted by flowcytometry in 96-well plates containing irradiated (50 Gy) allogeneic PBMCs (0.5*105/well) in 100 μL Iscove’s Modified Dulbecco’s Medium (Lonza, Verviers, Belgium) supplemented with 5% human serum (HS), 5% fetal calf serum (FCS; Lonza), IL-2 (120 IU/mL) (Chiron, Amsterdam, The Netherlands), and phytohemagglutinin (0.8 μg/mL) (Murex Biotec Limited, Dartford, United Kingdom). Proliferating T-cell clones were restimulated every 10 to 20 days.
Stimulator cells for functional analysis
Epstein-Barr virus–transformed lymphoblastoid cell lines (EBV-LCL) were generated using standard procedures. Donor EBV-LCL were retrovirally transduced with the patient-mismatched HLA class II alleles, as described previously.28 Monocyte-derived dendritic cells were generated as outlined in supplemental Methods. Patient T cells from samples before CD4+ DLI were isolated after staining with fluorescein isothiocyanate-labeled CD3 (Pharmingen) moAb and sorting by flowcytometry. Patient CMV-specific T cells were generated from patient PBMC samples before CD4+ DLI by stimulating PBMCs with CMV-pp65 and -IE1 protein-spanning peptide pools (Peptivator; Miltenyi Biotec GmbH; and PepMixTM; JPR Peptide Technologies, Berlin, Germany), as described previously.29 Activated CMV-specific T cells were generated by restimulation with peptide pool–pulsed donor EBV-LCL. Patient fibroblasts were generated from skin biopsies and cultured without or with 200 IU/mL Interferon (IFN)-γ (Boehringer Ingelheim, Alkmaar, The Netherlands) for 5 days in Dulbecco’s Modified Eagle Medium (Lonza) supplemented with 10% FCS.
Characterization of isolated T-cell clones
To analyze their specificity, isolated T-cell clones (2000 cells) were cultured with patient or donor stimulator cells (5000-20 000) in 40 μL Iscove’s Modified Dulbecco’s Medium supplemented with 5% HS, 5% FCS, and 10 IU/mL IL-2 in 384-well plates (Greiner Bio-One, Alphen a/d Rijn, The Netherlands). After overnight incubation at 37°C, IFN-γ production by T-cell clones was analyzed in 10 μL of supernatant by IFN-γ enzyme-linked immunosorbent assay (ELISA) (Sanquin, Amsterdam, The Netherlands). For detection of CMV-specific T cells, donor EBV-LCL were loaded with 5 μg/mL CMV lysate (Advanced Biotechnologies Inc., Columbia, MD) 1 hour before the addition of T cells.
HLA-restriction molecules for allo-reactive CD4+ T-cell clones were determined using moAb against HLA class I (W6/32), HLA class II (PdV5.2), HLA-DR (B8.11-2), HLA-DQ (SPVL3), and HLA-DP (B7.21) (Department of Immunohematology and Blood Transfusion, Leiden, The Netherlands). Stimulator cells were preincubated with saturating concentrations of moAb for 30 minutes before the addition of T cells.
To determine T-cell receptor (TCR)-Vβ usage of allo-reactive T-cell clones, a TCR-Vβ kit (Beckman Coulter, Fullerton, CA) was used according to the manufacturer’s instructions.
To determine whether patient-derived T cells before CD4+ DLI and CMV-specific T cells after isolation could activate the HLA-DPB1–specific CD4+ T cells, CD4+ T-cell clones (5000 cells) were labeled with PKH26 (Sigma-Aldrich, Steinheim, Germany) and coincubated with stimulator cells (5000-150 000). After 24 hours, CD137 expression on T-cell clones was determined by staining with APC-labeled CD137 (Becton Dickinson) and analysis by flowcytometry.
Fluorescent immunohistochemistry and fluorescence in situ hybridization analysis
Multicolor immunofluorescent analysis and fluorescence in situ hybridization (FISH) analysis were performed as outlined in supplemental Methods.
Results
Isolation and characterization of activated T cells after CD4+ DLI
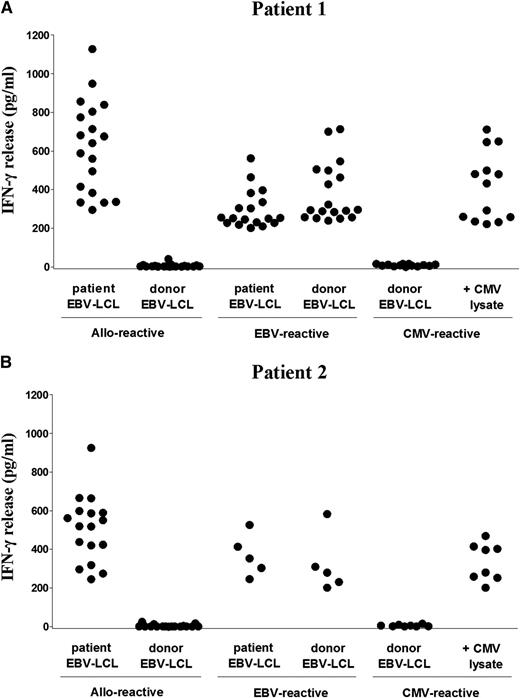
We investigated the specificity of T cell immune responses in 2 patients with AML in whom acute GVHD developed after prophylactic CD4+ DLI was administered 3 months after HLA-DPB1–mismatched TCD-alloSCT (Figure 1). On the basis of coexpression of HLA-DR, activated CD4+ and CD8+ T cells were clonally isolated from PB samples during acute skin and colonic GVHD after CD4+ DLI. Expansion was obtained for 775 CD4+ and 288 CD8+ T-cell clones from patient 1 and 204 CD4+ and 27 CD8+ T-cell clones from patient 2. In total, 19 (2.5%) and 16 (5.6%) CD4+ T-cell clones from patient 1 and patient 2, respectively, were allo-reactive based on specific production of IFN-γ (>200 pg/mL) upon stimulation with patient, but not donor, EBV-LCL (Figure 2). Similar results were obtained using patient- and donor-derived monocyte-matured dendritic cells as stimulator cells (supplemental Figure 1). Allo-reactivity was demonstrated for one CD8+ T-cell clone (0.3%) from patient 2. In addition to allo-reactive T-cell clones, 18 (2.3%) CD4+ T-cell clones from patient 1 and 5 (1.7%) CD4+ and 1 (3.8%) CD8+ T-cell clones from patient 2 recognized both patient and donor EBV-LCL, indicating isolation of EBV-reactive T-cell clones. Furthermore, 13 (1.7%) CD4+ and 7 (3.4%) CD8+ T-cell clones from patient 1 and 8 (2.8%) CD4+ T-cell clones from patient 2 recognized donor EBV-LCL pulsed with CMV lysate, indicating isolation of CMV-reactive T-cell clones.
Functional analysis of isolated CD4+ T-cell clones. Reactivity of all CD4+ T-cell clones isolated during acute skin and colonic GVHD from patient 1 (A) and patient 2 (B) was tested against patient and donor EBV-LCL with IFN-γ ELISA. Allo-reactive CD4+ T-cell clones produced significant levels of IFN-γ (>200 pg/mL) upon incubation with patient, but not donor, EBV-LCL, whereas EBV-reactive CD4+ T-cell clones produced IFN-γ upon incubation with both patient and donor EBV-LCL, and CMV-reactive CD4+ T-cell clones upon incubation with donor EBV-LCL pulsed with CMV lysate. Each symbol represents the release of IFN-γ (pg/mL) in 10 µL supernatants by a single CD4+ T-cell clone.
Functional analysis of isolated CD4+ T-cell clones. Reactivity of all CD4+ T-cell clones isolated during acute skin and colonic GVHD from patient 1 (A) and patient 2 (B) was tested against patient and donor EBV-LCL with IFN-γ ELISA. Allo-reactive CD4+ T-cell clones produced significant levels of IFN-γ (>200 pg/mL) upon incubation with patient, but not donor, EBV-LCL, whereas EBV-reactive CD4+ T-cell clones produced IFN-γ upon incubation with both patient and donor EBV-LCL, and CMV-reactive CD4+ T-cell clones upon incubation with donor EBV-LCL pulsed with CMV lysate. Each symbol represents the release of IFN-γ (pg/mL) in 10 µL supernatants by a single CD4+ T-cell clone.
These results illustrate that activated T cells in the patients at the time of acute GVHD after HLA-DPB1–mismatched CD4+ DLI contained allo-reactive and virus-specific T cells, and that allo-reactivity was almost exclusively exerted by CD4+ T cells.
HLA specificity of allo-reactive T-cell clones
To determine the HLA restriction molecules for allo-reactive T-cell clones, blocking studies were performed with moAb specific for HLA class I or HLA class II. All allo-reactive CD4+ T-cell clones from patient 1 (n = 19) and patient 2 (n = 16) showed HLA-DP–restricted recognition of patient EBV-LCL (Figure 3A-B). Because both patients and their URDs were only mismatched for HLA-DP (Table 1), all allo-reactive CD4+ T-cell clones were tested for recognition of donor EBV-LCL retrovirally transduced with patient-mismatched HLA-DP alleles (Table 1). For patient 1, specific recognition of donor EBV-LCL transduced with HLA-DPB1*0101/A1*0201 or HLA-DPB1*0301/A1*0201 was observed for 8 and 11 CD4+ T-cell clones, respectively (Figure 3C). For patient 2, all HLA-DP–restricted CD4+ T-cell clones (n = 16) recognized donor EBV-LCL transduced with HLA-DPB1*0301/A1*0103 (Figure 3D). The allo-reactive CD8+ T-cell clone from patient 2 was shown to be HLA-C–restricted (data not shown). TCR-Vβ analysis demonstrated T cells expressing a variety of TCRs, illustrating polyclonality of the T-cell response (data not shown).
HLA specificity of allo-reactive CD4+ T-cell clones. HLA restriction of all isolated allo-reactive CD4+ T-cell clones from patient 1 (n = 19) (A) and patient 2 (n = 16) (B) was analyzed using blocking moAb specific for HLA class I, HLA class II, and HLA-DR, -DQ, and -DP with IFN-γ ELISA. Each dot represents the release of IFN-γ (pg/mL) in 10-µL supernatants by a single allo-reactive CD4+ T-cell clone in response to patient EBV-LCL in the absence or presence of blocking moAb and donor EBV-LCL. All CD4+ T-cell clones showed HLA-DP–restricted recognition, as illustrated by abrogated IFN-γ production upon preincubation with moAb specific for HLA class II and HLA-DP, but not with moAb against HLA class I, HLA-DR, or HLA-DQ. (C-D) All allo-reactive HLA-DP–restricted CD4+ T-cell clones were tested for recognition of patient and donor EBV-LCL, and donor EBV-LCL retrovirally transduced with patient HLA-DPB1 and -DPA1 alleles (HLA-DPB1*0101 and -DPB1*0301 with DPA1*0201 for patient 1 [C] and HLA-DPB1*0301 with -DPA1*0103 for patient 2 [D]) with IFN-γ ELISA. HLA-DPB1–restricted CD4+ T-cell clones from patient 1 recognized either HLA-DPB1*0101 (○) or HLA-DPB1*0301 (●) transduced donor EBV-LCL. Each dot represents the release of IFN-γ (pg/mL) in 10 µL supernatant by a single CD4+ T-cell clone.
HLA specificity of allo-reactive CD4+ T-cell clones. HLA restriction of all isolated allo-reactive CD4+ T-cell clones from patient 1 (n = 19) (A) and patient 2 (n = 16) (B) was analyzed using blocking moAb specific for HLA class I, HLA class II, and HLA-DR, -DQ, and -DP with IFN-γ ELISA. Each dot represents the release of IFN-γ (pg/mL) in 10-µL supernatants by a single allo-reactive CD4+ T-cell clone in response to patient EBV-LCL in the absence or presence of blocking moAb and donor EBV-LCL. All CD4+ T-cell clones showed HLA-DP–restricted recognition, as illustrated by abrogated IFN-γ production upon preincubation with moAb specific for HLA class II and HLA-DP, but not with moAb against HLA class I, HLA-DR, or HLA-DQ. (C-D) All allo-reactive HLA-DP–restricted CD4+ T-cell clones were tested for recognition of patient and donor EBV-LCL, and donor EBV-LCL retrovirally transduced with patient HLA-DPB1 and -DPA1 alleles (HLA-DPB1*0101 and -DPB1*0301 with DPA1*0201 for patient 1 [C] and HLA-DPB1*0301 with -DPA1*0103 for patient 2 [D]) with IFN-γ ELISA. HLA-DPB1–restricted CD4+ T-cell clones from patient 1 recognized either HLA-DPB1*0101 (○) or HLA-DPB1*0301 (●) transduced donor EBV-LCL. Each dot represents the release of IFN-γ (pg/mL) in 10 µL supernatant by a single CD4+ T-cell clone.
These results show that both patients with acute GVHD after CD4+ DLI after HLA-DPB1–mismatched alloSCT developed profound allo-reactive CD4+ T-cell responses directed against the mismatched HLA-DPB1 alleles of the patient.
Recognition of nonhematopoietic cells by allo-reactive T-cell clones
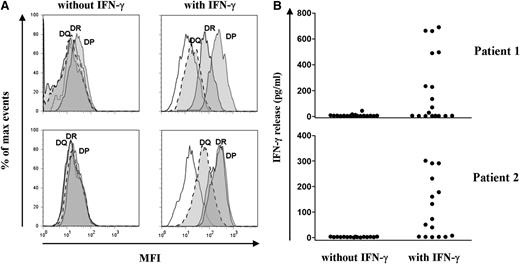
To analyze the reactivity of allo-reactive T-cell clones against nonhematopoietic cells, fibroblasts cultured from skin biopsies from the 2 patients were tested for recognition. Flowcytometric analysis showed that skin fibroblasts were HLA class I–positive, and that HLA class II expression was induced after pretreatment with IFN-γ, mimicking an inflammatory environment (Figure 4A). IFN-γ–pretreated fibroblasts were recognized by 9 HLA-DPB1–specific CD4+ T-cell clones from patient 1 and 10 HLA-DPB1*0301–specific CD4+ T-cell clones from patient 2 (Figure 4B). None of the CD4+ T-cell clones recognized skin fibroblasts in the absence of IFN-γ pretreatment, in line with the lack of HLA class II. The HLA-C–restricted CD8+ T-cell clone from patient 2 failed to recognize (IFN-γ pretreated) fibroblasts (data not shown).
Recognition of skin-derived fibroblasts by allo-reactive HLA-DPB1–specific CD4 T-cell clones. (A) Expression of HLA-DR, -DQ, and -DP on skin fibroblasts from patient 1 (upper) and patient 2 (lower) cultured for 5 days with and without IFN-γ (200 IU/mL) was analyzed by flow cytometry. Histograms show the mean fluorescence intensity after staining with HLA-DR– (solid lines), HLA-DQ– (dashed lines), and HLA-DP– (dotted lines) specific moAb (filled histograms). Nonfilled histograms represent the MFI of unstained cells. (B) Reactivity of all allo-reactive HLA-DPB1–specific CD4+ T-cell clones from patient 1 (upper) and patient 2 (lower) was tested for cytokine production against skin-derived fibroblasts from the patients cultured with and without IFN-γ in ELISA. Each dot represents the release of IFN-γ (pg/mL) in 10 µL supernatants by a single CD4+ T-cell clone. None of the HLA-DPB1–specific CD4+ T-cell clones recognized skin fibroblasts in the absence of IFN-γ pretreatment, in line with the observed lack of HLA class II expression. From patient 1, 7 CD4+ T-cell clones specific for HLA-DPB1*0101 and 2 CD4+ T-cell clones specific for HLA-DPB1*0301 recognized skin-derived fibroblasts cultured with IFN-γ. From patient 2, 10 CD4+ T-cell clones specific for HLA-DPB1*0301 recognized skin-derived fibroblasts cultured with IFN-γ.
Recognition of skin-derived fibroblasts by allo-reactive HLA-DPB1–specific CD4 T-cell clones. (A) Expression of HLA-DR, -DQ, and -DP on skin fibroblasts from patient 1 (upper) and patient 2 (lower) cultured for 5 days with and without IFN-γ (200 IU/mL) was analyzed by flow cytometry. Histograms show the mean fluorescence intensity after staining with HLA-DR– (solid lines), HLA-DQ– (dashed lines), and HLA-DP– (dotted lines) specific moAb (filled histograms). Nonfilled histograms represent the MFI of unstained cells. (B) Reactivity of all allo-reactive HLA-DPB1–specific CD4+ T-cell clones from patient 1 (upper) and patient 2 (lower) was tested for cytokine production against skin-derived fibroblasts from the patients cultured with and without IFN-γ in ELISA. Each dot represents the release of IFN-γ (pg/mL) in 10 µL supernatants by a single CD4+ T-cell clone. None of the HLA-DPB1–specific CD4+ T-cell clones recognized skin fibroblasts in the absence of IFN-γ pretreatment, in line with the observed lack of HLA class II expression. From patient 1, 7 CD4+ T-cell clones specific for HLA-DPB1*0101 and 2 CD4+ T-cell clones specific for HLA-DPB1*0301 recognized skin-derived fibroblasts cultured with IFN-γ. From patient 2, 10 CD4+ T-cell clones specific for HLA-DPB1*0301 recognized skin-derived fibroblasts cultured with IFN-γ.
These results show that a significant number of HLA-DPB1–specific allo-reactive CD4+ T-cell clones recognized patient-derived nonhematopoietic cells under inflammatory conditions, indicating that HLA-DPB1–specific CD4+ T cells may have played a direct role as mediators of acute GVHD after HLA-DPB1–mismatched CD4+ DLI.
Residual patient-derived hematopoietic cells in PB at the time of CD4+ DLI
At the time of CD4+ DLI, both patients were in complete remission, but were mixed chimeric as demonstrated by BM chimerism analysis (Figure 1). Lineage-specific chimerism analysis of PB samples before CD4+ DLI demonstrated (nearly) complete donor chimerism in all hematopoietic subsets, except for the T-cell compartments, which contained (almost) entirely T cells of patient origin (supplemental Table 1). Because both patients had an episode of CMV reactivation early after alloSCT, which was accompanied by significant T-cell expansion (Figure 1), we investigated the phenotype and specificity of these residual patient-derived T cells before CD4+ DLI. Flowcytometric analysis demonstrated that patient T cells displayed an effector-memory (CD45RO+/CD45RA-/CCR7-) phenotype (data not shown) and contained high numbers of CMV-specific T cells (supplemental Table 2).
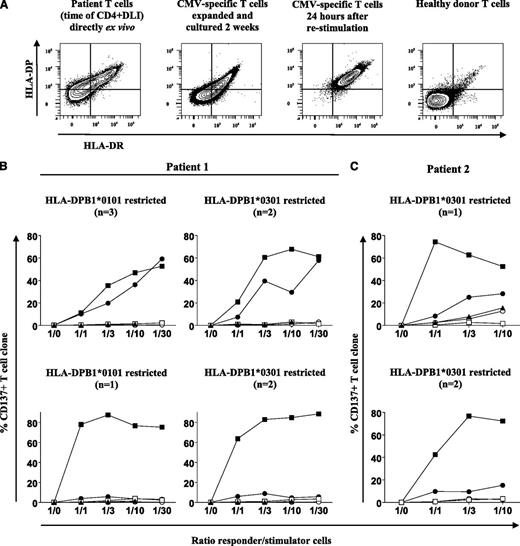
We next investigated whether residual patient-derived T cells at the time of CD4+ DLI were able to activate HLA-DPB1–specific CD4+ T cells. Flowcytometric analysis (Figure 5A) demonstrated that a fraction of residual patient-derived T cells was activated (HLA-DR+) and expressed HLA-DP in vivo. Isolated patient-derived CMV-specific T cells displayed a similar phenotype after 2 weeks of in vitro expansion, but, upon restimulation with CMV antigens, these in vitro expanded CMV-specific T cells upregulated expression of HLA-DP. In line with high expression levels of HLA-DP on EBV-LCL (data not shown), all analyzed HLA-DPB1–specific CD4+ T-cell clones from patient 1 (n = 8) and patient 2 (n = 3) were strongly activated upon stimulation with patient-derived, but not donor-derived, EBV-LCL, as measured by expression of the activation marker CD137 (Figure 5B-C). No or only limited activation of HLA-DPB1–specific T-cell clones was observed upon stimulation with patient-derived T cells ex vivo isolated from cryopreserved PBMCs, as well as patient-derived CMV-specific T cells after 2 weeks of in vitro expansion (Figure 5B-C). However, once activated by CMV antigens, patient-derived CMV-specific T cells could stimulate 6 of the 11 HLA-DPB1–specific CD4+ T-cell clones tested (Figure 5B-C).
Recognition of residual patient-derived T-cells in PB before CD4+ DLI by allo-reactive HLA-DPB1–specific CD4 T-cell clones. (A) Coexpression of HLA-DR and HLA-DP on CD3+ T cells in cryopreserved PBMCs from patient 1 before CD4+ DLI and on patient-derived CMV-specific T cells after 2 weeks of in vitro expansion and 24 hours after restimulation with CMV antigens was determined by flow cytometry. As a control, expression of HLA-DR and -DP is shown for healthy donor CD3+ T cells. (B-C) Activation of HLA-DPB1–specific CD4+ T-cell clones from patient 1 (B) and patient 2 (C) was measured upon stimulation with patient-derived T cells after direct ex vivo isolation from cryopreserved PBMCs (-▲-), patient-derived CMV-specific T cells after 2 weeks of in vitro expansion (-○-), and 24 hours after in vitro restimulation with CMV antigens (-●-) and against patient (-▪-) and donor (-□-) EBV-LCL. Activation was measured 24 hours after stimulation by flow cytometric staining for CD137. Percentages of activated CD137+ cells are depicted for different CD4+ T-cell clones at different responder/stimulator cell ratios. Responder/stimulator cell ratio 1/30 could not be tested for patient-derived T cells after ex vivo isolation from cryopreserved PBMCs because of insufficient material. In total, 4 HLA-DPB1*0101– and 4 HLA-DPB1*0301–specific CD4+ T-cell clones from patient 1 (B) and 3 HLA-DPB1*0301–specific CD4+ T-cell clones from patient 2 (C) were analyzed. No or only minimal activation of HLA-DPB1–restricted CD4+ T-cell clones was observed upon stimulation with patient-derived T cells after direct ex vivo isolation and patient-derived CMV-specific T cells after 2 weeks of in vitro expansion. Upon stimulation with patient-derived CMV-specific T cells 24 hours after in vitro restimulation with CMV antigens, however, 3 HLA-DPB1*0101–specific and 2 HLA-DPB1*0301–specific CD4+ T-cell clones from patient 1 (B, upper) and 1 HLA-DPB1*0301–specific CD4+ T-cell clone from patient 2 (C, upper) were activated, whereas the remaining HLA-DPB1–restricted CD4+ T-cell clones from patient 1 (B, lower) and patient 2 (C, lower) were not or were only minimally activated.
Recognition of residual patient-derived T-cells in PB before CD4+ DLI by allo-reactive HLA-DPB1–specific CD4 T-cell clones. (A) Coexpression of HLA-DR and HLA-DP on CD3+ T cells in cryopreserved PBMCs from patient 1 before CD4+ DLI and on patient-derived CMV-specific T cells after 2 weeks of in vitro expansion and 24 hours after restimulation with CMV antigens was determined by flow cytometry. As a control, expression of HLA-DR and -DP is shown for healthy donor CD3+ T cells. (B-C) Activation of HLA-DPB1–specific CD4+ T-cell clones from patient 1 (B) and patient 2 (C) was measured upon stimulation with patient-derived T cells after direct ex vivo isolation from cryopreserved PBMCs (-▲-), patient-derived CMV-specific T cells after 2 weeks of in vitro expansion (-○-), and 24 hours after in vitro restimulation with CMV antigens (-●-) and against patient (-▪-) and donor (-□-) EBV-LCL. Activation was measured 24 hours after stimulation by flow cytometric staining for CD137. Percentages of activated CD137+ cells are depicted for different CD4+ T-cell clones at different responder/stimulator cell ratios. Responder/stimulator cell ratio 1/30 could not be tested for patient-derived T cells after ex vivo isolation from cryopreserved PBMCs because of insufficient material. In total, 4 HLA-DPB1*0101– and 4 HLA-DPB1*0301–specific CD4+ T-cell clones from patient 1 (B) and 3 HLA-DPB1*0301–specific CD4+ T-cell clones from patient 2 (C) were analyzed. No or only minimal activation of HLA-DPB1–restricted CD4+ T-cell clones was observed upon stimulation with patient-derived T cells after direct ex vivo isolation and patient-derived CMV-specific T cells after 2 weeks of in vitro expansion. Upon stimulation with patient-derived CMV-specific T cells 24 hours after in vitro restimulation with CMV antigens, however, 3 HLA-DPB1*0101–specific and 2 HLA-DPB1*0301–specific CD4+ T-cell clones from patient 1 (B, upper) and 1 HLA-DPB1*0301–specific CD4+ T-cell clone from patient 2 (C, upper) were activated, whereas the remaining HLA-DPB1–restricted CD4+ T-cell clones from patient 1 (B, lower) and patient 2 (C, lower) were not or were only minimally activated.
In summary, the data showed that mixed patient chimerism at the time of CD4+ DLI primarily resided in the T-cell compartments, and that residual patient-derived T cells contained high numbers of CMV-specific T cells, illustrating profound expansion of patient-derived CMV-specific T cells caused by active CMV infection after alloSCT. The data also showed that patient-derived CMV-specific T cells, once activated by CMV antigens, were capable of stimulating a substantial number of HLA-DPB1–specific CD4+ T-cell clones, indicating that residual patient-derived T cells may become stimulators and targets for HLA-DPB1–specific CD4+ T cells when locally activated by CMV infection in vivo.
Composition of the T-cell infiltrates and expression of HLA class II in colonic tissue biopsies
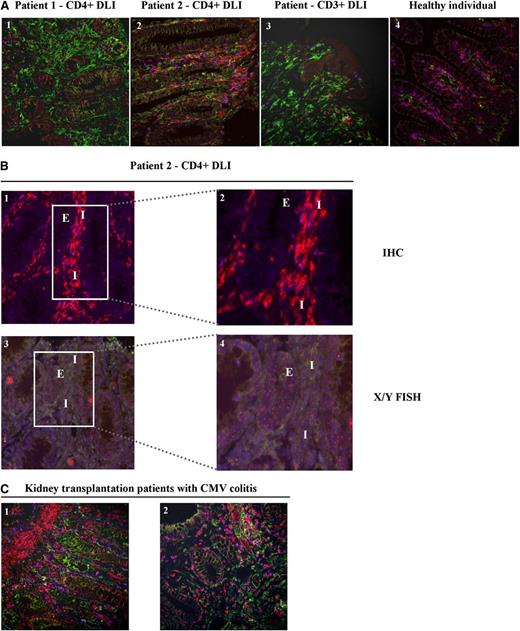
Because our data suggested that HLA-DPB1–directed CD4+ T cells mediated acute GVHD, we analyzed the composition of the T-cell infiltrates and determined HLA class II expression in colonic biopsies from both patients at the time of GVHD diagnosis. Colonic biopsies from a healthy individual and a patient with colonic GVHD after CD3+ DLI were analyzed for comparison. Immunofluorescent staining and enumeration of CD4+ and CD8+ T cells in normal colonic mucosa showed a CD4/CD8 ratio of 0.8. The T-cell infiltrate from patient 1 treated with CD4+ DLI displayed a mixed T-cell composition (ratio CD4/CD8:0.9), whereas the colonic biopsy from patient 2 showed predominantly CD4+ T cells (ratio CD4/CD8:1.5) (Figure 6A). The biopsy from the patient treated with CD3+ DLI contained more CD8+ T cells (ratio CD4/CD8:0.4). By performing FISH analysis for the X and Y chromosomes on the colonic biopsy from patient 2, who was transplanted with cells from a sex-mismatched URD, we demonstrated that the CD4+ T-cell infiltrate was of donor origin (Figure 6B). Furthermore, colonic epithelial cells in biopsies from both patients after CD4+ DLI and from the patient after CD3+ DLI were demonstrated to express HLA class II, which was absent in normal colon (Figure 6A).
T-cell infiltrate and HLA class II expression in colonic biopsies. (A) Multicolor immunofluorescent stainings for CD3 (clone PS1, IgG2a + IgG2a ALEXA 546) (red), CD8 (clone 4B11, IgG2b + IgGb ALEXA 647) (blue), and HLA class II (clone CR3/43, IgG1 + IgG1 ALEXA 488) (green) were performed on colonic biopsies from patient 1 (1) and patient 2 (2) obtained at the time of GVHD diagnosis after CD4+ DLI, a patient diagnosed with colonic GVHD after CD3+ DLI (3), and a normal colon from a healthy individual (4). Double CD3+CD8+ cells (purple) were defined as CD8+ T cells, whereas CD3+CD8-expressing cells (red) were defined as CD4+ T cells. Images were captured with a confocal laser scanning microscope (LSM510; Zeiss, Sliedrecht, The Netherlands) (original magnification ×200) (supplemental Methods). Numbers of CD4+ and CD8+ T cells were enumerated by a third party in a blinded fashion, and tissue sections showed CD4/CD8 ratios of 0.9, 1.5, 0.4, and 0.8 for images 1, 2, 3, and 4, respectively. (B) Combined immunofluorescent stainings for CD3 (clone PS1, IgG2a + IgG2a ALEXA 546) (red) and CD8 (clone 4B11, IgG2b + IgGb ALEXA 647) (blue) (1-2), and FISH analysis for detection of X (green) and Y (red) chromosomes (XX/XY) (3-4) were performed on serial sections of the colonic biopsy from patient 2 (male; URD = female). Immunofluorescent staining: original magnification ×200 (1) and framed area, original magnification ×400 (2). Corresponding fields in X/Y FISH analysis: original magnification ×200 (3) and framed area, original magnification ×400 (4). Interstitium (I) and colonic epithelial cells (E) are indicated in the stainings. Colonic epithelial cells show recipient-specific XY karyotype, whereas interstitial T-cell infiltrate predominantly shows donor-specific XX karyotype. Control normal male colonic biopsy was also examined by X/Y FISH (data not shown). All slides were digitized using a Mirax slide scanner (3DHISTEC, Budapest, Hungary) and analyzed using Panoramic Viewer (3DHistec, version 1.14.50 RTM) (supplemental Methods). (C) Immunofluorescent stainings (original magnification ×200) for CD3 (red), CD8 (blue), and HLA class II (green) on colonic biopsies from 2 kidney transplantation patients (1-2) with CMV colitis. Enumeration of CD4+ and CD8+ T cells showed CD4/CD8 ratios of 2.3 and 0.5 for images 1 and 2, respectively.
T-cell infiltrate and HLA class II expression in colonic biopsies. (A) Multicolor immunofluorescent stainings for CD3 (clone PS1, IgG2a + IgG2a ALEXA 546) (red), CD8 (clone 4B11, IgG2b + IgGb ALEXA 647) (blue), and HLA class II (clone CR3/43, IgG1 + IgG1 ALEXA 488) (green) were performed on colonic biopsies from patient 1 (1) and patient 2 (2) obtained at the time of GVHD diagnosis after CD4+ DLI, a patient diagnosed with colonic GVHD after CD3+ DLI (3), and a normal colon from a healthy individual (4). Double CD3+CD8+ cells (purple) were defined as CD8+ T cells, whereas CD3+CD8-expressing cells (red) were defined as CD4+ T cells. Images were captured with a confocal laser scanning microscope (LSM510; Zeiss, Sliedrecht, The Netherlands) (original magnification ×200) (supplemental Methods). Numbers of CD4+ and CD8+ T cells were enumerated by a third party in a blinded fashion, and tissue sections showed CD4/CD8 ratios of 0.9, 1.5, 0.4, and 0.8 for images 1, 2, 3, and 4, respectively. (B) Combined immunofluorescent stainings for CD3 (clone PS1, IgG2a + IgG2a ALEXA 546) (red) and CD8 (clone 4B11, IgG2b + IgGb ALEXA 647) (blue) (1-2), and FISH analysis for detection of X (green) and Y (red) chromosomes (XX/XY) (3-4) were performed on serial sections of the colonic biopsy from patient 2 (male; URD = female). Immunofluorescent staining: original magnification ×200 (1) and framed area, original magnification ×400 (2). Corresponding fields in X/Y FISH analysis: original magnification ×200 (3) and framed area, original magnification ×400 (4). Interstitium (I) and colonic epithelial cells (E) are indicated in the stainings. Colonic epithelial cells show recipient-specific XY karyotype, whereas interstitial T-cell infiltrate predominantly shows donor-specific XX karyotype. Control normal male colonic biopsy was also examined by X/Y FISH (data not shown). All slides were digitized using a Mirax slide scanner (3DHISTEC, Budapest, Hungary) and analyzed using Panoramic Viewer (3DHistec, version 1.14.50 RTM) (supplemental Methods). (C) Immunofluorescent stainings (original magnification ×200) for CD3 (red), CD8 (blue), and HLA class II (green) on colonic biopsies from 2 kidney transplantation patients (1-2) with CMV colitis. Enumeration of CD4+ and CD8+ T cells showed CD4/CD8 ratios of 2.3 and 0.5 for images 1 and 2, respectively.
To investigate whether the inflammatory conditions as created by an ongoing CMV infection may have contributed to the development of GVHD after CD4+ DLI, we determined HLA class II expression in colonic biopsies from kidney transplantation patients (n = 2) in whom CMV colitis developed and demonstrated that HLA class II was also expressed on these colonic epithelial cells (Figure 6C). Becasue nonhematopoietic cells are major targets of CMV infection in vivo,30 we investigated whether CMV infection in vitro induced HLA class II expression on patient-derived skin fibroblasts (supplemental Methods). Similar to that described by others,31-34 CMV infection by itself did not induce surface expression of HLA class II on fibroblasts (data not shown).
In summary, the data show evident infiltration of donor-derived CD4+ T cells in the colonic biopsy from one patient with colonic GVHD after CD4+ DLI, and that colonic epithelial cells of both patients expressed HLA class II. HLA class II expression was also demonstrated on colonic epithelial cells from kidney transplantation patients with active colonic CMV infection, whereas CMV infection in vitro failed to induce HLA class II on CMV-infected fibroblasts. These data provide evidence that HLA class II expression on nonhematopoietic cells can be upregulated in vivo as a consequence of inflammatory conditions created by the anti-CMV immune response, and therefore support our finding that GVHD was induced by HLA-DPB1–directed CD4+ T cells attacking HLA class II–expressing nonhematopoietic tissues.
Discussion
In this study, we analyzed the clinical course and specificity of T-cell immune responses in 2 patients with AML who converted to full-donor chimerism and in whom acute GVHD developed after prophylactic CD4+ DLI administered 3 months after HLA-DPB1–mismatched TCD-alloSCT. Our results show induction of profound polyclonal CD4+ T-cell immune responses directed against mismatched HLA-DPB1 alleles in both patients. Allo-reactive HLA-DPB1–specific CD4+ T-cells were shown to recognize HLA class II–expressing patient hematopoietic cells as well as skin-derived fibroblasts pretreated with proinflammatory cytokines. Our data illustrate that allo-reactive HLA-DPB1–specific CD4+ T cells can induce and mediate profound GVHD after prophylactic HLA-DPB1–mismatched CD4+ DLI.
Evidence for direct involvement of HLA-DPB1–specific CD4+ T cells in GVHD pathogenesis has been provided by studies showing isolation of HLA-DPB1–specific CD4+ T-cell clones from skin biopsies of patients at the onset of acute GVHD after HLA-DPB1–mismatched partial TCD-alloSCT.35,36 We previously demonstrated high frequencies of HLA-DPB1–specific CD4+ T cells in patients who responded to unmodified DLI administered late after HLA-DPB1–mismatched TCD-alloSCT with and without development of GVHD, indicating involvement of HLA-DPB1–specific CD4+ T cells in both GVL and GVHD.12,37 Interestingly, previous studies in patients who received CD8+ TCD DLI late after HLA-identical alloSCT have suggested that donor CD8+ T cells derived from the stem cell graft or residual donor CD8+ T cells present in the DLI were the dominant effector T cells in GVL and GVHD in these patients.38,39 Our present data, however, demonstrate that highly purified CD4+ DLI, without contaminating CD8+ T cells, can induce GVHD, and that GVHD was mediated by allo-reactive HLA-DPB1–specific CD4+ T cells. Furthermore, our findings demonstrate that clinical circumstances strongly influence the activation state of nonhematopoietic tissues and their susceptibility for CD4+ T-cell attack. Increased susceptibility of nonhematopoietic tissues to HLA-DPB1–specific CD4+ T cells may occur not only in the early transplantation period but also at a later stage when a proinflammatory environment is created by infections with pathogens and concurrent immune responses. As a consequence of inflammatory triggers, HLA class II expression on nonhematopoietic cells can be upregulated making them targets for allo-reactive CD4+ T cells.
At the time of prophylactic CD4+ DLI, the 2 patients analyzed in this study were in complete remission but demonstrated (nearly) complete patient chimerism in their T-cell compartments, whereas other hematopoietic subsets were of donor origin. In both patients, residual patient-derived T cells contained high numbers of CMV-specific T cells as a consequence of CMV reactivation early after alloSCT. We showed that these patient-derived T cells after direct ex vivo isolation from cryopreserved patient PBMCs, as well as patient-derived CMV-specific T cells after 2 weeks of in vitro expansion, displayed modest expression of HLA-DP and failed to stimulate allo-reactive HLA-DPB1–specific CD4+ T cells. Upon restimulation with CMV antigens, however, these in vitro expanded CMV-specific T cells upregulated HLA-DP expression and acquired the capacity to stimulate HLA-DPB1–specific CD4+ T cells. On the basis of these data, and the knowledge that both patients initially developed only limited skin GVHD after CD4+ DLI, we postulate that residual patient-derived CMV-specific T cells have been activated as a result of CMV reactivation after CD4+ DLI, and that these activated T cells became stimulators and targets for HLA-DPB1–specific CD4+ T cells. Proinflammatory cytokines released by activated CMV-specific T cells may have induced HLA class II expression on other residual patient-derived T cells as well as on nonhematopoietic cells, which then also became targets for HLA-DPB1–specific CD4+ T cells, as illustrated by subsequent conversion to full-donor hematopoiesis and development of severe colonic GVHD. As a consequence of elimination of patient-derived CMV-specific T cells by HLA-DPB1–specific CD4+ T cells, CMV reactivations were reinitiated, which elicited donor-derived CMV-specific T-cell responses, as illustrated by the isolation of donor-derived CMV-specific T-cell clones after CD4+ DLI. These donor-derived anti-CMV T-cell responses may have further stimulated the inflammatory environment and HLA class II expression on patient nonhematopoietic tissues, thereby enhancing GVHD. In both patients, T cells were the only patient-derived hematopoietic cells in PB at the time of CD4+ DLI, and, once activated by CMV infection, these T cells may have induced or amplified HLA-DPB1–specific allo-reactive CD4+ T-cell responses in vivo. Alternatively, or in addition, HLA-DPB1–specific CD4+ T cells may have been induced by residual patient-derived tissue-resident APCs, because human studies have shown that recipient skin-resident Langerhans cells persist beyond 3 months after nonmyeloablative alloSCT,24,40 and murine studies demonstrated the relevance of tissue-resident APC in GVHD development.41,42 This is supported by our data demonstrating recognition of patient-derived, monocyte-matured dendritic cells by all HLA-DPB1–specific CD4+ T-cell clones tested.
Nonhematopoietic cells have been identified as major targets of acute colonic CMV infection in vivo.30 In our study, colonic CMV infection was documented for 1 patient during GVHD. We demonstrated HLA class II expression on colonic epithelial cells in biopsies from kidney transplantation patients with active colonic CMV infection, whereas normal colonic epithelial cells were HLA class II–negative, and we confirmed previous reports that CMV infection by itself does not induce HLA class II expression on nonhematopoietic cells.32-34 Therefore, we postulate that CMV-specific T cells elicited in patients upon CMV reactivations after alloSCT and CD4+ DLI may have induced a local inflammatory response in CMV-infected colonic tissues, and that soluble factors elaborated by activated CMV-specific T cells upregulated HLA class II expression on non–CMV-infected nonhematopoietic cells, as shown by others.34 This intense local inflammation can induce expression of HLA class II, adhesion, and costimulatory molecules on nonhematopoietic cells, as was recently shown,43 and therefore may have amplified donor-derived HLA-DPB1–specific CD4+ T-cell responses against HLA class II–expressing nonhematopoietic cells, resulting in local exacerbation of GVHD in our patients.
Recently, Elmaagacli et al44 reported that early CMV reactivation after non–TCD-alloSCT was associated with a reduced relapse risk in patients with AML and speculated that CMV-specific T cells directly mediated this GVL effect by eliminating CMV-infected AML blasts. Others, however, have not documented the protective effect of adoptively transferred CMV-specific T cells administered after TCD-alloSCT.45 Based on our study, we postulate that the protective effect of CMV reactivation against leukemic relapses44 may be ascribed to an enhanced capacity of AML blasts to process and present antigens as a consequence of proinflammatory cytokines released during CMV infection, thereby making AML blasts better targets for allo-reactive T cells. This is supported by our study demonstrating that leukemic cells can acquire a professional APC phenotype in vivo upon interaction and release of proinflammatory cytokines by allo-reactive CD4+ T cells.46 Moreover, we previously demonstrated that not only AML blasts,47,48 but also other leukemic cells,46-50 upregulated expression of HLA, adhesion, and costimulatory molecules in the presence of soluble factors and acquired an enhanced capacity to stimulate allogeneic T cells in vitro.
In summary, we showed in 2 patients that prophylactic CD4+ DLI administered early after HLA-DPB1–mismatched TCD-alloSCT led to conversion to donor hematopoiesis with concomitant GVHD caused by allo-reactive CD4+ T cells directed against patient-mismatched HLA-DPB1 alleles. Our results suggest that active CMV infection and subsequent expansion and cytokine release by residual patient-derived CMV-specific T cells may have provided essential inflammatory triggers for development of GVHD by HLA-DPB1–specific CD4+ T cells. Therefore, despite significant drug-related toxicities, antiviral prophylaxis may be considered to prevent CMV reactivations in high-risk patients scheduled to receive DLI early after TCD-alloSCT. Moreover, the timing of prophylactic CD4+ DLI based on concomitant clinical circumstances may contribute to a reduced incidence and severity of GVHD after HLA class II–mismatched alloSCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood commentary on this article in this issue.
Acknowledgments
The authors thank Prof Dr M. Masserle (Hannover Medical School) for kindly providing HCMV-TB40/E-GFP viral construct, Dr R. Arens and A. Redeker (Leiden University Medical Center, Department of Immunohematology and Blood Transfusion) for technical assistance with fibroblast infection with HCMV-TB40/E-GFP, Dr A. Mulder (Leiden University Medical Center, Department of Immunohematology and Blood Transfusion) for providing essential reagents, Dr M. Eefting for clinical data (Leiden University Medical Center, Department of Hematology), and E.J. Dreef (Leiden University Medical Center, Department of Pathology) for technical assistance with immunohistochemistry.
This study was supported by grant 2008-4263 from the Dutch Cancer Society.
Authorship
Contribution: S.S., I.J., M.G., and J.H.F.F. designed experiments; S.S., C.A.M.v.B., S.A.P.v.L.-H., B.v.d.Z., E.S.J., A.B.K., M.v.d.M., and J.C.H. performed experiments; F.H.J.C. provided essential reagents; E.W.A.M., J.J.Z., and C.J.M.H. provided clinical data; and S.S., M.G., and J.H.F.F. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sanja Stevanović, Department of Hematology, Leiden University Medical Center, P.O. Box 9600, 2300 RC Leiden, The Netherlands; e-mail: s.stevanovic@lumc.nl.

![Figure 1. Clinical immune responses in patients treated with prophylactic CD4+ DLI after HLA-DPB1–mismatched URD alloSCT. After a nonmyeloablative conditioning regimen consisting of fludarabine, busulfan, alemtuzumab, and rabbit-derived antithymocyte globulin, patients received a stem cell transplant graft depleted of T cells by in vitro incubation with alemtuzumab. Percentage of donor chimerism (-▪-) in BM (left, Y-axis), absolute numbers of CD3+CD4+ (-●-), and CD3+CD8+ (-○-) T cells (first right, Y-axis [black]) in PB and CMV-DNA () (log copies/mL) (second right, Y-axis [gray]) after alloSCT for patient 1 (A) and patient 2 (B) are shown (supplemental Methods). Arrows indicate CD4+ DLI infusion. Asterisks and stars indicate the time of diagnosis of acute skin GVHD (21 and 16 days after CD4+ DLI for patients 1 and 2, respectively) and colonic GVHD (35 and 58 days after CD4+ DLI for patients 1 and 2, respectively). § indicates time of detection of CMV infection in colonic biopsy of patient 1 (133 days after CD4+ DLI). Both patients developed CMV reactivation within the first month after alloSCT, which was treated with antiviral drugs (valganciclovir). Both patients cleared CMV reactivation at 2 months after alloSCT but again experienced several episodes of CMV reactivations requiring antiviral treatment after CD4+ DLI. Longitudinal bars indicate duration of antiviral therapy. Patient 2 experienced 1 episode of Epstein-Barr virus reactivation (black bar), which was treated with Rituximab (anti-CD20 monoclonal antibody). The intensity and duration of systemic immunosuppressive treatment is indicated by longitudinal bars. CsA, cyclosporine; MMF, mycofenolate mofetil; PNL, prednisolone.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/11/10.1182_blood-2012-12-470872/4/m_1963f1.jpeg?Expires=1767721626&Signature=WMK9vwTcjjcwVjITdSQgyRLmQSQzLaFBS1D-~mGZnQke5njVSLPYrhSNMg9spu~gaglvHDaipxuduCiGmCW582VStlViSGABtx6lrDDAG8TmkLcDTH2SZ~uiussstCGsg9qmFLP0lUb7xYZoYiiqnoJKKV1ZCRK2S0plLQ1kjxPZn8hkTWVByOQqWJqDrlJ3RYH~ibiaV0O~ZQyNm1CqX4H6-q6GfcsVEDU-RyJuZirRY1OmrWIe64qTk5PT7~auGgBS3GgQm1yNebXmCGVeB8MfsbCmK-AlDlla1g4Z9RUEH5U7QCGQb998MZso1tjA9MlfBY9jVlls5yJd3Tr93A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. HLA specificity of allo-reactive CD4+ T-cell clones. HLA restriction of all isolated allo-reactive CD4+ T-cell clones from patient 1 (n = 19) (A) and patient 2 (n = 16) (B) was analyzed using blocking moAb specific for HLA class I, HLA class II, and HLA-DR, -DQ, and -DP with IFN-γ ELISA. Each dot represents the release of IFN-γ (pg/mL) in 10-µL supernatants by a single allo-reactive CD4+ T-cell clone in response to patient EBV-LCL in the absence or presence of blocking moAb and donor EBV-LCL. All CD4+ T-cell clones showed HLA-DP–restricted recognition, as illustrated by abrogated IFN-γ production upon preincubation with moAb specific for HLA class II and HLA-DP, but not with moAb against HLA class I, HLA-DR, or HLA-DQ. (C-D) All allo-reactive HLA-DP–restricted CD4+ T-cell clones were tested for recognition of patient and donor EBV-LCL, and donor EBV-LCL retrovirally transduced with patient HLA-DPB1 and -DPA1 alleles (HLA-DPB1*0101 and -DPB1*0301 with DPA1*0201 for patient 1 [C] and HLA-DPB1*0301 with -DPA1*0103 for patient 2 [D]) with IFN-γ ELISA. HLA-DPB1–restricted CD4+ T-cell clones from patient 1 recognized either HLA-DPB1*0101 (○) or HLA-DPB1*0301 (●) transduced donor EBV-LCL. Each dot represents the release of IFN-γ (pg/mL) in 10 µL supernatant by a single CD4+ T-cell clone.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/11/10.1182_blood-2012-12-470872/4/m_1963f3.jpeg?Expires=1767721626&Signature=meVSrOuLW-H9voKhAUGrrHpdMvBCx~Haey9JOCiCKJl5U4lbAdMmvVBux~1NFYjpgG2kO3ikPnk7KyebQogeEf2Qr0YXC4S2XDcQ2j5TmTmnr6F9Ck7JZqJUb9I6R~mlt3QoLAVKsbg4mxk7hNDBIXz-Xy9bkpyYXKcvn0WYvamfrVqC0Nvv1Kxz8e1itJAe7XEe-GFs1~715R5cUXTp1NXA5boVAtb8rlXlT4efzNh6bVT3on1y~kKdNI7VkH1eGhe4VWLgnywxq1B~2Q~Xpm~u34xWM2xDWzg54~nSNgFwFwS5KVHgPAFbVY~vFkspZPKiLDn-sNCMM21rq-zoww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal