In this issue of Blood, Stevanović et al demonstrate a possible link between cytomegalovirus (CMV) infection and the induction of CD4-dependent HLA-DP–directed colonic graft-versus-host disease (GVHD).1 Their data suggest that virus-specific T cells mediate this effect via cytokine-induced upregulation of HLA class II molecules, providing further evidence of the plasticity of CD4 T cells in both orchestrating and effecting immune responses, the potential importance of DP matching when choosing a transplant donor, and the possible role of viral infections in providing the inflammatory cues that induce GVHD.
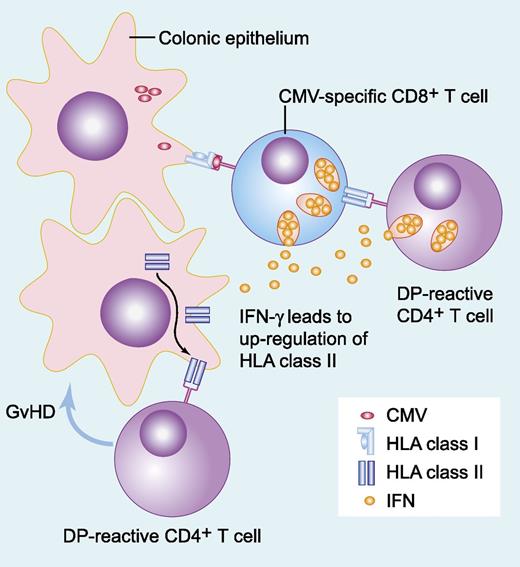
CMV-specific T cells recognizing virus in the context of MHC-I molecules secrete inflammatory cytokines, resulting in upregulation of MHC-II molecules both on the potential colonic targets of alloreactive DP-mismatched CD4+ T cells and on the CMV-specific T cells themselves. Recognition of these mismatched MHC-II molecules results in eradication of recipient-derived CMV-specific immunity and induction of local tissue damage (GVHD), with or without recruitment of further immune effector populations. IFN, interferon. Professional illustration by Paulette Dennis.
CMV-specific T cells recognizing virus in the context of MHC-I molecules secrete inflammatory cytokines, resulting in upregulation of MHC-II molecules both on the potential colonic targets of alloreactive DP-mismatched CD4+ T cells and on the CMV-specific T cells themselves. Recognition of these mismatched MHC-II molecules results in eradication of recipient-derived CMV-specific immunity and induction of local tissue damage (GVHD), with or without recruitment of further immune effector populations. IFN, interferon. Professional illustration by Paulette Dennis.
Major histocompatibility complex (MHC) class II proteins (HLA-DR, HLA-DP, and HLA-DQ) play a fundamental role in the regulation of immune responses in the context of recognition by CD4+ T cells. The expression of these transmembrane glycoproteins is largely restricted to thymic epithelial cells and to cells specialized in the capture and presentation of extracellular antigens, such as B cells, cells of monocyte-macrophage lineage, and dendritic cells. There are 2 general cell-type–specific modes of MHC-II expression: constitutive and inducible. Both constitutive and inducible MHC-II expression can be modulated in a cell-type–specific manner by various secondary stimuli. Constitutive expression is mainly restricted to thymic epithelial cells and antigen-presenting cells. Other types of cells do not usually express MHC-II molecules, although they can often be induced to express them after exposure to interferon gamma and other cytokines, dependent on the status of class II trans-activator protein, a coactivator of the MHC-II gene promoter. Therefore, inflammatory cues may be important in promoting the direct activation of CD4+ T cells, as well as being indirectly relevant in influencing the activation status of antigen-presenting cells.
The complex relationships that exist between CMV infection and clinical outcomes after allogeneic transplantation have been recognized for some time.2,3 The observation that increased immune compromise resulting both from the process and the treatment of GVHD results in an increased propensity to CMV infection is intuitively understandable. The converse, that CMV serostatus or infection correlates with an increased incidence of GVHD, is also well recognized, although the mechanistic explanation has remained less clear.3,4 The study by Stevanović et al provides one possible mechanism linking these prior observations (see figure). Their findings suggest that CMV-reactive T cells can directly induce upregulation of MHC-II molecules on tissues via the release of inflammatory cytokines, and that these tissues then become targets for alloreactive T-cell clones. Furthermore, they demonstrate that in the setting of persistent mixed T-cell chimerism, as more commonly occurs after reduced intensity and T-cell–depleted transplantation, recipient-derived virus-specific T cells may themselves upregulate MHC-II molecules after recognition of a cognate antigen, which can in itself act as a trigger for allorecognition by class II-mismatched CD4+ T cells. Elimination of the recipient T cells and a switch to full-donor chimerism might further fuel the process by eradicating CMV-specific T-cell immunity, allowing further uncontrolled proliferation of CMV, particularly in the context of CMV-seronegative donors or enhanced immune suppression given to treat GVHD.
What are the implications of these findings? Could this be part of the explanation for the failure of reduced intensity-conditioning regimens to deliver the expected decrease in the incidence of GVHD despite a reduction in conditioning-related inflammation? Should we be considering evaluation of viral suppression at the time of DP-mismatched donor lymphocyte infusions (DLIs)? It is notable that the 2 patients outlined in this study underwent T-cell–depleted transplantation and received prophylactic CD8-depleted DLIs at early time points after transplantation, when CMV reactivation is more commonly detected. Therefore, questions arise about the broader relevance of the findings in the contexts of unrelated donor allogeneic transplantation, DLIs delivered at later time points, and full-donor chimeras. Certainly, the findings lead to a number of testable hypotheses. Although there is no a priori reason to suspect that other viruses, such as norovirus, could not have a similar impact, at least in the more direct influence of upregulation of MHC-II molecules on target tissues within the gut, the numerical preponderance of CMV makes it feasible to consider epidemiological analyses aimed at determining whether the excess GVHD incidence noted in DP-mismatched transplant cohorts correlates with recipient CMV serostatus or, perhaps more importantly, CMV reactivation. These trends may help to show whether the findings are broadly relevant to donor choice and events earlier posttransplantation, when inflammation secondary to transplant conditioning may override any influence of cytokines released by virus-specific T cells. Stratification for conditioning intensity and T-cell depletion would clearly be important in any correlative epidemiological studies. Because other factors clearly drive GVHD incidence, the background noise in the system may act to obscure any true influence of DP mismatching and CMV infection, requiring careful interrogation of large-registry data sets. If these findings do have broader relevance, do they help us to choose an unrelated donor for an individual patient? This issue brings us back to ongoing debate regarding “permissive” and “nonpermissive” MHC mismatches. A mismatch defined as nonpermissive in the risk for GVHD may associate with a lower risk for relapse and appear permissive regarding progression-free or overall survival. In other words, the permissibility of a given MHC mismatch is, in part, defined by the locus and, in part, by the disease risk regarding survival outcomes. Whether we could ever usefully modulate donor choice in this regard, according to DP matching and CMV serostatus, remains debatable.
Finally, the data highlight a growing appreciation of the potential importance of CD4+ T cells in mediating direct cytolytic activity.5,6 Although the data do not fully exclude a pathogenic role of CD8+ cytotoxic T-cell lymphocytes, which might be recruited after activation of CD4+ helper subsets, they do illustrate a direct role of “cytotoxic” CD4+ T cells in the process. Tumor-specific cytotoxic CD4+ T cells have been demonstrated in murine models, apparently functioning by interferon gamma–dependent induction of class II MHC upregulation, followed by granzyme-dependent cytotoxicity.7 It is perhaps no coincidence that cells with similar phenotype have previously been described both in the settings of viral infection and GVHD in humans.6 Harnessing the activity of these populations may provide powerful anti-tumor immunity, and further study of their biological characteristics in model settings such as DP-mismatched transplants may greatly inform such efforts.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal