In this issue of Blood, Vincent et al report on their seminal finding of the contribution of mast cell activation to neurogenic inflammation, and thus to the pathobiology of pain in sickle cell disease (SCD).1
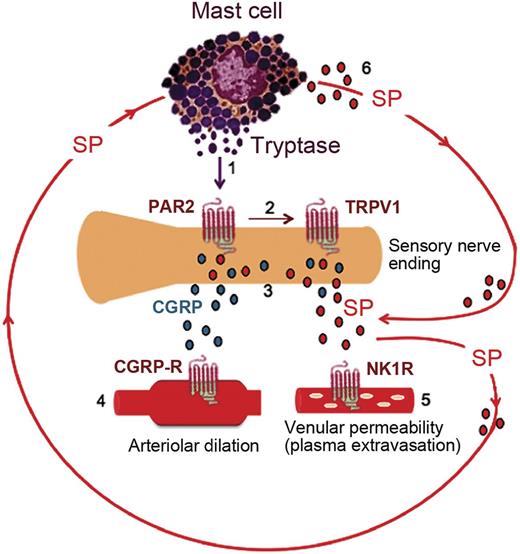
Activated mast cells contribute to a cycle of neuropeptide release in the skin of sickle mice. (1) Tryptase from mast cells activates protease activated receptor 2 (PAR2) on the peripheral nerve endings. (2) Activation of PAR2 sensitizes transient receptor potential vanilloid 1 (TRPV1). (3) Excited nociceptors stimulate the release of calcitonin gene-related peptide (CGRP) and substance P (SP) from the sensory nerve endings. (4) CGRP interacts with the type 1 CGRP receptor (CGRP-R) on arterioles to induce dilatation. (5) SP activates plasma extravasation via neurokinin 1 receptors (NK1R). (6) SP released from nerve endings and from mast cells also acts on the mast cells themselves, thus promoting a cycle of mast cell activation.
Activated mast cells contribute to a cycle of neuropeptide release in the skin of sickle mice. (1) Tryptase from mast cells activates protease activated receptor 2 (PAR2) on the peripheral nerve endings. (2) Activation of PAR2 sensitizes transient receptor potential vanilloid 1 (TRPV1). (3) Excited nociceptors stimulate the release of calcitonin gene-related peptide (CGRP) and substance P (SP) from the sensory nerve endings. (4) CGRP interacts with the type 1 CGRP receptor (CGRP-R) on arterioles to induce dilatation. (5) SP activates plasma extravasation via neurokinin 1 receptors (NK1R). (6) SP released from nerve endings and from mast cells also acts on the mast cells themselves, thus promoting a cycle of mast cell activation.
“Painful crises,” or vaso-occlusive episodes, are considered the “hallmark” of SCD and are responsible for >90% of health care encounters in this patient population at an annual hospitalization cost of approximately $1 billion, by 2004 figures.2,3 More than a century after the first description of SCD by Herrick,4 our understanding of pain in this disease is far from complete. However, despite this lack of a thorough understanding, progress has been made in defining the scope of pain. The landmark PISCES study by Smith et al5 established that roughly 30% of SCD patients have daily chronic pain, thus dispelling the long-held view that adult SCD patients have only episodes of pain (the so-called “crises”) interspersed with varying periods of normalcy or “freedom from pain.” Thus, the emerging view of the spectrum of pain in SCD ranges from nociceptive, owing to tissue injury from vaso-occlusion, especially in children and young adults, to increasing frequency of chronic pain, with neuropathic and centralized components in older adults. The lack of a better understanding of the various underpinnings of SCD pain has contributed to stigmatization and disparities in health care.
Vincent et al have conducted a series of elaborate experiments using a sickle cell mouse model (Berkeley mice), and they provide unequivocal evidence about the contribution of mast cell activation to neurogenic inflammation, hyperalgesia, and pain by the elaboration of tryptase, which activates protease activated receptor 2 receptors, as well as by the secretion of substance P and calcitonin gene-related peptide (see figure) as mediators of neurogenic inflammation. As proof of their hypothesis, they clearly demonstrate that inhibitors of mast cell activity by imatinib or stabilization of mast cells by cromolyn sodium leads to a decrease in neurogenic inflammation and results in the reversal of hyperalgesia and the restoration of the analgesic effect of morphine. As a supportive line of experiments, they show that Berkeley SS mice backcrossed with WBB6FM mice, with a point mutation that inactivates c-kit, leads to the generation of Berkeley sickle mice that lack mast cells. These mice behave in a manner similar to control (Hb AA) mice, suggesting an important role of mast cells in neurogenic inflammation and hyperalgesia.
These findings are certainly important contributions to our understanding of the complex mechanisms in SCD pain. In addition, they signal a significant translational potential for treatment of chronic SCD pain by mast cell inactivation or prevention of mast cell degranulation by the repurposing of 2 existing drugs—imatinib and cromolyn sodium, respectively. Of note also is a recently published case report of a patient with SCD in whom chronic myeloid leukemia developed; during treatment with imatinib, the patient remained pain free.6
Conflict-of-interest disclosure: The author declares no competing financial interests.