Key Points
Products of ATP hydrolysis, 5′AMP, and adenosine orchestrate the dual regulatory activity of B cells.
B cells emerge as a key regulatory component of T cell–B cell interactions, which are under environmental control.
Abstract
Antibody-independent role of B cells in modulating T-cell responses is incompletely understood. Freshly isolated or cultured B cells isolated from the peripheral blood of 30 normal donors were evaluated for CD39 and CD73 coexpression, the ability to produce adenosine 5′-monophosphate (AMP) and adenosine (ADO) in the presence of exogenous adenosine triphosphate (ATP) as well as A1, A2A, A2B, and A3 adenosine receptor (ADOR) expression. Human circulating B cells coexpress ectonucleotidases CD39 and CD73, hydrolyze exogenous ATP to 5′-AMP and ADO, and express messenger RNA for A1R, A2AR, and A3R. 2-chloroadenosine inhibited B-cell proliferation and cytokine expression, and only A3R selective antagonist restored B-cell functions. This suggested that B cells use the A3R for autocrine signaling and self-regulation. Mediated effects on B-cell growth ± ADOR antagonists or agonists were tested in carboxyfluorescein diacetate succinimidyl ester assays. In cocultures, resting B cells upregulated functions of CD4+ and CD8+ T cells. However, in vitro–activated B cells downregulated CD73 expression, mainly produced 5′-AMP, and inhibited T-cell proliferation and cytokine production. These B cells acquire the ability to restrict potentially harmful effects of activated T cells. Thus, B cells emerge as a key regulatory component of T cell–B cell interactions, and their dual regulatory activity is mediated by the products of ATP hydrolysis, 5′-AMP, and ADO.
Introduction
It is known that B cell functions are necessary for the development and maintenance of immune responses.1 Early studies in B cell–deficient mice showed that the absence of B cells had adverse effects on CD4+ as well as CD8+T cell responses.2 Mice lacking B cells during embryonic development exhibited a variety of immunologic abnormalities and defects in the structure of various organs.3,4 It has been widely acknowledged that B cells are necessary for the development of T-cell immunity because they serve as excellent antigen-presenting cells, providing costimulatory signals and producing cytokines necessary for effector functions of T cells.5 More recently, it has been reported that B-cell depletion is an effective therapy for several human autoimmune diseases, suggesting that B cells contribute to the disease process and do so independently of autoantibody production.6,7 A novel paradigm that implicates B cells in regulating peripheral tolerance by modulating development, expansion and function of regulatory T cell (Treg) has been recently introduced.8 In patients with autoimmune syndromes who were responsive to rituximab therapy, depletion of B cells was associated with the significantly increased frequency of Treg producing interleukin (IL)-10 and transforming growth factor-β.9,10 In this instance, B-cell depletion allowed for Treg expansion and suppression of autoreactive effector T cells, presumably accounting, at least in part, for therapeutic benefits of rituximab in autoimmune diseases.11 However, other studies suggest that B cells are necessary for proliferation and expansion not only of antigen-primed effector CD4+T cells but also of Treg. For example, coculturing of CD19+ human B cells with CD4+CD25+ alloreactive T cells in the presence of IL-2 and CD28-specific antibody (Ab) was reported to induce a 40-fold expansion of Treg.12,13 The current hypothesis is that B cells exert dual and potentially opposing effects on T-cell responses. On the one hand, they can promote primary T-cell responses and the generation of memory T helper (Th)1 and Th2 cells through antigen-dependent but Ab-independent mechanisms. On the other hand, B cells can modulate functions of Treg.12,13 The concept that B cells can both suppress and enhance T-cell responses has led to the conclusion that functionally different subsets of B cells exist, some serving as effector B cells and others as regulatory B cells.14 Mechanisms used by regulatory B cells to mediate suppression are unknown, although it has been reported that they are able to produce IL-10.15
While studying expression of the adenosinergic pathway components in human CD4+T cells, we observed that human naturally occurring Treg (nTreg) and inducible Treg (iTreg, Tr1) express CD39, an ectonucleoside triphosphate diphosphohydrolase-1 and CD73, an ecto-5′-nucleotidase, and use these enzymes to hydrolyze exogenous adenosine triphosphate (ATP) to adenosine 5′-monophosphate (AMP) and finally to adenosine (ADO).16,17 Recently, we also showed that human peripheral blood B cells also express these ectonucleotidases. In this report, we describe the phenotypic and functional properties of human B cells that express these enzymes and produce immunosuppressive ADO. We test the hypothesis that B cell–derived ADO, binding to ADO receptors expressed on T cells as well as B cells, exerts immunosuppressive paracrine and autocrine effects, respectively. Through these regulatory mechanisms, CD39+CD73+ human B cells may self-regulate and also be able to downregulate potentially harmful effects of activated T cells.
Materials and methods
Collection of PBMC
Peripheral blood was obtained from normal volunteers who, in accordance with the Declaration of Helsinki, signed an informed consent approved by the University of Pittsburgh IRB (IRB # 991206). Blood was drawn into heparinized tubes and centrifuged on Ficoll-Hypaque gradients (GE Healthcare Life Sciences). Peripheral blood mononuclear cells (PBMC) were recovered, washed twice in RPMI medium, counted in a trypan blue dye, and immediately used for experiments. For a large-scale B-cell separation, buffy coats collected from normal donors were purchased from the Central Blood Bank of Pittsburgh.
Antibodies and reagents
The following anti-human monoclonal antibodies (mAbs) were purchased from Beckman Coulter and used for flow cytometry: anti-CD19-ECD, anti-CD19-PC5, anti-CD4-PC5, anti-CD25-PC5, and anti-CD26-PE Abs. In addition, anti-CD39-fluorescein isothiocyanate Ab was purchased from eBioscience. Anti-CD73-PE was purchased from Biolegend. The relevant isotypes, which served as negative controls for surface staining, were also purchased and were used in all experiments. 2-chloroadenosine (CADO) was purchased from Sigma-Aldrich and used in place of ADO, because of its greater stability. The following ADO receptor (ADOR) antagonists were purchased from Tocris Bioscience: PSB-36, an ADO A1R antagonist and PSB-10, an ADO A3R antagonist. Also, ZM241385, an ADO A2AR antagonist, was purchased from Sigma-Aldrich. The following ADOR agonists were purchased from Tocris Bioscience: 2-chloro-N6-cyclopentyladenosine, an A1R agonist; CGS 21680 hydrochloride, an A2AR agonist; and 2-Cl-IB-MECA, an A3R agonist.
Cell separation
CD19+ B cells were separated from PBMC using human B-cell enrichment separation kits (STEMCELL Technologies) or anti-CD19 Ab-coated magnetic beads (Miltenyi Biotec) in some experiments. CD4+T cells were separated from PBMC by negative selection using the Miltenyi reagents as previously described.18 CD8+T cells were separated using CD8 immunobeads (Miltenyi Biotec). All assays were performed according to the manufacturers’ instructions. The purity of separated cells was monitored by flow cytometry. The purity of separated subsets ranged from 93% to 98%.
Surface staining
Cells were stained for flow cytometry of surface markers as previously described.19 Briefly, cells were incubated with mAbs specific for surface markers for 30 minutes at 4°C in the dark and then fixed with 2% (w/v) paraformaldehyde in phosphate-buffered saline (PBS) for flow cytometry analysis. Before use, all Abs were titrated using resting as well as activated PBMC obtained from normal controls to determine the optimal staining dilutions. Appropriate isotype controls were included for each sample.
Flow cytometry
Flow cytometry was performed using an EPICS XL-MCL flow cytometer equipped with Expo32 software or Gallios 10 color flow cytometer equipped with Kaluza Flow Cytometry Software (Beckman Coulter). The acquisition and analysis gates were restricted to the lymphocyte gate based on characteristic properties of the cells in forward and side scatter. The forward scatter and side scatter were set in a linear scale, and at least 105 cells were acquired for analysis.
Image analysis
Expression of CD39 and CD73 in B cells was evaluated by confocal microscopy. Freshly harvested B cells were placed in Eppendorf tubes (106 cells/tube) and fixed with 4% (w/v) paraformaldehyde in PBS. Cells were permeabilized with 0.1% (v/v) Triton-X in PBS for 25 minutes at room temperature, washed twice and blocked with 2% (w/v) bovine serum albumin in PBS for 45 minutes. Primary Abs (mouse anti-human CD39 purchased from Ancell at 1:100 dilution and polyclonal rabbit anti-human CD73 purchased from Santa Cruz at 1:500 dilution) were added and cells were incubated for 1 hour at room temperature, washed with the bovine serum albumin/PBS buffer, and incubated with fluorescein isothiocyanate–labeled anti-mouse immunoglobulin (Invitrogen) plus phycoerythrin-labeled anti-rabbit immunoglobulin (Jackson Laboratories) for 45 minutes in the dark at room temperature. Finally, cells were washed, stained with the Hoescht dye for 30 seconds, washed again, and layered by cytospin onto slides. Cells were imaged using a confocal microscope (Olympus Fluoview 500 Confocal Micro scope; Olympus). A 60× or 100× objective lens was used for all images. Images were analyzed with MetaMorph/Fluor (Universal Imaging Corporation; Molecular Devices) and Adobe Photoshop (Adobe Systems, Inc).
Western blots
Whole-cell protein extracts were prepared using freshly separated B-cell and T-cell subsets. Cells were lysed at 4°C in a lysis buffer containing 0.5% NP40, 150 mM NaCl, and 50 mM Tris base (Sigma). Laemmli loading buffer (4% sodium dodecyl sulfate, 10% b-mercaptoethanol 20% glycerol, 0.004% bromophenol blue, 0.125 M Tris HCl) was added to the cell lysates at the 1:1 ratio (v/v), and the lysates were boiled for 5 minutes. Protein extracts were subjected to electrophoresis on 4% to 15% Tris-HCl gradient gels (BioRad) and were subsequently transferred to polyvinylidene fluoride membranes Millipore). The membranes were treated with rabbit polyclonal primary antibodies specific for human CD73 or CD39 (Santa Cruz; 1:400) followed by goat anti-rabbit secondary Ab conjugated to horseradish peroxidase (goat, 1:150 000, Pierce). SuperSignal West Femto Maximum Sensitivity Substrate (Pierce) and Kodak BioMax MR Film were used to visualize the target proteins.
B-cell culture
Freshly separated CD19+B cells were seeded in wells of flat bottom 96-well plates (105 cells/well) and cultured in RPMI containing 10% fetal bovine serum, 4 mM l-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (all from GIBCO). B cells were stimulated with 5% solution of UltraCD40L (Multimeric Biotherapeutics) and 200 IU/mL of IL-4 and cultured for up to 5 days at 37°C in the atmosphere of 5% CO2 in air. Proliferation was measured in carboxyfluorescein diacetate succinimidyl ester (CFSE)-based assays.19 Supernatants of proliferating B cells were collected for cytokine measurements.
Cytokine production and expression by B cells
Cytokine levels in supernatants of 5-day cultured B cells were measured using Luminex technology. Human cytokine 10-plex Ab kits were purchased from Biosource/Invitrogen, and assays were performed according to the manufacturer’s instructions. Expression of proinflammatory cytokines by cultured B cells was also measured by flow cytometry after cell permeabilization and staining with labeled anti-cytokine antibodies purchased from eBioscience.
Coculture of B with T cells
To establish B- and T-cell cocultures, CD4+ or CD8+T cells were labeled with 1.5 μM CFSE (Molecular Probes; Invitrogen) and incubated at 37°C for 10 minutes as previously described.19 Various numbers of CD19+B cells were titrated into CFSE-labeled autologous CD4+ or CD8+T cells (1 × 105) serving as responders and incubated in the presence of plate-bound anti-CD3 Ab and anti-CD28 Ab (2 μg/mL each) for 4 days. Proliferation of CFSE-labeled responder T cells was measured by flow cytometry as previously described.19 All CFSE data were analyzed using the ModFit software provided by Verity Software House as previously described.19
Effects of exogenous ADO on B-cell proliferation
Freshly separated CD19+ B cells were labeled with CFSE and cultured in the presence of soluble CD40L and IL-4 as described previously. B cells were incubated in the presence or absence of 2.5 μM or 5.0 μM CADO. Various antagonists or agonists selective for ADOR were added to B cells 1 hour before the addition of CADO. All antagonists, agonists, and CADO were initially titrated to determine the concentrations that were not toxic for B cells (ie, did not adversely affect B-cell proliferation or viability). Annexin V binding to B cells was used to confirm B-cell viability by flow cytometry. After 5 days in culture ± antagonists or agonists, B cells were harvested, washed with PBS, and tested for suppression of proliferation as previously described.18,19 Briefly, the percentage of suppression in the presence of CADO was calculated by using the mean proliferation index of B cells alone compared with the proliferation index of cells cultured in the presence of CADO. The ModFit program determines the percentage of cells within each detected peak; the sum of all peaks in the control culture is set to be 100% proliferation or 0% suppression.
RT-PCR for ADOR
Total RNA was extracted from B or T cells using RNeasy Mini Spin Kit (Qiagen). Quantitative reverse transcription polymerase chain reaction (RT–-PCR) was performed using SYBR Green PCR Master Mix (Applied Biosystems), and was carried out on an AB7300 Real-Time PCR System (Applied Biosystems). Relative quantification of the target gene messenger RNA (mRNA) expression was calculated with the comparative counts method. For all assays, the expression levels of target genes were normalized to the levels of an endogenous control (the β-actin housekeeping gene). The following gene-specific PCR primers were used: human A1R, cctccatctcagctttccag (forward) and agtaggtctgtggcccaatg (reverse); human A2aR, aggcagcaagaacctttcaa (forward) and ctaaggagctccacgtctgg (reverse); human A2bR, ctccatcttcagccttctgg (forward) and acaaggcagcagctttcatt (reverse); human A3, agctggcagaaagattgcat (forward) and ttcaggggtgtttcaggaag (reverse); and human β-actin, actcttccagccttccttc (forward) and atctccttctgcatcctgtc (reverse).
Mass spectrometry
B cells, CD4+, and CD8+ T-cell subsets were separated using magnetic immunobeads as described previously. For detection of ATP and 5′-AMP hydrolysis, 25 000 cells from each subset were incubated in 200 µL PBS in 96-well plates in the presence of 20 µM ATP for various time periods. Control wells contained cells alone. All experiments were performed in duplicate. Cells and supernatants were collected, centrifuged for 2 minutes at 6000 × g, boiled for 2 minutes to inactive ADO-degrading enzymes, and stored at −80°C for subsequent analysis. Purines were measured using liquid chromatography-tandem mass spectrometer by selected reaction monitoring with 13C10-ADO as the internal standard. In this regard, samples were injected into a Acuity ultra-performance liquid chromatographic system (Waters) and were separated with a C18 column (Waters UPLC BEH C18; 1.7 micron; 2.1 × 100 mm) using the following elution conditions: mobile phase A, 1% acetic acid in H2O; mobile phase B, methanol; flow rate, 0.3 mL/min; elution gradient (A/B) was 99.5%/0.5% (0 to 2 minutes), 98%/2% (2 to 3 minutes), 85%/15% (3 to 4 minutes), and 99.5%/0.5% (4 to 5 minutes). Purine levels were analyzed with a TSQ Quantum-Ultra triple-quadruple mass spectrometry equipped with a heated electrospray ionization source. The mass spectrometer was operated in the positive-ion mode and the following mass-to-charge transitions were monitored: 348→136 for 5′-AMP; 268→136 for ADO.
Statistical analysis
All data are presented as means of at least 3 experiments ± 1 SD. The data were analyzed using the Student t test or paired t tests, and P values < .05 were considered to be significant. To test proliferation stimulation or inhibition of T cells, the Page test for ordered alternatives was used.20
Results
Human peripheral B cells coexpress CD39 and CD73
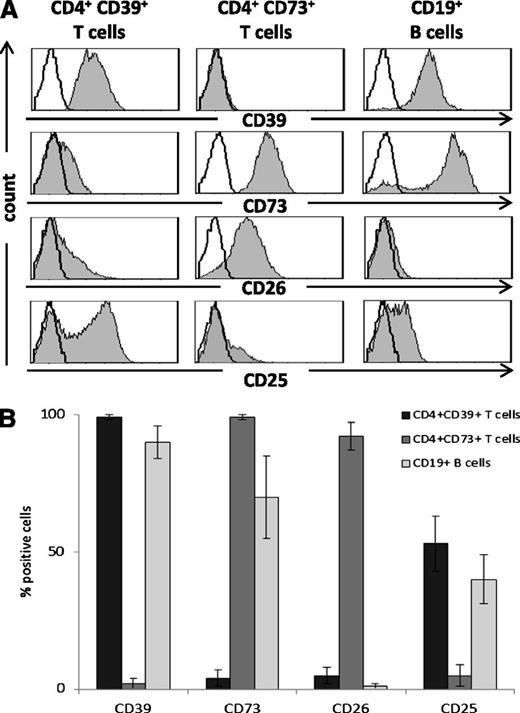
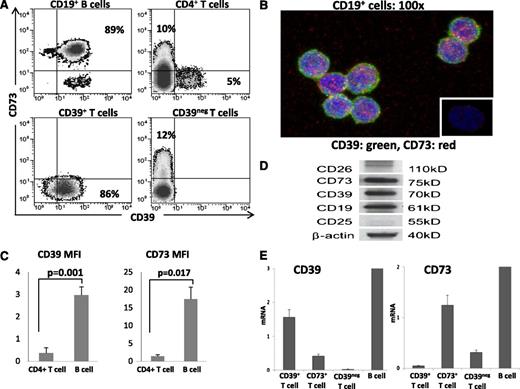
PBMC obtained from normal donors were tested by flow cytometry for expression of CD39 and CD73 ectonucleotidases as well as CD26 and CD25 on CD4+CD39+Treg,18 CD4+CD39negCD73+T cells, and CD19+B cells. Figure 1 shows that the B-cell phenotype was distinct from that of either T-cell subset. Up to 90% of circulating B cells coexpressed CD39 and CD73 on the cell surface. B cells were negative for CD26 and about 50% of B cells expressed CD25. All Treg were CD39+ but CD73neg/lowCD26neg/low and 50% stained for CD25, as previously reported.18 All of the CD4+CD39negCD73+ T cells were CD26+ and none or very few expressed CD25. These phenotypic differences suggest that the 3 lymphocyte subsets might differ functionally. Importantly, because B cells coexpressed CD39 and CD73, they presumably were capable of exogenous (e)ATP hydrolysis to ADP, AMP, and ADO. Indeed, coexpression of these 2 enzymes in freshly isolated, unstimulated B cells was confirmed by flow cytometry and confocal microscopy (Figure 2A-B). Further, CD39 and CD73 expression levels measured as mean fluorescence intensity (MFI) were found to be higher in B cells compared with CD4+T cells (Figure 2C). Coexpression of the 2 enzymes in B cells was also confirmed at the protein and mRNA levels by western blots (Figure 2D) and RT-PCR analysis (Figure 2E), respectively. In agreement with high CD39 and CD73 expression levels, B cells contained higher levels of mRNA for both the ectonucleidases (Figure 2E) relative to CD39+Treg or CD4+CD73+T cells.
Phenotypic characterization of human CD19+ B cells relative to CD4+CD39+ and CD4+CD73+ T cells. Freshly isolated PBMC were stained for flow cytometry and examined for surface expression of ectonucleotidases, CD39 and CD73, ADO deaminase–associated protein, CD26, and CD25. (A) Representative expression data for CD4+ T and CD19+ B cells of a normal donor. (B) Percentages of positive cells in each cell subset shown as means ± SD for 6 different normal donors tested. B cells are CD39+ and CD73+ but negative for CD26.
Phenotypic characterization of human CD19+ B cells relative to CD4+CD39+ and CD4+CD73+ T cells. Freshly isolated PBMC were stained for flow cytometry and examined for surface expression of ectonucleotidases, CD39 and CD73, ADO deaminase–associated protein, CD26, and CD25. (A) Representative expression data for CD4+ T and CD19+ B cells of a normal donor. (B) Percentages of positive cells in each cell subset shown as means ± SD for 6 different normal donors tested. B cells are CD39+ and CD73+ but negative for CD26.
Expression of CD39 and CD73 proteins and mRNA in isolated human B cells. (A) Flow cytometry shows coexpression of CD39 and CD73 in the CD19+, CD4+, CD39+, and CD39neg subsets of human lymphocytes. Percentages of positive cells are indicated in relevant quadrants. (B) Confocal images of CD19+ B cells coexpressing CD39 and CD73. Magnification ×100. (C) The MFI values for surface expression of the 2 ectonucleotidases are higher in B cells than CD4+ T cells. Data are means ± standard deviation determined for all CD4+ T cells and CD19+ B cells. The cells were obtained from 5 normal donors. (D) Western blots of freshly isolated human CD19+ B cells. (E) RT-PCR results for CD39 and CD73 mRNA expression in the isolated subsets of human peripheral blood T cells and CD19+ B cells. B cells express significantly higher levels of mRNA specific for CD39 and CD73 as compared with various T-cell populations. The data are mean relative levels ± standard deviation from 3 independent experiments with cells of different donors.
Expression of CD39 and CD73 proteins and mRNA in isolated human B cells. (A) Flow cytometry shows coexpression of CD39 and CD73 in the CD19+, CD4+, CD39+, and CD39neg subsets of human lymphocytes. Percentages of positive cells are indicated in relevant quadrants. (B) Confocal images of CD19+ B cells coexpressing CD39 and CD73. Magnification ×100. (C) The MFI values for surface expression of the 2 ectonucleotidases are higher in B cells than CD4+ T cells. Data are means ± standard deviation determined for all CD4+ T cells and CD19+ B cells. The cells were obtained from 5 normal donors. (D) Western blots of freshly isolated human CD19+ B cells. (E) RT-PCR results for CD39 and CD73 mRNA expression in the isolated subsets of human peripheral blood T cells and CD19+ B cells. B cells express significantly higher levels of mRNA specific for CD39 and CD73 as compared with various T-cell populations. The data are mean relative levels ± standard deviation from 3 independent experiments with cells of different donors.
5′-AMP and ADO production by human B cells
To show that the ectonucleotidases coexpressed on B cells are functional, freshly isolated B cells were incubated in the presence/absence of extracellular ATP (eATP) for various time periods. Levels of 5′-AMP, ADO, and inosine were measured in the cell supernatants by mass spectrometry. B cells produced higher levels of both 5′-AMP and especially ADO than CD4+CD39+ T cells and a little less inosine (Figure 3). These data indicate that CD39 and CD73, abundantly expressed on the surface of freshly isolated B cells, are enzymatically active and efficiently hydrolyze eATP to ADO. The decline in 5′-AMP and ADO levels seen in Figure 3 after 45 minutes of incubation could be attributed to the depletion of eATP in this enzymatic reaction or potentially to experimental variability in this highly sensitive mass spectrometry-based assay.
Production of 5′AMP, ADO, and inosine by human CD19+ B cells and CD4+CD39+ T cells. B cells and T cells were isolated from PBMC as described in Methods and materials and incubated in the presence of 20 µM ATP for various time periods before collecting supernatants for mass spectrometry. The data are means ± standard deviation from 3 independent experiments with cells of different donors.
Production of 5′AMP, ADO, and inosine by human CD19+ B cells and CD4+CD39+ T cells. B cells and T cells were isolated from PBMC as described in Methods and materials and incubated in the presence of 20 µM ATP for various time periods before collecting supernatants for mass spectrometry. The data are means ± standard deviation from 3 independent experiments with cells of different donors.
ADO-mediated suppression of B-cell proliferation and cytokine expression
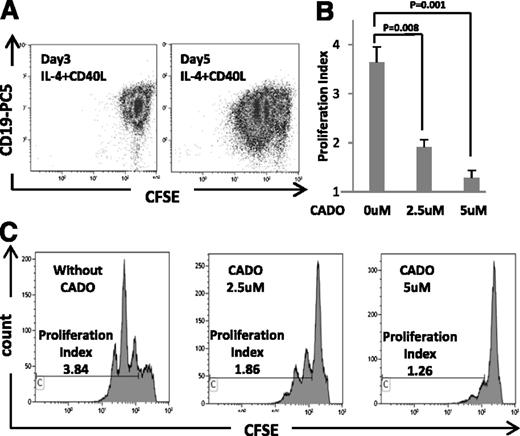
B cells stimulated by CD40L and supplemented with IL-4 expand well in 5-day cultures (Figure 4A). CADO, a well-known immunosuppressive agent, added to these B-cell cultures at concentrations ranging from 1 to 10 μM on days 0 and 3, inhibited B cell proliferation (Figure 4C). At the concentrations ranging from 1.0 to 5.0 μM, CADO was not toxic to B cells (data not shown); at 5.0 μM, it almost completely blocked their proliferation (P < .001; Figure 4B).
Proliferation of B cells in the presence and absence of eCADO. CD19+ B cells were isolated from PBMC of normal donors, labeled with CFSE, and cultured in the presence of IL-4 and CD40L as described in Methods and materials. Proliferation index was determined on days 3 and 5 of culture. (A) Flow cytometry of cells from a representative proliferating B-cell culture on days 3 and 5. (B) Inhibitory CADO effects on B-cell proliferation are shown for 3 different B-cell cultures. Data are means ± standard deviation. (C) CADO at the indicated concentrations was added to B cells at the start of culture. It inhibited B-cell proliferation. Data are from a representative experiment of 3 performed with cells of different donors.
Proliferation of B cells in the presence and absence of eCADO. CD19+ B cells were isolated from PBMC of normal donors, labeled with CFSE, and cultured in the presence of IL-4 and CD40L as described in Methods and materials. Proliferation index was determined on days 3 and 5 of culture. (A) Flow cytometry of cells from a representative proliferating B-cell culture on days 3 and 5. (B) Inhibitory CADO effects on B-cell proliferation are shown for 3 different B-cell cultures. Data are means ± standard deviation. (C) CADO at the indicated concentrations was added to B cells at the start of culture. It inhibited B-cell proliferation. Data are from a representative experiment of 3 performed with cells of different donors.
Table 1 shows cytokine production by activated B cells. IL-6 was produced at the highest level, and granulocyte macrophage–colony-stimulating factor, IL-8, tumor necrosis factor-α, and IL1-β at lower levels. Intracytoplasmic expression of IL-6 and IL-8 was measured in these B cells upon incubation in the presence of CADO and was found to be significantly reduced (supplemental Figure 1). Thus, CADO downregulated cytokine expression in activated B cells.
Levels of cytokines produced by activated B cells
| Cytokines . | Concentration . |
|---|---|
| pg/mL . | |
| IL-1β | 23 ± 12 |
| IL-2 | 5 ± 1.3 |
| IL-5 | 2 ± 0.5 |
| IL-6 | 5970 ± 3885 |
| IL-8 | 37 ± 18 |
| IL-10 | 13 ± 5 |
| GM-CSF | 144 ± 211 |
| IFN-γ | 2 ± 0.6 |
| TNF-α | 29 ± 4 |
| Cytokines . | Concentration . |
|---|---|
| pg/mL . | |
| IL-1β | 23 ± 12 |
| IL-2 | 5 ± 1.3 |
| IL-5 | 2 ± 0.5 |
| IL-6 | 5970 ± 3885 |
| IL-8 | 37 ± 18 |
| IL-10 | 13 ± 5 |
| GM-CSF | 144 ± 211 |
| IFN-γ | 2 ± 0.6 |
| TNF-α | 29 ± 4 |
B cells obtained from PBMC of 4 normal donors were activated by ultra-CD40L and IL-4 in RPMI for 5 days. Supernatants were collected and cytokine concentrations were analyzed by Luminex. The data are mean values ± standard deviation.
GM-CSF, granulocyte macrophage–colony-stimulating factor; TNF, tumor necrosis factor.
ADOR expression on B cells
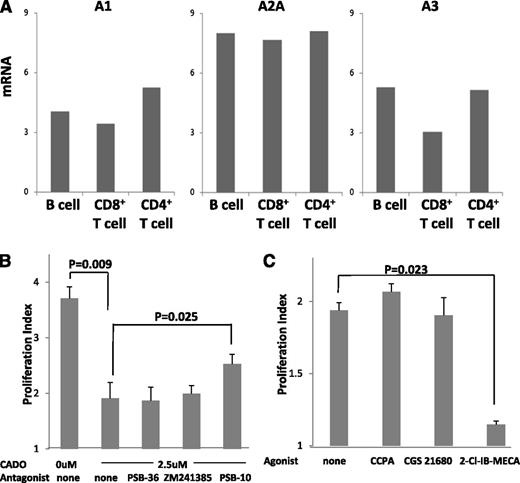
Because human B cells are capable of producing ADO and eCADO inhibits B-cell proliferation, we expected that ADOR are expressed on B cells and are involved in autocrine signaling. To confirm this, RT-PCR was performed in which mRNAs obtained from B cells, CD4+ T cells and CD8+ T cells were amplified with gene-specific PCR primers for the 4 known ADOR. Similar to other lymphocytes, B cells were found to express messages for A1R, A2AR, and A3R but not for A2BR (Figure 5A).
ADOR expression on B cells and signaling in the presence of ADOR agonists or antagonists. (A) RT-PCR results are shown for relative expression of A1R, A2AR, A2BR, and A3R. RT-PCR was performed as described in Methods and materials using the indicated subsets of T cells and B cells. No message for A2BR was detected in T or B lymphocytes. The data are from 1 representative experiment of 3 performed with cells of different normal donors. (B) Effects of ADOR antagonists or ADOR agonists (all used at 1 μM after initial titrations) on proliferation of B cells cultured in the presence of IL-4, CD40L, and CADO are shown. (C) Effects of ADOR agonists on proliferation of B cells cultured in the presence of IL-4 and CD40L. The viability of B cells incubated with agonists or antagonists was always >95%. Data in B and C are means ± SD from 3 independent experiments with cells of different normal donors.
ADOR expression on B cells and signaling in the presence of ADOR agonists or antagonists. (A) RT-PCR results are shown for relative expression of A1R, A2AR, A2BR, and A3R. RT-PCR was performed as described in Methods and materials using the indicated subsets of T cells and B cells. No message for A2BR was detected in T or B lymphocytes. The data are from 1 representative experiment of 3 performed with cells of different normal donors. (B) Effects of ADOR antagonists or ADOR agonists (all used at 1 μM after initial titrations) on proliferation of B cells cultured in the presence of IL-4, CD40L, and CADO are shown. (C) Effects of ADOR agonists on proliferation of B cells cultured in the presence of IL-4 and CD40L. The viability of B cells incubated with agonists or antagonists was always >95%. Data in B and C are means ± SD from 3 independent experiments with cells of different normal donors.
A3R mediates inhibitory signals in B cells
Suppressive signals delivered by CADO could be processed by 1 or more of ADOR present on B cells. To determine which ADOR is involved, we tested the antagonists selective for each of the 3 ADOR for the ability to block inhibitory CADO signaling and to restore B-cell proliferation. B cells were cultured in the presence of CADO ± each antagonist added on days 0 and 3 of culture. Neither PSB-36 (1 μM), a selective A1R antagonist, nor ZM241385 (0.3 μM), a selective A2AR antagonist, was able to reverse CADO-mediated suppression of proliferation (Figure 5B). However, in the presence of PSB-10 (1 μM), a selective A3R antagonist, a partial but significant (P ≤ .025) restoration of B-cell proliferation was observed. These experiments indicated that in B cells, the A3R is responsible for the transfer of inhibitory ADO signals. When PBS-10 and ZM241385 were combined, CADO-induced inhibition of B-cell proliferation was in part restored, but this effect was not greater than that induced by the A3R antagonist alone.
To confirm that signaling via the A3R is involved in suppression of B-cell proliferation, the ADOR agonists were next added to cultured B cells. As shown in Figure 5C, only the A3R agonist 2-Cl-IB MECA significantly inhibited B-cell proliferation, indicating that in B cells, only A3R is responsible for delivery of suppressive signals. These experiments demonstrate that B-cell expansion can be regulated by autocrine ADO signaling via the A3R.
B-cell and T-cell interactions
Because freshly isolated B cells produced ADO, we expected that their coincubation with T cells would suppress T-cell proliferation. Instead, and as previously observed, freshly purified B cells cocultured with separated autologous CD4+ or CD8+T cells stimulated via the T-cell receptor (TCR) augmented proliferation of these T cells (P < .012; Figure 6A, middle, and 6B) as well as their cytokine production (data not shown). These cocultures did not contain CD4+FOXP3+CD25high Treg as determined by flow cytometry (data not shown). However, when B cells were first cultured in the presence of CD40L and IL-4 for 5 days and then added to CFSE-labeled autologous CD4+ or CD8+ T cells, significant inhibition of T-cell proliferation was observed (P = .007) as shown in Figure 6A, right, and 6B. Interestingly, stimulated (ie, precultured) B cells showed significantly increased expression levels of CD39 relative to fresh B cells (MFI = 18.7 vs 2.9), although CD73 expression was downregulated (MFI = 5.7 vs 15.6). Further, activated B cells produced significantly more 5′-AMP than fresh B cells (Figure 6C) upon incubation in the presence of eATP, as measured by mass spectrometry. These activated B cells also produced inosine and hypoxanthine, whereas fresh B cells did not (Table 2). The data show that upon activation, human B cells acquire the capability to downregulate proliferation of autologous CD4+ or CD8+ T cells, and that 5′-AMP and perhaps also other components of the ADO pathway produced by activated B cells are potentially responsible for this inhibitory activity.
The dual ability of B cells to stimulate and inhibit T-cell proliferation. Cocultures of CFSE-labeled, activated T cells with resting or activated B cells were established at different T:B cell ratios. (A) On the left, proliferation of T cells alone. In the middle, freshly harvested resting B cells promoted proliferation of activated T cells in a representative coculture. On the right, activated B cells inhibited T-cell proliferation in a representative coculture. (B) Results for 3 independent coculture experiments with T cells and resting B cells and 3 independent coculture experiments with T cells and activated B cells. All cocultures were established with autologous T and B cells. (C) 5′-AMP production by resting or activated B cells in the presence of eATP. 25 000 B cells were incubated in 200 μL PBS in wells of 96-well plates in the presence of 20 μL ATP for various time periods. 5′-AMP production was measured by mass spectrometry. Control wells contained ATP alone. Representative data from 1 of 3 experiments performed with cells of different normal donors.
The dual ability of B cells to stimulate and inhibit T-cell proliferation. Cocultures of CFSE-labeled, activated T cells with resting or activated B cells were established at different T:B cell ratios. (A) On the left, proliferation of T cells alone. In the middle, freshly harvested resting B cells promoted proliferation of activated T cells in a representative coculture. On the right, activated B cells inhibited T-cell proliferation in a representative coculture. (B) Results for 3 independent coculture experiments with T cells and resting B cells and 3 independent coculture experiments with T cells and activated B cells. All cocultures were established with autologous T and B cells. (C) 5′-AMP production by resting or activated B cells in the presence of eATP. 25 000 B cells were incubated in 200 μL PBS in wells of 96-well plates in the presence of 20 μL ATP for various time periods. 5′-AMP production was measured by mass spectrometry. Control wells contained ATP alone. Representative data from 1 of 3 experiments performed with cells of different normal donors.
5′-AMP and ADO production by B cells in the presence of exogenous ATP
| . | 5′AMP . | ADO . | Inosine . | Hypoxanthine . |
|---|---|---|---|---|
| Cells tested . | ng/mL . | |||
| Control | 49; 34 | 0; 0 | 0; 0 | 0; 0 |
| Fresh B cells | 123; 69 | 14; 6 | 0; 0 | 1; 0 |
| Activated B cells | 475; 258 | 8; 4 | 3; 2 | 4; 1 |
| . | 5′AMP . | ADO . | Inosine . | Hypoxanthine . |
|---|---|---|---|---|
| Cells tested . | ng/mL . | |||
| Control | 49; 34 | 0; 0 | 0; 0 | 0; 0 |
| Fresh B cells | 123; 69 | 14; 6 | 0; 0 | 1; 0 |
| Activated B cells | 475; 258 | 8; 4 | 3; 2 | 4; 1 |
For detection of ATP and 5′-AMP hydrolysis by mass spectrometry, 25 000 B cells were incubated in 200 µL PBS in 96-well plates in the presence of 20 µM ATP for 60 minutes. Control wells contained ATP alone. The data are from 2 independent experiments.
Because of a possibility that 5′-AMP, which is an A1R agonist, might also have autocrine effects on B cells, we measured B-cell proliferation in the presence of 2-chloro-N6-cyclopentyladenosine, an A1R agonist. In preliminary experiments performed with activated B cells obtained from 3 different donors, this A1R agonist increased B-cell proliferation (P < .02), suggesting that autocrine signals mediated by A1R could also regulate functions of B cells.
Discussion
Mechanisms responsible for the regulation of immune responses to pathogens or autoantigens have long been of great scientific and clinical interest. An intense focus on adaptive immunity, and primarily on T-cell responses, has led to the identification of multiple regulatory mechanisms, including Treg, myeloid-derived suppressor cells and various inhibitory soluble factors, that are responsible for maintaining the immune homeostasis in health and disease.21,22 The role of B cells, essential for humoral immunity, has been generally considered to be limited to differentiation into plasma cells and Ab production. Recently, however, B cells have emerged as important contributors to a variety of T-cell responses,23,24 and a new paradigm of regulatory B cells “B-reg” has been introduced.1,14,15 B-reg in mice and man are thought to be able to control CD4+T-cell responses either by IL-10 or transforming growth factor-β production or by promoting expansion of Treg.12-15,25,26 However, the nature of interactions between B cells and T cells that leads to suppression of T-cell functions is not clear, although its understanding is important for elucidating the B-cell involvement in autoimmune or infectious diseases and cancer.
While studying human Treg in the peripheral circulation, we observed that the phenotype of CD19+B cells was in some respects similar to that of Treg: B cells expressed CD39, CD73, CD25 but not CD26. In the presence of eATP, B cells also produced 5′-AMP and ADO but in a much larger quantity than human Treg (data not shown). The presence of ectonucleotidases and the ability to produce 5′-AMP and ADO by a lymphoblastoid B-cell line was previously reported.27 However, it has not been known that these features also characterize normal circulating human B cells. Our findings suggested that ADO-producing B cells could suppress functions of other immune cells expressing ADOR, including CD4+ or CD8+ T effector cells (Teff) which are known to express A2AR considered to be largely responsible for delivering suppressive signals to these cells.16,28 However, in our hands and those of others, coincubation of freshly harvested B cells with autologous T cells usually results not in suppression but rather in expansion of Teff cells, consistent with potent costimulatory effects known to be mediated by human or murine B cells.1,5
Because B cells express ADORs and produce ADO in the presence of eATP, the potential effects of autocrine self-regulation in B cells were considered. In the presence of CADO, functions of B cells (eg, their activation and proliferation) were downregulated and, based on studies with ADOR agonist and antagonists, we concluded that this suppression was mediated via the A3R. Thus, autocrine ADO signaling in B cells could be responsible for maintaining these cells in a resting state, preventing their functional activation when eATP became available in the microenvironment. The role of A3R in the delivery of inhibitory signals to B cells to prevent their activation is not consistent with the previously reported view of A3R as a Gi/0-coupled inhibitor of adenylate cyclase.29 Nevertheless, in B cells, signaling via A3R reproducibly resulted in suppression of B-cell functions. The biologic significance of this ability of B cells to self-regulate via ADO production can be appreciated in the context of their interactions with T cells responding to TCR mediated signals (Figure 7). Resting B cells promoted responses of activated T cells. However, activated B cells upregulated CD39 ectonucleotidase expression and 5′-AMP as well as inosine production and became strongly suppressive to activated T cells. Because 5′-AMP was recently reported to be an A1R agonist30 and T cells express A1R (Figure 5), it is likely that their suppression in cocultures with activated B cells was mediated by A1R-directed signaling. Further, proliferation of B cells was also shown to be modulated via A1R, suggesting that 5′-AMP generated by B cells could also have autocrine effects. Importantly, the ability of B cells to downregulate T-cell functions depends on the state of their activation and the microenvironmental context.
A schematic of signaling between a resting (freshly harvested) B cell or an activated (precultured with IL-4 and CD-40L) B cell and a T cell stimulated with anti-CD3/anti-CD28 Abs via the TCR. Note that ADO produced by resting B cells primarily exerts autocrine regulation, downmodulating B-cell functions via the A3R. Activated Teff cells rich in ADO deaminase hydrolyze ADO to inosine (INO) and thus avoid inhibition. In contrast, activated B cells enriched in ADO deaminase hydrolyze ADO to inosine and largely produce 5′-AMP, which binds to A1R on activated Teff and inhibits their functions.
A schematic of signaling between a resting (freshly harvested) B cell or an activated (precultured with IL-4 and CD-40L) B cell and a T cell stimulated with anti-CD3/anti-CD28 Abs via the TCR. Note that ADO produced by resting B cells primarily exerts autocrine regulation, downmodulating B-cell functions via the A3R. Activated Teff cells rich in ADO deaminase hydrolyze ADO to inosine (INO) and thus avoid inhibition. In contrast, activated B cells enriched in ADO deaminase hydrolyze ADO to inosine and largely produce 5′-AMP, which binds to A1R on activated Teff and inhibits their functions.
The dual ability of B cells to promote and curtail immune responses is known,1,14,15 but it has been considered to be a feature of a small subset of B cells (B10 cells) capable of producing immunosuppressive IL-10.31 Further, it has been recently proposed that signaling via the IL-21R drives B10 expansion and promotes their regulatory functions in vitro and in vivo in mice.15 This further emphasizes the role of cytokines in shaping the microenvironment populated by immune cells. Evidence also suggests that there is a link between IL-10 and ADO, because in IL-10–deficient (IL10−/−) mice, Treg have impaired ADO production and lack the ability to mediate suppression.32 Thus, it seems reasonable to suggest that there may be multiple mechanisms contributing to regulatory activity of B cells and that the existing redundancy may be necessary for providing a sufficient response magnitude when it is needed.
The potential role of B cell–derived ADO or other components of the ADO pathway in attenuating or promoting T-cell functions is of special interest, because it implies that all B cells, and not only a small B10-like cell subset,15 could participate in downregulation of activated T cells when required to do so. This focuses an even greater attention on B-cell/T-cell interactions long known to be critical for the outcome of immune responses. The participation of the ADO pathway in shaping these interactions and potential signals involved is illustrated in Figure 7. Although it is likely that other immune and nonimmune cells expressing ADOR in the microenvironment can also modulate these responses, their contextual nature involving a ubiquitous ADO pathway fits well with the current paradigm of immune cell plasticity that is environmentally determined. We are in the process of further examining the molecular mechanisms responsible for the role of this pathway in regulating B-cell/T-cell cooperation in human health and disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Institutes of Health (grant P01CA109688) (T.L.W.) and grants HL-109002, DK-091190, HL-069846, DK-068575, and DK-079307 (E.K.J.). HL grants are from the National Heart, Lung and Blood Institute; and DK grants are from the National Institute of Diabetes and Digestive and Kidney Diseases.
Authorship
Contribution: Z.S., P.J.S., C.-S.H., and D.C. performed experiments; Z.S. analyzed results and prepared the figures; and Z.S., E.K.J., and T.L.W. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Theresa L. Whiteside, University of Pittsburgh Cancer Institute, Research Pavilion at the Hillman Cancer Center, 5117 Centre Ave, Suite 1.27, Pittsburgh, PA 15232; e-mail: whitesidetl@upmc.edu.
References
Author notes
Z.S. and P.J.S. contributed equally to this study.