Key Points
Loss of PHD2 induces HIF-2α–induced erythrocytosis.
HIF-1α protects conditional PHD2-deficient mice.
Abstract
Erythropoiesis must be tightly balanced to guarantee adequate oxygen delivery to all tissues in the body. This process relies predominantly on the hormone erythropoietin (EPO) and its transcription factor hypoxia inducible factor (HIF). Accumulating evidence suggests that oxygen-sensitive prolyl hydroxylases (PHDs) are important regulators of this entire system. Here, we describe a novel mouse line with conditional PHD2 inactivation (cKO P2) in renal EPO producing cells, neurons, and astrocytes that displayed excessive erythrocytosis because of severe overproduction of EPO, exclusively driven by HIF-2α. In contrast, HIF-1α served as a protective factor, ensuring survival of cKO P2 mice with HCT values up to 86%. Using different genetic approaches, we show that simultaneous inactivation of PHD2 and HIF-1α resulted in a drastic PHD3 reduction with consequent overexpression of HIF-2α-related genes, neurodegeneration, and lethality. Taken together, our results demonstrate for the first time that conditional loss of PHD2 in mice leads to HIF-2α–dependent erythrocytosis, whereas HIF-1α protects these mice, providing a platform for developing new treatments of EPO-related disorders, such as anemia.
Introduction
Erythropoietin (EPO) is the primary regulator of red blood cell formation and its expression level is extremely responsive to fluctuations in tissue oxygenation. Dysregulation of EPO production can lead to either anemia, if levels are inadequately low, such as in terminal kidney failure or to erythrocytosis if levels are inappropriately high. Erythrocytosis will develop when EPO is continuously overproduced, causing the generation of new erythrocytes at a rate exceeding the removal of senescent red blood cells.1 In adult animals the main physiologic source of EPO is the kidney, although the liver as well as the central nervous system (CNS) have also been shown to produce EPO but to a much lesser extent.2,3 The main physiologic stimulus of EPO production is tissue hypoxia in the interstitium of the kidney.4 Already 2 decades ago it was shown that this effect was dependent on binding of the transcription factor hypoxia inducible factor (HIF) to 5′ and 3′-enhancer regions of the EPO gene, known as the hypoxia responsive element (HRE).5
HIF is a heterodimeric complex composed of an oxygen-sensitive HIFα and a constitutive HIFβ subunit. Of the most intensively studied HIF-α genes, HIF-1α has a ubiquitous pattern of expression in all tissues,6 whereas expression of the paralogue HIF-2α is restricted to certain cell types.7,8 However, both factors actively promote oxygen delivery and adaptive processes to hypoxia such as erythropoiesis, angiogenesis, anaerobic glycolysis, and hematopoiesis.1,9-11 Oxygen-sensing is therefore indispensable as it enables the cells to instantaneously adapt to an inappropriately low pO2. This machinery relies on the HIF-prolyl hydroxylases (PHD1-3), enzymes that hydroxylate, and consequently, lead to the inactivation of HIFα in the presence of oxygen.
PHD2 is believed to be the crucial oxygen sensor during normoxia and mild hypoxia,12 which is underscored by the fact that inactivation of PHD2 severely deregulates normal embryonic development resulting in embryonic lethality by E14.5, whereas PHD1−/− or PHD3−/− mice develop normally.13 Moreover, even haplodeficient PHD2+/− mice show normalization of the endothelial lining during tumor development compared with wild-type controls.14 In humans, several heterozygous point mutations in the PHD2 gene have been described, which lead to an absolute increase in red blood cell mass. These are in some cases associated with severe clinical conditions, such as hemorrhage, stroke, and increased risk of thrombosis,15-18 although the latter was shown to be independent of elevated hemoglobin/HCT.19 PHD2 is therefore the main HIF prolyl hydroxylase isoform which, next to HIF-2α and the von Hippel-Lindau protein (VHL), controls the expression of EPO in humans.20 Despite this, the role of PHD2 in the etiology of erythrocytosis and related disorders has not been extensively studied. Indeed, systemic PHD2 heterozygosity in mice shows only a very mild induction of hematocrits (HCTs),14 whereas somatic inactivation shortly before or after birth leads to severe erythrocytosis and early lethality.21,22
In this report, we describe a new conditional PHD2 mouse line expressing cre-recombinase under the control of the modified human CD68 promoter, commonly defined as a monocyte/macrophage marker.23 In addition, we found PHD2-loss in other cell lineages, resulting in excessive HIF-2α–induced EPO production in kidney and brain, extreme HCTs up to 86%, thrombocytopenia, and splenomegaly, but survival of the mice in a HIF-1α–dependent manner. Additional inactivation of HIF-1α (PHD2/HIF-1α double-knockout) leads to early lethality around adulthood, which is confined to PHD3-reliant HIF-2α–over-stabilization and consequent neurodegeneration in the brain.
Taken together, our results establish a principal role for PHD2 as an oxygen sensor in CD68-positive cells for the HIF-2α–dependent regulation of EPO and highlight a protective role of HIF-1α in polycythemic mice.
Methods
Generation of a CD68:cre line
The modified hCD68-IVS1 promoter,23 which combines the 2940 bp of sequence 5′ to the ATG and the 83-bp first intron of the human CD68 gene was cloned just in front a codon-improved Cre recombinase sequence (1091 bp)24 and a polyA signal. Different founders were produced and tested on expression levels in isolated peritoneal F4/80+ cells. Finally, 2 lines were selected of which 1 expressed cre-recombinase already in sperm as well as egg cells. The other transgenic line was intercrossed with PHD2f/f mice and used in the current report.
Mice
All mice were housed at the Experimental Center at the University of Technology Dresden (Medical Faculty, University Hospital Carl-Gustav Carus), under specific pathogen-free conditions. Experiments were performed with male and female mice at the age of 8 to 12 weeks or as stated in the text. All mice described in this report were born in a normal Mendelian manner. HIF-2αf/f mice were obtained from The Jackson Laboratory and originally produced in the research group of Dr C. Simon (Cell and Developmental Biology, University of Pennsylvania School of Medicine, Philadelphia, PA).25 All mouse strains were at least 9 generations backcrossed to C57BL/6. Mice were genotyped using primers described in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The construction and identification of the PHD2f/f mouse line will be described elsewhere (R.P.S, K.F., J.K., S.M., A.M., and B.W., unpublished results, June 2008).
More than 60% of all CD68:cre-PHD2f/f (cKO P2), CD68:cre-PHD2/HIF-1αff/ff (cKO P2/H1) and CD68:cre-PHD2/PHD3ff/ff (cKO P2/P3) mice showed redness of snout/paws associated with high HCT (HCT; > ∼ 70%) and were used for the described experiments. Nonerythrocytotic cKO, cKO P2/H1 and P2/P3 mice all showed HCTs comparable with WT littermates (< 55%), which was always related with lower penetrance of cre-recombinase activity and were only used for breeding. No cKO mice were found with HCTs between 55 and 70%. Penetrance in cKO P2/H1, CD68:cre-PHD2/HIF-2αff/ff (cKO P2/H2), CD68:cre-PHD2/PHD3ff/ff (cKO P2/P3), CD68:cre-HIF-1αf/f (cKO H1) and CD68:cre-HIF-2αf/f (cKO H2) mice was defined via qRT-PCR on BM mRNA and/or genomic PCR on ear biopsies. Knockdown efficiencies for HIF-1α and HIF-2α in these genotypes were comparable with expression levels for PHD2. It is noteworthy that only a low percentage (∼ 20%) of cKO P2/H2 mice showed cre activity with high penetrance on both alleles simultaneously. Only these mice were considered for the presented experiment. All other mice, carrying CD68:cre-PHD2f/f and HIF-2αf/f, showed no targeting or only of 1 of the alleles. All animal experiments were in accordance with the facility guidelines on animal welfare and were approved by the Landesdirektion Dresden, Germany.
Blood analysis
Blood parameters (HCT, RBCs, Hb, and thrombocytes) were measured using a Sysmex automated blood cell counter (Sysmex XE-2100 and XE-5000). Plasma erythropoietin and thrombopoietin concentrations were determined using the Quantikine Mouse/Rat EPO immunoassay and the Quantikine Mouse Tpo immunoassay (R&D Systems).
Blood pressure and heart rate were measured noninvasively with the tail cuff method (UGO BASILE). After training of the animals for 2 weeks, the average of at least 2 independent measurements was calculated.
Cells
The human kidney cell line REPC was cultivated as previously described.26 Astrocytes were isolated from the cerebral cortex of 5-day-old pups by incubation with 0.025% Trypsin for 30 minutes, followed by enrichment with the biotinylated anti-GLAST (ACSA-1) antibody (Miltenyi Biotec) and magnetic beads. The highly enriched astrocyte fraction (purity > 90%) was confirmed via FACS (data not shown).
Isolation of individual cell types
Primary mouse keratinocytes and fibroblasts were isolated from newborn mice by incubating the skin with 250 mg/mL Dispase (Roche) overnight at 4°C. The epidermal layer was separated from the dermal layer and incubated in TrypLE select enzyme (Invitrogen) for 30 minutes at room temperature. The resulting single-cell suspension was cultured in CnT medium (Cell-n-Tech). The dermal layer was finely minced and then incubated in a 0.25% trypsin/EDTA solution (Gibco) for 30 minutes at 37°C. The resulting cell suspension was cultured in DMEM (Lonza) containing 10% FBS (Biochrom), 1% L-Glutamine (Lonza), and 1% penicillin/streptomycin (Lonza). Identity of keratinocytes was confirmed via Keratin6 (K6) staining (data not shown). Enterocytes were isolated from 2 cm of colon starting from the cecum. The colon was cut into 5 to 6 pieces and incubated in Cell Recovery solution (BD Bioscience) for 1.5 hours at 4°C. After vigorous shaking, the cell suspension was filtered, centrifuged, and the resulting cell pellet frozen. Identity of the enterocytes (colonocytes) was confirmed via Villin1 staining (data not shown). Endothelial cells were isolated from the lung via FACS (CD31+CD34+) and confirmed based on CD31 mRNA expression (data not shown).
Expression analysis
RNA from organs and sorted cells was isolated using RNeasy Mini Kit (QIAGEN), NucleoSpin RNA XS (Machery-Nagel), Trizol (Invitrogen), Universal RNA Purification Kit (roboklon), or DNA+RNA+PROTEIN Purification Kit (roboklon). cDNA was synthesized using random primers (Roche) and SuperScript II (Invitrogen). Expression levels were determined by performing quantitative real-time PCR using the Maxima SYBR Green QPCR Master Mix (Fermentas) on an iCycler iQ (Biorad). Sequences of primers used are given in supplemental Table 2. Expression levels were normalized with the ΔΔCt method using primers given in supplemental Table 3.
Histology and immunofluorescence
For all samples, organs were placed in 4% formaldehyde at 4°C overnight, dehydrated, embedded in paraffin and cut. Sections were rehydrated and subjected to hematoxylin and eosin staining or acidic Fuchsin orange G-staining. For IHC, antigen retrieval (citrate buffer-PH 6.0) at 95°C for 20 minutes was performed on rehydrated sections. Incubation with primary antibody (Ab; Iba1, WAKO; cleaved-Caspase3,Cell Signaling; NeuN, Abcam) was 1.5 hours at 37°C. Secondary Abs were Alexa-488 or Alexa-568 (Molecular Probes) incubated for 30 minutes at room temperature. Cell nuclei were stained with DAPI. Slides were mounted with fluorescent mounting media (Dako). For frozen sections, samples were embedded in OCT, cut and stored at −20°C. For immunofluorescence staining, sections were first fixed for 10 minutes in cold acetone and blocked with 5% goat serum in TNT buffer (20mM Tris pH 7.6, 0.9% NaCl, 0.05% Tween in PBS). Thereafter, sections were incubated with mouse anti-NeuN and rabbit anti-PHD2 at 37°C for 1 hour (custom-made polyclonal rabbit anti–mouse antibody against a C-terminal peptide of PHD2; EKGVRVELKPNSVSKDV) and purified via a peptide sulfoLink immobilization column (Pierce, ThermoScientific). Secondary antibody as described.
Microscopy
Fluorescent and light microscopy was done with an Axioplan-2 imaging microscope and plan Apochromat lenses (Carl Zeiss). The cameras and acquisition software were either Q Imaging Retiga 2000R and Image pro MC6.0 (fluo) or Axiocam MRc5 and Axiovision (light). Image processing and analysis was done using ImageJ 1.45s and the ImageJ distribution Fiji (http://pacific.mpi-cbg.de/wiki/index.php/Fiji).
Flow cytometry
FACS analysis was performed on LSRII (Becton Dickinson), sorting was done on Aria II (Becton Dickinson). Cell numbers were counted on MACS quant (Miltenyi). Data were analyzed on DIVA 7.0 (Becton Dickinson), MACS quantify Version 3.0 (Miltenyi), or FlowJo Version 9.6.1 (TreeStar) software. BM single-cell suspensions were made by flushing the marrow using 23G needles. Single-cell suspensions from spleen and lymph nodes were prepared by manual disruption using frosted slide ends, additionally for spleen cells enzymatic digestion was performed with 0.3 U/mL Collagenase D (Roche) for 40 minutes at 37°C. The lineage cocktail included CD3 (145-2C11, eBioscience), CD19 (eBio1D3, eBioscience), NK1.1 (PK136, eBioscience), Ter119 (Terr119, eBioscience), CD11b (M1/70, eBioscience), Gr1 (RB6-8C5, eBioscience), B220 (RA3-6B2, eBioscience), CD127 (A7R34, eBioscience), and CD11c (N418, eBioscience). For MEP analysis, spleen and bone marrow cells along with lineage cocktail were also incubated with c-kit (APC, 2B8, eBioscience), Sca1 (Pe-Cy7, D7, eBioscience), CD16/32 (A700, 93, eBioscience), CD34 (FITC, RAM34, eBioscience), and SA (PerCP, BD Bioscience). One × 106 cells from bone marrow and 2 × 106 cells from spleen were acquired. For EB analysis, spleen and bone marrow cells were stained with CD71 (FITC, R17217, eBioscience) and Ter119 (Pe-Cy7, Ter119, eBioscience). Two × 105 cells were acquired. For lymphoid cell analysis, spleen, and lymph node cells were incubated with CD3 (APC, 145-2c11, eBioscience) and CD19 (FITC, MB19-1, eBioscience). For myeloid cell analysis, spleen cells were incubated with CD11b (PE, M1/70, eBioscience), F4/80 (PerCP-cy5.5, BM8, eBioscience), and/or Gr1 (A700, RB6-8C5, eBioscience).
Blood volume determination
Blood was collected in EDTA vials, washed twice with 0.9% NaCl, and stained with 15uM Vybrant DiI Cell-labeling solutions (Invitrogen) for 2.5 hours at room temperature on a tilt shaker. After washing and resuspension in PBS 100 μL was injected intravenously. One hour later a blood sample was taken from the mouse, diluted, and analyzed for DiI containing cells.
Statistical analysis
Data and graphs represent mean ± SEM of representative experiments. Statistical significance was calculated as 2-tailed by the Mann-Whitney U test (GraphPad Prism Version 5.04), with P < .05 considered statistically significant.
Results
Conditional PHD2-deficient mice display severe HIF-2α–dependent erythrocytosis
PHD2 is the main HIF prolyl hydroxylase isoform, which controls the expression of EPO in humans.20 Somatic inactivation of PHD2 shortly before or after birth leads to severe polycythemia probably in a HIF-1α–dependent manner but is accompanied by early lethality and has therefore not been extensively studied.21,22 To conditionally ablate PHD2, we first generated a PHD2f/f mouse line by gene targeting using a construct in which exons 2 and 3 are flanked by loxP sites (R.P.S, K.F., J.K., S.M., A.M., and B.W., unpublished results, June 2008). We combined this with a newly designed mouse line expressing cre-recombinase under the control of the modified human CD68 promoter, known as a monocyte/macrophage marker (supplemental Figure 1A). Thorough analyses revealed that these cKO P2 mice are not only significantly deficient for PHD2 in macrophages, but in the entire hematopoietic system (bone marrow; supplemental Figure 1B). In addition, we also found a profound PHD2 reduction in a few subsets of epithelial cells (eg, keratinocytes and colonocytes), but not in other cell lineages, such as endothelial cells (ECs) and fibroblasts (supplemental Figure 1C). In the lysates of nonhematopoietic organs studied in the current report, we found no significant reduction of PHD2 expression (supplemental Figure 1D). Moreover, no significant compensation by one of the other PHDs could be shown (supplemental Figure 1E)
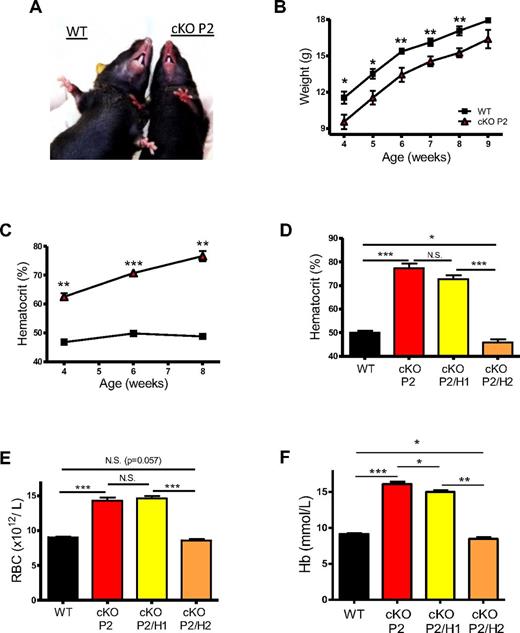
Interestingly, the majority of these cKO P2 mice showed obvious redness of paws and snout starting around 4 weeks of age, which was accompanied by significant growth retardation until adulthood (Figure 1A-B). This striking phenotype was because of elevated HCTs which increased with age to > 85% in some individuals (Figure 1C). However, although the blood viscosity was substantially increased, we observed no difference in the blood pressure, heart rate, or cardiac hypertrophy, as has been shown before for other erythrocytotic mice (supplemental Figure 2A-B).27 As PHD2 can control the activity of both HIF-1α and HIF-2α we determined which of the HIFs contributed to the erythrocytosis. The notion that HIF-2α acts as the main regulator of EPO is based on histologic and genetic approaches in HIF-2α–deficient mice.10,25,28 Moreover, several studies in patients with idiopathic erythrocytosis revealed the presence of different heterozygous missense mutation in the coding sequence of HIF-2α, but not HIF-1α, leading to impaired PHD2-induced hydroxylation.29,30 On the other hand, a somatic knockout of PHD2, suggested that HIF-1α, rather than HIF-2α, contributed to increased renal EPO synthesis.21 To unequivocally determine the major regulator in the erythrocytotic cKOs, we therefore generated mice double deficient for PHD2 and HIF-1α or HIF-2α. Our data demonstrate that the erythrocytosis phenotype as shown in cKO P2 mice was abolished in CD68:cre-PHD2/HIF-2αff/ff mice (cKO P2/H2), displaying even significantly lower HCTs and hemoglobin levels than in WT mice (Figure 1D-F). On the other hand, HCTs in CD68:cre-PHD2/HIF-1αff/ff mice (cKO P2/H1) were indistinguishable from cKO P2, showing that PHD2-induced severe erythrocytosis is exclusively dependent on HIF-2α but not on HIF-1α. These results are emphasized by the fact that conditional knock-down of HIF-2α alone (CD68:cre-HIF-2αf/f [cKO H2]), but not HIF-1α (CD68:cre-HIF-1αf/f [cKO H1]), already resulted in a mild anemia (supplemental Figure 3A-C).
Conditional deficient PHD2 mice display severe HIF-2α–induced erythrocytosis. (A) Compared with WT mice, cKO P2 show profound redness of the snout and paws. (B) Body weight of WT and cKO P2 mice was measured on a weekly basis until adulthood. No significant difference was observed after week 8 (n = 5-11). (C) Percentage of red blood cell volume per blood volume (HCT-hct) from mice was measured every 2 weeks until adulthood, displaying highly significant differences from week 4 after birth (n = 5-10). (D) HCTs in cKO P2/H1 remained as high as in cKO P2 mice whereas cKO P2/H2 mice showed even slightly but significantly lower HCTs than WT (n = 6-15). (E-F) RBC count (n = 7-32) and hemoglobin (n = 6-22) concentration in freshly isolated blood samples demonstrate that HIF-2α but not HIF-1α induces erythrocytosis because of loss of PHD2. All data are mean ± SEM (*P < .05, **P < .01, ***P < .001).
Conditional deficient PHD2 mice display severe HIF-2α–induced erythrocytosis. (A) Compared with WT mice, cKO P2 show profound redness of the snout and paws. (B) Body weight of WT and cKO P2 mice was measured on a weekly basis until adulthood. No significant difference was observed after week 8 (n = 5-11). (C) Percentage of red blood cell volume per blood volume (HCT-hct) from mice was measured every 2 weeks until adulthood, displaying highly significant differences from week 4 after birth (n = 5-10). (D) HCTs in cKO P2/H1 remained as high as in cKO P2 mice whereas cKO P2/H2 mice showed even slightly but significantly lower HCTs than WT (n = 6-15). (E-F) RBC count (n = 7-32) and hemoglobin (n = 6-22) concentration in freshly isolated blood samples demonstrate that HIF-2α but not HIF-1α induces erythrocytosis because of loss of PHD2. All data are mean ± SEM (*P < .05, **P < .01, ***P < .001).
Erythrocytosis is EPO dependent and associated with increased EPO expression in kidney and brain
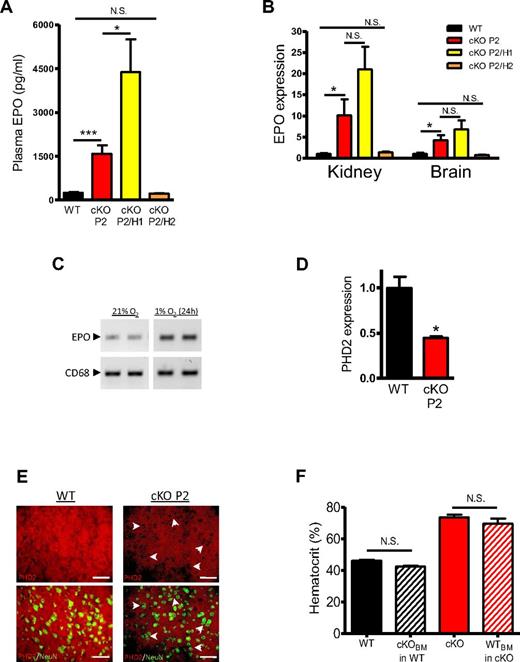
Because excessive secondary erythropoiesis is mainly mediated by EPO,27 we measured EPO plasma levels in both erythrocytotic mouse lines (cKO P2 and cKO P2/H1) and found a highly significant induction of EPO expression in cKO P2 mice compared with WT littermates. Surprisingly, although cKO P2/H1 mice showed similar HCTs to cKO P2 mice, they contained on average 2.5-fold higher plasma EPO values than cKO P2 mice (Figure 2A). The main source of EPO in adult mice is the kidney,3 where we found a 10-fold and 20-fold increase of EPO in extracts from cKO P2 and from cKO P2/H1 mice, respectively. In addition, the expression of EPO in the brain was significantly up-regulated (Figure 2B). In contrast, hepatic EPO expression levels were very low, and no increase could be observed in any of the mice (data not shown).
Loss of PHD2 in cKO P2 mice leads to induction of EPO in kidney and brain. (A) EPO concentration in plasma measured by ELISA in the plasma of WT, cKO P2, cKO P2/H1, and cKO P2/H2 mice (n = 6-22). cKO mice contain on average 6 times more EPO in the circulation than their WT littermates, whereas cKO P2/H1 mice have more than 17 times more EPO. No difference between WT and cKO P2/H2 was found. (B) mRNA levels in total extracts from kidney and brain (n = 5-15) is markedly induced compared with WT but not significantly different between cKO P2 and cKO P2/H1 mice because of the high variation between individual samples (n = 7-11). No difference between WT and cKO P2/H2 could be shown. (C) The renal-derived human cell line (REPC)26 was tested for the expression of EPO and CD68 grown under normoxic and hypoxic conditions via RT-PCR, showing the expected EPO induction and expression of CD68 under both conditions, suggesting a potential link between cre-expression, PHD2 inactivation and subsequent EPO expression in these cell types. (D) Astrocytes isolated from the cerebral cortex of 5 day old pups show a significantly reduced PHD2 content in cKO P2 versus WT mice (n = 3). (E) Typical IHC on brain sections for PHD2 (red) with or without NeuN (green) show a majority of PHD2-negative neurons in the brain of cKO P2 mice (depicted by arrow heads) but not in WT. (F) WT and cKO P2 mice were lethally irradiated and received cKO P2 or WT bone marrow (BM) respectively. HCTs were measured 4 months after transfer. BM from either genotype did not change HCTs in the recipient (n = 6-23). Scale bar in (E) represents 50 μm. All data are mean ± SEM (NS indicates not significant; *P < .05, ***P < .001).
Loss of PHD2 in cKO P2 mice leads to induction of EPO in kidney and brain. (A) EPO concentration in plasma measured by ELISA in the plasma of WT, cKO P2, cKO P2/H1, and cKO P2/H2 mice (n = 6-22). cKO mice contain on average 6 times more EPO in the circulation than their WT littermates, whereas cKO P2/H1 mice have more than 17 times more EPO. No difference between WT and cKO P2/H2 was found. (B) mRNA levels in total extracts from kidney and brain (n = 5-15) is markedly induced compared with WT but not significantly different between cKO P2 and cKO P2/H1 mice because of the high variation between individual samples (n = 7-11). No difference between WT and cKO P2/H2 could be shown. (C) The renal-derived human cell line (REPC)26 was tested for the expression of EPO and CD68 grown under normoxic and hypoxic conditions via RT-PCR, showing the expected EPO induction and expression of CD68 under both conditions, suggesting a potential link between cre-expression, PHD2 inactivation and subsequent EPO expression in these cell types. (D) Astrocytes isolated from the cerebral cortex of 5 day old pups show a significantly reduced PHD2 content in cKO P2 versus WT mice (n = 3). (E) Typical IHC on brain sections for PHD2 (red) with or without NeuN (green) show a majority of PHD2-negative neurons in the brain of cKO P2 mice (depicted by arrow heads) but not in WT. (F) WT and cKO P2 mice were lethally irradiated and received cKO P2 or WT bone marrow (BM) respectively. HCTs were measured 4 months after transfer. BM from either genotype did not change HCTs in the recipient (n = 6-23). Scale bar in (E) represents 50 μm. All data are mean ± SEM (NS indicates not significant; *P < .05, ***P < .001).
In the kidney, EPO is produced by specialized interstitial cells localized in the deep cortex between the proximal tubular and vascular endothelial cells. These renal EPO producing (REP) cells also express neuronal-specific markers but isolation of these primary cells has been a real challenge so far.31 Therefore, we tested the recently described human REP cell line, isolated from the tumor-free tissue of a male patient26 and demonstrate that these cells coexpressed both CD68 and EPO, supporting the link between CD68-driven cre expression, PHD2 inactivation and subsequent EPO expression in our cKO P2 mice (Figure 2C). In the brain, astrocytes and neurons are known to be sources of EPO.32 We therefore isolated astrocytes from the cerebral cortex of 5-day-old pups and found a significant PHD2 reduction in cKO P2 cells versus WT (Figure 2D). The latter result was similar to the knockdown efficiency found in other cell types (supplemental Figure 1B-C). Via IHC we demonstrate that sections from adult cKO P2 brains contain a majority of PHD2-negative cells that were positive for the neuronal marker NeuN (Figure 2E).
Moreover, because cKO P2 mice are also deficient for PHD2 in the BM, we transplanted BM cells from cKO P2 mice into lethally irradiated WT mice and vice-versa. However, we found no significant difference in HCT compared with nontransplanted animals, thus excluding the possibility that the phenotype could be transferred with PHD2-deficient hematopoietic cells (Figure 2F).
Taken together, the erythrocytosis phenotype in cKO P2 and cKO P2/H1 mice is correlated with an enormous induction of EPO in the kidney and the brain, related to PHD2 inhibition in REP cells, astrocytes, and neurons.
Erythrocytosis is mainly caused by extramedullary erythropoiesis.
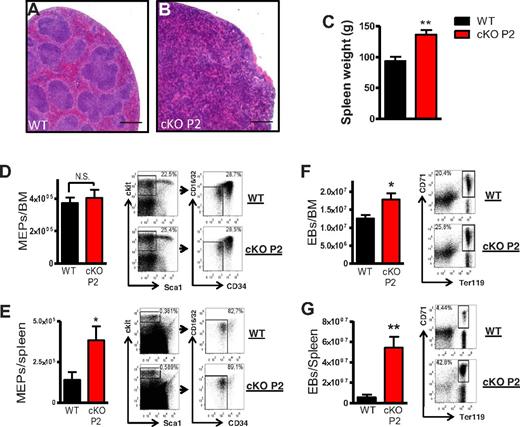
Basal erythropoiesis mainly takes place in the bone marrow, whereas the spleen can increase its capability to produce RBCs under conditions of stress erythropoiesis.33 In the cKO P2, polycythemia resulted in splenomegaly accompanied by loss of splenic architecture, with an increase in nucleated cells and total weight (Figure 3A-C). Remarkably, the megakaryocyte-erythroid progenitors (MEPs) were found to be significantly induced (> 2.5-fold) in the spleen of cKO P2 mice but not in BM (Figure 3D-E). In addition, splenic CD71+/Ter119+ erythroblasts (EBs) were increased more than 10-fold in cKO P2 mice compared with WT littermates, whereas only a mild increase in EBs was found in BM (Figure 3F-G). The fraction of B and T cells were markedly decreased in the cKO P2 spleen, whereas lymph nodes contained an excess of both populations, suggesting that there is no impairment of their production but only a difference in localization because of the EB excess in the spleen (supplemental Figure 4A-D). Splenic CD11b+F4/80+ macrophages and neutrophils (PMNs) were not significantly altered, although red pulp macrophages, a distinct subset of macrophages involved in the removal of senescent erythrocytes, were highly induced in the cKO P2 spleen (supplemental Figure 4E-G). These results imply that the spleen is primarily responsible for the overproduction of RBCs in cKO P2 mice.
Erythrocytosis is mainly caused by extramedullary erythropoiesis in the spleen. (A-B) Representative sections of WT and cKO P2 spleens (H&E) show an extensive structural loss and an augmentation of nucleated cells in erythrocytotic cKO P2 mice. (C) Spleen weight of WT and cKO P2 mice (n = 10-16). (D-G) Representative FACS staining profiles of (D and E) megakaryocyte-erythrocyte progenitors (MEP; n = 6) and (F-G) erythroblasts (EB; n = 7) in BM and spleen of WT and cKO P2 showing that the overproduction of RBCs is mainly executed in the spleen. Scale bars represent 500 μm. All data are mean ± SEM (*P < .05, ***P < .001).
Erythrocytosis is mainly caused by extramedullary erythropoiesis in the spleen. (A-B) Representative sections of WT and cKO P2 spleens (H&E) show an extensive structural loss and an augmentation of nucleated cells in erythrocytotic cKO P2 mice. (C) Spleen weight of WT and cKO P2 mice (n = 10-16). (D-G) Representative FACS staining profiles of (D and E) megakaryocyte-erythrocyte progenitors (MEP; n = 6) and (F-G) erythroblasts (EB; n = 7) in BM and spleen of WT and cKO P2 showing that the overproduction of RBCs is mainly executed in the spleen. Scale bars represent 500 μm. All data are mean ± SEM (*P < .05, ***P < .001).
Thrombocytopenia is directly related to the higher blood volume
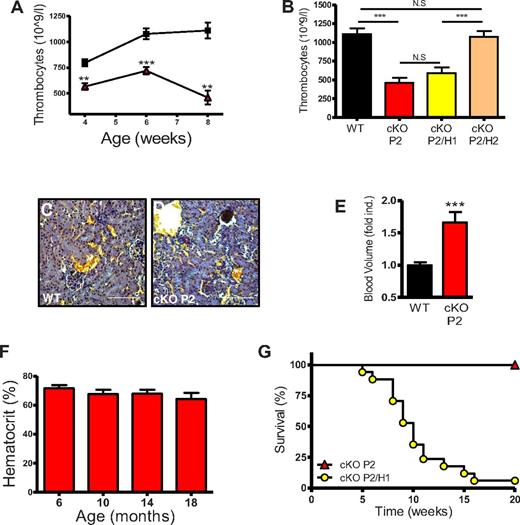
Further hematologic analysis revealed a significant decrease in platelet numbers in cKO P2 mice compared with WT mice, as early as at 4 weeks of age (Figure 4A). This effect was also related to HIF-2α but not HIF-1α, because the phenotype was rescued only in the cKO P2/H2 mice (Figure 4B). To examine the molecular background of this effect we analyzed different parameters but found no relative difference in thrombopoietin (TPO), the number of megakaryocytes in the BM or spleen (supplemental Figure 5A-C), nor did we detect microvascular thrombosis, which would have indicated increased consumption of platelets (Figure 4C-D). However, using flow cytometry on labeled RBCs, we found that the total blood volume in cKO P2 mice was significantly increased by approximately 65% (Figure 4E); strongly suggesting that the reduced platelet count is a dilution effect.
cKO P2 mice show thrombocytopenia but no early lethality, whereas cKO P2/H1 die prematurely. (A) Thrombocyte concentrations in the blood of WT and cKO P2 mice until adulthood reveal a significant reduction of circulating platelets per volume of blood in cKO P2 mice (n = 5-10). WT is represented by black squares and cKO P2 by red triangles. (B) Eight- to 10-week-old mice were bled and thrombocytes measured in a fixed volume of blood showing thrombocytopenia in cKO and cKO P2/H1 mice compared with WT and cKO P2/H2 mice (n = 6-7). (C-D) WT and cKO P2 kidneys stained with acid Fuchsin orange-G show no signs of microvascular thrombosis suggesting that platelets are not trapped within blood clots. (E) Instead, the total blood volume of cKO P2 mice was significantly increased compared with WT mice (n = 9-10). (F) 9 cKO P2 mice (5 females and 4 males) were kept for 20 months. Mice were bled every 4 months to measure HCTs. No significant differences in HCTs were found and none of the mice died before the end of the experiment. (G) cKO P2/H1 (n = 17) and cKO P2 (n = 15) mice were followed for 20 weeks. cKO P2/H1 mice began to die from week 5 after birth. By week 16, 16 of 17 mice were deceased. No cKO P2 mice died during the course of this experiment. Scale bars represent 100 μm. All data are mean ± SEM (**P < .01, ***P < .001).
cKO P2 mice show thrombocytopenia but no early lethality, whereas cKO P2/H1 die prematurely. (A) Thrombocyte concentrations in the blood of WT and cKO P2 mice until adulthood reveal a significant reduction of circulating platelets per volume of blood in cKO P2 mice (n = 5-10). WT is represented by black squares and cKO P2 by red triangles. (B) Eight- to 10-week-old mice were bled and thrombocytes measured in a fixed volume of blood showing thrombocytopenia in cKO and cKO P2/H1 mice compared with WT and cKO P2/H2 mice (n = 6-7). (C-D) WT and cKO P2 kidneys stained with acid Fuchsin orange-G show no signs of microvascular thrombosis suggesting that platelets are not trapped within blood clots. (E) Instead, the total blood volume of cKO P2 mice was significantly increased compared with WT mice (n = 9-10). (F) 9 cKO P2 mice (5 females and 4 males) were kept for 20 months. Mice were bled every 4 months to measure HCTs. No significant differences in HCTs were found and none of the mice died before the end of the experiment. (G) cKO P2/H1 (n = 17) and cKO P2 (n = 15) mice were followed for 20 weeks. cKO P2/H1 mice began to die from week 5 after birth. By week 16, 16 of 17 mice were deceased. No cKO P2 mice died during the course of this experiment. Scale bars represent 100 μm. All data are mean ± SEM (**P < .01, ***P < .001).
cKO P2 mice display a normal life span whereas cKO P2/H1 die early.
One common feature of the somatic PHD2-deficient mice21,22 as well as other EPO-related erythrocytotic mice34,35 is that these mice die prematurely. Surprisingly, we never observed any premature death in our cKO P2 mice. Moreover, we did not encounter any lethality in a group of 9 erythrocytotic cKO P2 mice that were maintained for more than 20 months. These mice were bled every 4 months but no significant drop in HCT could be observed (Figure 4F). Finally, all mice were killed and several organs examined in great detail. Except for a regressing cyst associated with the ovary of 1 mouse (supplemental Figure 6A), no obvious pathologic defects or tumors were detected (supplemental Figure 6B). On the other hand, the cKO P2/H1 mice started to die very sudden from week 5 onward, and by week 16, 95% of these mice were dead (mean survival time: 11 weeks; Figure 4G). In contrast, neither cKO P2/H2 nor cKO H1 or H2 mice showed any premature death. Importantly, these results show for the first time that extremely high HCTs do not necessarily lead to lethality or pathologic abnormalities in part because of a protective function of HIF-1α.
Simultaneous loss of PHD2 and HIF-1α in the brain leads to neurodegeneration
To investigate the biologic background of the early lethality of cKO P2/H1 mice, we conducted a thorough examination of 8 to 10-week-old living as well as recently deceased cKO P2/H1 mice. No acute cardiovascular complications or obvious evidence for thrombosis or degeneration in different examined organs were observed (data not shown). However, via IHC staining we detected a dramatic induction of activated (Iba1+) microglia cells in the brain of cKO P2/H1 mice compared with WT and cKO P2 mice (Figure 5A-C). This strongly suggests that pathologic changes in cKO P2/H1 brains led to the activation of these resident macrophages. Consistent with this, we found numerous patches of neurons (NeuN+) in cKO P2/H1 brain sections, especially from freshly deceased mice, that showed strong expression of cytoplasmic cleaved-caspase3 (Cl-Casp3), indicative of apoptosis (Figure 5D-F). In contrast, no Cl-Casp3 staining could be detected in cKO P2 or WT brains (data not shown). In line with the observed phenotype, we found that some (3 of 16) of the cKO P2/H1 mice even displayed a dome-shaped skull accompanied by a dramatic hydrocephalus (supplemental Figure 7A-B).
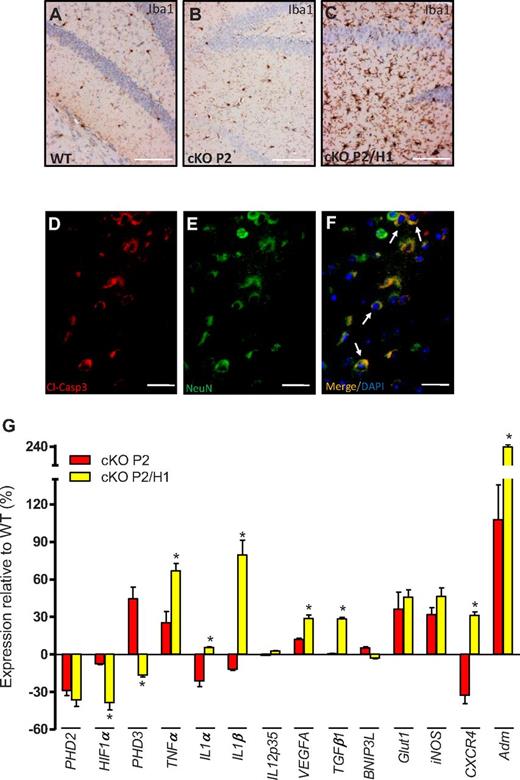
HIF1a serves as a protective factor to prevent brain damage. (A-C) IHC on brain sections from WT, cKO P2, and cKO P2/H1 for Iba1 (brown) showing more activated microglia cells in brain sections of a cKO P2/H1 mouse. (D-F) IHC on brain sections from a freshly deceased cKO P2/H1 mouse for (D) cleaved-caspase3 (cl-Casp3; red), (E) neurons (NeuN; green), and (F) a merged picture combined with DAPI (white arrows depict typical examples of cl-Casp3+ neurons). No obvious cl-Casp3 staining was detected in WT or cKO P2 brains (data not shown). (G) Expression profile (qRT-PCR) of different genes in the lysate of the entire brain of cKO P2 and cKO P2/H1 mice in relation to their respective WT littermates (n = 5-9). All data are mean ± SEM (*P < .05). Scale bars in panels A through C, 100 μm; panels D through F, 50 μm.
HIF1a serves as a protective factor to prevent brain damage. (A-C) IHC on brain sections from WT, cKO P2, and cKO P2/H1 for Iba1 (brown) showing more activated microglia cells in brain sections of a cKO P2/H1 mouse. (D-F) IHC on brain sections from a freshly deceased cKO P2/H1 mouse for (D) cleaved-caspase3 (cl-Casp3; red), (E) neurons (NeuN; green), and (F) a merged picture combined with DAPI (white arrows depict typical examples of cl-Casp3+ neurons). No obvious cl-Casp3 staining was detected in WT or cKO P2 brains (data not shown). (G) Expression profile (qRT-PCR) of different genes in the lysate of the entire brain of cKO P2 and cKO P2/H1 mice in relation to their respective WT littermates (n = 5-9). All data are mean ± SEM (*P < .05). Scale bars in panels A through C, 100 μm; panels D through F, 50 μm.
To unravel the molecular background of this neurodegeneration, we compared the gene profiles of cKO and cKO P2/H1 brains. Remarkably, PHD3, a HIF-1α–regulated gene and formerly identified as a very potent negative regulator of HIF-2α,36 was drastically down-regulated in cKO P2/H1 brains compared with cKO P2 counterparts. Conversely, we found a significant induction of proinflammatory cytokines, such as TNFα, Il-1α, and IL-1β, as well as VEGFA, TGFβ1, CXCR4, and adrenomedullin (Adm; Figure 5G), many of which have been shown to be HIF-2α–dependent genes.37 These data therefore suggest that knocking out HIF-1α in addition to PHD2 in a subset of brain cells (eg, astrocytes and microglia cells) leads to an additional stabilization of HIF-2α and consequent transcriptional activation of a variety of genes, including detrimental inflammatory cytokines that can contribute to neurodegeneration and lethality of the cKO P2/H1 mice.
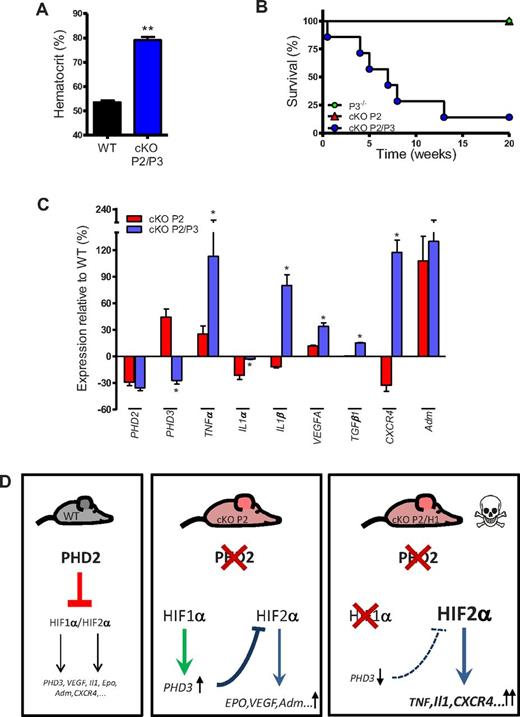
To evaluate whether HIF-1α–induced PHD3-expression is directly related to the protection of cKO P2 mice, we developed mice lacking PHD2 and PHD313 in CD68+ cells (CD68:cre-PHD2/PHD3ff/ff [cKO P2/P3]). Interestingly, these mice did not only display severe erythrocytosis (Figure 6A); a majority of them suddenly died starting shortly after birth (Figure 6B), whereas cKO P2 as well as PHD3−/− mice never died during this period. Furthermore, we isolated the brains of these cKO P2/P3 mice and analyzed the same array of genes as we studied for the cKO P2/H1 animals. Interestingly, we found that virtually all differentially expressed genes in brain lysates from cKO P2/H1 mice are also significantly changed in the brains of cKO P2/P3 mice, compared with cKO P2 (Figure 6C). These data strengthen our hypothesis that PHD3 is a not only a protective factor in the brain of cKO P2 mice, but acts downstream of HIF1α. Taken together, these results reveal a tight and essential balance between HIF-1α and HIF-2α activity in the brain, which also involves the PHD3 regulation.
Mice deficient for PHD2 and PHD3 display erythrocytosis and die prematurely. (A) cKO P2/P3 mice show enhanced HCTs compared with their WT littermates (n = 5). (B) WT, PHD3−/− and cKO P2/P3 mice were followed for 20 weeks. cKO P2/P3 mice began to die shortly after birth (n = 7). (C) Expression profile (qRT-PCR) of different genes in the lysate of the entire brain of cKO P2 and cKO P2/P3 mice in relation to their respective WT littermates. *P < .05 is significantly different from cKO P2 samples (expression relative to their WT littermates; n = 5-7). (D) Schematic overview of the different genetic mouse models that were generated in this study showing nonlethal erythrocytotic cKO P2 mice that display a perfect balance between the protective HIF-1α activity and detrimental HIF-2α activity in the brain. In cKO P2/H1 mice, this balance is disturbed in favor of the HIF-2α activity leading to early lethality in the mice. All data are mean ± SEM (*P < .05, **P < .01).
Mice deficient for PHD2 and PHD3 display erythrocytosis and die prematurely. (A) cKO P2/P3 mice show enhanced HCTs compared with their WT littermates (n = 5). (B) WT, PHD3−/− and cKO P2/P3 mice were followed for 20 weeks. cKO P2/P3 mice began to die shortly after birth (n = 7). (C) Expression profile (qRT-PCR) of different genes in the lysate of the entire brain of cKO P2 and cKO P2/P3 mice in relation to their respective WT littermates. *P < .05 is significantly different from cKO P2 samples (expression relative to their WT littermates; n = 5-7). (D) Schematic overview of the different genetic mouse models that were generated in this study showing nonlethal erythrocytotic cKO P2 mice that display a perfect balance between the protective HIF-1α activity and detrimental HIF-2α activity in the brain. In cKO P2/H1 mice, this balance is disturbed in favor of the HIF-2α activity leading to early lethality in the mice. All data are mean ± SEM (*P < .05, **P < .01).
Discussion
In this study, we used a genetic approach to investigate in detail the biologic role of PHD2 in CD68-positive cells and the relation of 2 of its substrates (HIF-1α and HIF-2α) with erythropoietin production and during erythropoiesis. We have demonstrated that conditional PHD2-deficient mice are able to cope with extreme numbers of RBCs, thrombocytopenia, and splenomegaly through a discrete interplay between HIF-1α and HIF-2α involving the regulation of PHD3.
Although CD68 has been described as a marker mainly limited to monocytic cells,23 our data demonstrate at least temporary expression in the hematopoietic compartment as well as in subsets of epithelial lineages, but not in ECs or fibroblasts. Moreover, our results demonstrate loss of PHD2 in a subset of renal and brain cells responsible for the production of EPO, which leads to massive stress erythropoiesis primarily executed by the adult spleen. Remarkably and in clear contrast to many other reports,3,21,22,35 our erythrocytotic cKO P2 mice show normal lifespan, demonstrating that an unusual high HCT is not per se lethal; a phenomenon that has also been described in a few healthy cobalt miners at high altitude that developed HCTs ranging from 75% to 91%.38 Genetically, several heterozygous PHD2 missense mutations have been defined in patients, which impair the binding to both HIF-1α and HIF-2α resulting in erythrocytosis.15-18 One patient with such a PHD2 mutation and consequent polycythemia developed a paraganglioma, suggesting that PHD2 may act as a tumor suppressor.1 However, our results strongly suggest that sustained exposure to high levels of EPO and permanent loss of PHD2 in different cell types does not necessarily lead to spontaneous tumor development over a period of 20 months in mice. This has also been underscored by recent work from our group showing that silencing of PHD2 in several tumor lines typically leads to reduced tumor growth.39
In mice, we (K.F. and B.W., unpublished data, October 2011) and others14 have shown that PHD2 heterozygosity only results in a very mild form of erythrocytosis whereas global inactivation just before or after birth leads to severe and lethal polycythemia.21,22 Based on expression levels in the kidney of the latter mice, it was proposed that HIF-1α, rather than HIF-2α, might be the central erythrocytosis mediator in relation to PHD2.21 However, our genetic approach (PHD2/HIFα double-deficient mice) clearly shows that this phenotype is driven by HIF-2α. The notion that only HIF-2α induces EPO is not new,25,28 and is in line with observations from Percy and colleagues in familial erythrocytosis.29,30 However, to the best of our knowledge this is the first report that shows that PHD2-induced severe erythrocytosis is exclusively dependent on HIF2α and not on HIF1α. In addition, the finding that CD68:cre-HIF-2αf/f mice were mildly anemic emphasizes the importance of this HIF-subunit and confirms the, at least temporary, activity of CD68, in specialized EPO-producing cells.
Simultaneous ablation of PHD2 and HIF-1α on the other hand led to early lethality, clearly demonstrating that HIF-1α acts as a crucial survival factor in the erythrocytotic cKO P2 mice. In particular, detailed analysis of mature cKO P2/H1 mice showed pathologic defects only in the brain, a feature that was underlined by the severe hydrocephalus we observed in a few of the cKO P2/H1 mice. We found that a majority of cKO P2/H1 mice contained activated microglia cells and patches of apoptotic neurons, the latter especially evident in recently deceased mice. A gene-expression profile of individual brains showed clear induction of different proinflammatory cytokines (eg, TNFα and IL-1β) in cKO P2/H1 mice compared with cKO P2 mice. These cytokines are known as main contributors in several neurodegenerative disorders and are typically produced by glia cells, including microglia and astrocytes, as well as neurons.40-43 In addition, the vascular growth factor VEGFA,44 the chemokine receptor CXCR4,45-47 and the multifunctional growth factor TGFβ1,48,49 were overexpressed in cKO P2/H1 brains, and are related to the initiation and/or secondary phase of neurodegeneration. Interestingly, many of these genes, including EPO, have been described as HIF-2α–related genes,25,37 suggesting that loss of HIF-1α enhances HIF-2α activity, causing brain damage as well as lethality of these mice. This idea is further supported by the observation that the HIF-1α–inducible gene, PHD3, and known to be an excellent negative regulator of HIF-2α,36 is down-regulated in the brain of cKO P2/H1 compared with cKO P2 mice. In addition, cKO P2/P3 mice also display severe erythrocytosis, die prematurely, and show a similar induction of the array of genes induced in the brain of cKO P2/H1. This strongly suggests that PHD3 is an essential gene downstream of HIF-1α that protects cKO P2 mice and is not just fine-tuning the HIF response.50 Taken together, this strongly suggests that HIF-1α in a subset of cells in the brain, including microglia, neurons, and astrocytes, protects cKO P2 mice through the induction of PHD3, and subsequent control of the HIF-2α-activity. Other evidence for a prominent role of HIF-2α in the PHD2/HIF-1α–deficient background was supported by the 2-fold increase of EPO in circulation in these mice compared with cKO P2 mice. Surprisingly, cKO P2/H1 mice did not show any significant change in HCT compared with cKO P2, again implying that the high RBC concentration alone was probably not the cause of their early death.
In conclusion, we have shown through several loss-of-function mutations that conditional PHD2 deficiency in kidney and brain results in an excessive HIF-2α–driven overproduction of EPO. However, HIF-1α serves as a protective factor in these mice, providing new insights to a potential interplay with HIF-2α and PHD3 (Figure 6D). In addition, these findings have implications for pharmacologic strategies that aim at inducing erythropoiesis. Whereas specific inhibition of PHD2 might be beneficial, compounds that simultaneously target all PHDs can ultimately be harmful.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the teams of Dr Roland Jung and Beate Gnauk for excellent technical support.
This work was supported by grants from the MeDDrive-Programm (TU Dresden, Germany) to B.W., the DFG (WI 3291/1-1 and 1-2 to B.W. and FA225/22 to J.F.), and the Swiss National Science Foundation to M.G. This work was also partially supported by National Institutes of Health grant 5R01EY019721 to G-H.F. The work was performed as collaborative project within the COST Action TD0901 “HypoxiaNet.“ K.F, J.K, R.P.S., S.M., and A.M. were supported by the Emmy Noether program (the Deutsche Forschungsgemeinschaft [DFG] Germany). B.W. is an Emmy Noether fellow.
National Institutes of Health
Authorship
Contribution: K.F., J.K, R.P.S, S.M., and A.M designed and performed the experiments, analyzed the data, and helped write the paper; A.W. provided tools and helpful discussions; V.I. performed experiments and helped write the paper; S.J, K.W., and K.G. did pathologic analysis and provided helpful discussions; M.M., A.M.S., D.M.P., and T.R provided helpful discussions and analyzed data; T.O. performed important experiments; S.B. supervised the hematologic analysis; G. Breier, G.F., D.R.G., and S.B, provided helpful tools; G. Baretton supervised all pathologic experiments and provided helpful discussions; T.C., J.F., and M.G. provided tools, helpful discussions, and helped write the paper; B.W. designed the study, supervised the overall project, performed experiments, analyzed the data,and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.K. is Department of Nephrology and Hypertension, University Clinic Erlangen, Erlangen, Germany.
Correspondence: Ben Wielockx, Emmy Noether group (DFG) Inst of Pathology, University of Technology Dresden, Schubertstrasse 15, D-01307 Dresden, Germany; e-mail: ben.wielockx@uniklinikum-dresden.de.
References
Author notes
K.F., J.K., S.M., and R.P.S. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal