In this issue of Blood, Nazi and colleagues have demonstrated that patients with immune thrombocytopenia (ITP) who receive rituximab display impaired responses to immunization with pneumococcal and Haemophilus influenzae type b (Hib) vaccines. Although similar observations have been reported in patients with other disorders who have received rituximab, this is the first study to examine this issue in patients with ITP.1
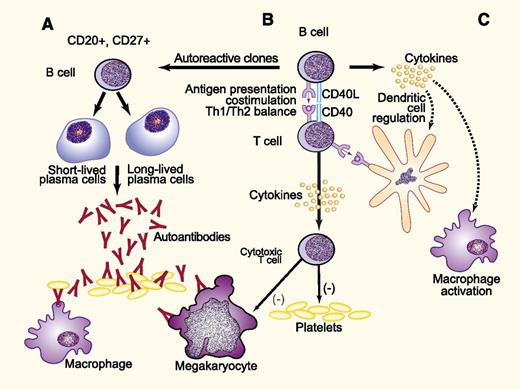
Proposed mechanisms for development of ITP may parallel those generated during vaccine responses. B cells are integral to the immune response in ITP through several mechanisms. These include the production of platelet and megakaryocyte-reactive antibodies (A), as well as antigen presentation (B), in which B-cell–T-cell interactions lead to costimulation resulting in an altered Th1/Th2 balance, clonal expansion of cytotoxic T cells, and release of inflammatory cytokines (C). The latter may stimulate macrophages and dendritic cells, enhancing clearance and destruction of antibody-coated platelets and altering the distribution of T-cell subsets. In the case of ITP, the antigen may be a viral peptide cross-reactive with platelet glycoproteins, platelet glycoproteins themselves following loss of tolerance, or others that have not been characterized. The response to vaccines likely parallels that to platelet glycoproteins depicted here. Adapted from Stasi9 with permission. Professional illustration by Paulette Dennis.
Proposed mechanisms for development of ITP may parallel those generated during vaccine responses. B cells are integral to the immune response in ITP through several mechanisms. These include the production of platelet and megakaryocyte-reactive antibodies (A), as well as antigen presentation (B), in which B-cell–T-cell interactions lead to costimulation resulting in an altered Th1/Th2 balance, clonal expansion of cytotoxic T cells, and release of inflammatory cytokines (C). The latter may stimulate macrophages and dendritic cells, enhancing clearance and destruction of antibody-coated platelets and altering the distribution of T-cell subsets. In the case of ITP, the antigen may be a viral peptide cross-reactive with platelet glycoproteins, platelet glycoproteins themselves following loss of tolerance, or others that have not been characterized. The response to vaccines likely parallels that to platelet glycoproteins depicted here. Adapted from Stasi9 with permission. Professional illustration by Paulette Dennis.
Rituximab is an effective therapy for ITP, with an overall response rate of ∼60%2 ; importantly, however, retrospective studies demonstrate that >20% of patients with ITP treated with rituximab achieve a long-term remission.3 Although the positioning of rituximab in the overall scheme of ITP therapy is a matter of debate, these response rates have led to wide acceptance of rituximab as a key component of ITP therapy that is often used prior to thrombopoietic agents, splenectomy, or other interventions.
Splenectomy offers the best chance for long-term remission of ITP, although its use has decreased over the last decade as more effective ITP therapies have become available. It is well appreciated that splenectomy is associated with an increased risk of infection, including overwhelming sepsis caused by encapsulated organisms such as pneumococcus; recent studies suggest the potential for additional long-term complications.4 The association of the asplenic state with infectious risk and the diminished response to vaccination following splenectomy has led to incorporation of recommendations for presplenectomy vaccination for pneumococcus, Hib, and meningococcus into ITP guidelines.5,6
What has not been as widely appreciated is the fact that treatment with rituximab may also inhibit responses to vaccination and that this effect may persist for 6 to 12 months; this observation has particularly important implications for patients with ITP who fail to respond to rituximab and are then referred for splenectomy. Even if such individuals receive vaccination prior to splenectomy, the study by Nazi and colleagues demonstrates that their response is likely to be significantly impaired.1 In this study, only 3 of 14 patients who received rituximab in a randomized clinical trial, vs 4 of 6 patients from the same trial who received placebo, achieved a fourfold increase in anti-pneumococcal antibodies (P = .12). Likewise, only 4 of 14 rituximab-treated patients vs 5 of 6 placebo-treated patients demonstrated a fourfold increase in anti-Hib antibodies (P < .05). The numbers of patients included in this report are small, and this is a notable weakness. However, the significance of these findings is supported by the fact that fewer rituximab-treated patients demonstrated Hib killing in a functional assay.
Why do rituximab-treated patients demonstrate an impaired response to vaccines? The explanation is likely more complex than a simple reduction in circulating and splenic CD20-positive B cells. Genetic analysis of platelet autoantibodies from patients with ITP suggests that these arise through a T-cell–dependent, antigen-driven process.7 The central role of T cells in the immunopathogenesis of ITP has been increasingly appreciated, reflecting the importance of B-cell–T-cell interactions in the generation of the humoral and cellular immune responses to platelets and megakaryocytes (see figure). Clinical correlates include the observations that the degree of B-cell depletion, particularly in the spleen,8 does not correlate directly with the response to rituximab, and that the use of rituximab leads to alterations in the T-cell compartment including restoration of regulatory T-cell number and suppressive capacity, normalization of the T-cell vβ repertoire, and normalization of Th1 and Th2 ratios.9 Rituximab may also reduce pathogenic Th17+ cell populations. Because vaccination responses are also antigen-driven and T-cell–dependent, these rituximab-induced changes in both B- and T-cell compartments likely contribute to the impaired response.
What is the relevance of this report to the management of patients with ITP? Although current dogma suggests that the use of rituximab is not associated with an increased incidence of infection in patients with ITP, some reports imply otherwise. In a large meta-analysis, Arnold et al observed a 2.3% incidence of serious infections in rituximab-treated patients.2 In another retrospective analysis, Moulis et al observed that bacterial pneumonia developed in 5 of 43 ITP patients treated with rituximab at a mean of 14.8 months after the first infusion; 3 of these patients had not been vaccinated against Streptococcus pneumoniae.10 However, other studies suggest no difference in rates of infection in ITP patients who have been treated with rituximab vs those who have not. Clearly, more information is needed to better quantify the infectious risk of rituximab in the ITP population, but in the meantime one should not assume that this risk is trivial.
Moulis et al also found that while >70% of French patients with ITP are appropriately immunized prior to splenectomy, only 34% of ITP patients were vaccinated prior to treatment with rituximab.10 It is likely that the incidence of vaccination prior to rituximab therapy is even lower in the United States and other countries, since the 2009 French ITP guidelines recommended prerituximab vaccination, while neither the American Society of Hematology nor International Working Group guidelines specifically do so (although the latter acknowledge the likelihood of decreased responses after rituximab treatment).
Vaccination against pneumoccus, H influenzae, and meningococcus should be strongly considered in patients with ITP prior to treatment with rituximab. In patients who have failed to respond to rituximab and are being considered for splenectomy, one might consider temporizing with thrombopoietic agents beforehand in order to allow the immunosuppressive effects of rituximab to abate and appropriate vaccination to be administered. While acknowledging that more information is needed concerning this issue, the report of Nazi et al suggests that in the case of vaccination of ITP patients after rituximab treatment, “better late” may not be better “than never.”
Conflict-of-interest disclosure: K.R.M. has received consulting fees from Amgen.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal