Key Points
Human hematopoietic cells develop within human iPSC-derived teratomas in immunodeficient mice.
Co-transplantation of OP9 stromal cells along with human iPSCs increases hematopoietic specification within teratomas.
Abstract
Lineage-restricted cells can be reprogrammed to a pluripotent state known as induced pluripotent stem (iPS) cells through overexpression of 4 transcription factors. iPS cells are similar to human embryonic stem (hES) cells and have the same ability to generate all the cells of the human body, including blood cells. However, this process is extremely inefficient and to date has been unsuccessful at differentiating iPS into hematopoietic stem cells (HSCs). We hypothesized that iPS cells, injected into NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ immunocompromised (NSG) mice could give rise to hematopoietic stem/progenitor cells (HSPCs) during teratoma formation. Here, we report a novel in vivo system in which human iPS cells differentiate within teratomas to derive functional myeloid and lymphoid cells. Similarly, HSPCs can be isolated from teratoma parenchyma and reconstitute a human immune system when transplanted into immunodeficient mice. Our data provide evidence that in vivo generation of patient customized cells is feasible, providing materials that could be useful for transplantation, human antibody generation, and drug screening applications.
Introduction
Somatic cells can be reprogrammed into pluripotent stem cells by overexpressing Oct4 and Sox2 in combination with either c-Myc and/or Klf4.1-6 induced pluripotent stem (iPS) cells exhibit many of the molecular and functional characteristics of embryonic stem (ES) cells7,8 including the capability to generate all the cells of the human body. The concept of producing autologous pluripotent stem cells from somatic cells has attracted the attention of investigators and clinicians who seek a feasible methodology for cell therapy. This treatment can be applied to the care of patients with degenerative diseases and organ failure, as well as novel applications for drug discovery, screening, and toxicology.8
Directed differentiation protocols already in use for ES cells were applied to iPS cells yielding numerous cell types, including neurons, cardiomyocytes, adipocytes, endothelial, and hematopoietic cells.7,8 In particular, hematopoietic lineage specification from pluripotent cells can be experimentally achieved using either embryoid body formation in the presence of hematopoietic cytokines, or co-culture with stroma cells.9,10 Several blood cell types have been efficiently generated from human ES or iPS cells. Hematopoietic cells derived from pluripotent stem cells that might be used for transplantation and human therapies include B11 and T-lineage,11,12 megakaryocytes13,14 myelomonocytic cells,15 natural killer,16 and erythroid cells.17,18 Here we present a novel approach for the generation of a human hematopoietic system in vivo from iPS cells through teratoma formation. In particular, we isolated hematopoietic stem/progenitor cells (HSPCs), myeloid, B, and T cells from teratomas demonstrating the presence of several critical components of a hematopoietic system. Interestingly, HSPCs were capable of multilineage reconstitution when transplanted in immunodeficient recipient mice, suggesting that teratoma is a permissive niche for hematopoietic differentiation. Using hematopoietic cells as a paradigm, we demonstrated that it is feasible to isolate functional cells from teratoma. We believe that using this approach allows for several specific cell types to be isolated from teratoma and cultured for further studies. This has relevance for those tissues/cell types that still lack of robust in vitro differentiation protocol from ES/iPS cells.
Methods
Retroviral transduction and iPS cell generation
Retroviral transduction was performed as previously described4 with the pMXs vectors. Human keratinocytes (Lonza) were infected with a pool of the 4 factors Oct4, Sox2, Klf4, and c-Myc in a ratio of 1:1:1:1 for a 72-hour period. At day 4 after infection, keratinocyte medium was switched to human ES media (Invitrogen) in presence of FGF (Peprotech) and 20% replacement serum (Invitrogen). After 10 to 12 days, human iPS colonies were morphologically identified, picked, and mechanically disaggregated on irradiated mouse embryonic fibroblasts (Globalstem) and cultured on human ES medium. Two iPS cell lines were established using this approach and used for the study.
Immunofluorescence and alkaline phosphatase analysis
Cells grown in 6-well plates were fixed with 4% paraformaldehyde. The following antibodies were used: OCT4, NANOG, TRA1-81, TRA1-60, SSEA4, smooth muscle actinin (SMA; all from Abcam), SSEA3 (Stemgent), Tuj1 (Covance), and α-fetoprotein (Dako). Secondary antibodies used were all from Jackson ImmunoResearch Laboratories and used according to the manufacturer's guidelines. Images were taken using an Axiovert 200M microscope (Zeiss). Direct alkaline phosphatase activity was analyzed using an alkaline phosphatase red membrane substrate solution kit (Stemgent). Isotype IgGs anti–mouse, anti–rat, and anti–rabbit were purchased from Santa Cruz Biotechnology.
Teratoma formation and analysis
Two to 10 × 106 iPS cells were mechanically scraped, pelleted, resuspended in 100 μL of hES media, and injected subcutaneously and intramuscularly into NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG), for 7 to 9 weeks. Later, teratomas were harvested, fixed overnight with 10% formaldehyde, embedded in paraffin, sectioned, H&E-stained, and analyzed using BX40 Microscope (Olympus). In experiments using the OP9 system, 106 OP9, OP9W3a, or OP9D stroma cells were co-injected with 2 × 106 iPS cells. iPS cells between passage 3 and 11 were injected in NSG mice for teratoma formation assay to avoid chromosomal abnormalities as previously reported,19 30 mice were injected with human iPS cells, and the efficiency of teratoma formation was 100%. Sixty teratomas were collected from NSG mice and used according to the aims of each experiment. Animal research was performed under the oversight of the Office of Animal Resources at BIDMC.
Immunostaining and laser scanning cytometry of teratomas and bone marrow
Teratomas and femoral bones were fixed in paraformaldehyde-lysine-periodate (PLP) for 8 hours, equilibrated in 30% sucrose/PBS solution for 48 hours, and snap frozen in OCT (TissueTek). Single-cell-thick (5 μm) cryosections of sections were obtained using a Leica Cryostat and the Cryojane tape transfer system (Leica Microsystems). Sections were blocked in PBS/Tween, stained with rat anti–human CD45 (Abcam) and mouse anti–human CD34 (both Abcam), followed by secondary staining with donkey DyLight anti rat IgG, and donkey DyLight anti–mouse IgG (Jackson ImmunoResearch Laboratories). The antibodies were tested for specificity on murine bone marrow to exclude interspecies cross-reactivity. Sections were stained with DAPI was for nuclear identification. Fluorescent signal was collected by scanning whole femoral or teratoma sections using a 4 laser (405/488/561/633 nm) iCys Research Imaging Cytometer with a 40× dry objective lens and a spatial resolution of 0.5-mm size of stage steps. Quantification of total cell numbers was performed by segmentation of DAPI+ signal and fluorescence in specific channels for individual cells was quantified with the iCys software and displayed in scatterplots. Negative staining cutoff was established individually for each dye based on isotype control for detection of CD45 and/or CD34 positive cells within the context of whole tissues. In femoral bone marrow sections, vascular structures were visualized by staining with rabbit anti–mouse Laminin (Sigma-Aldrich), followed by staining with Dylight649 donkey anti–rabbit IgG (Jackson ImmunoResearch Laboratories).
Teratoma formation and single-cell preparation
Two to 3 × 106 of iPS cells were injected subcutaneously and intramuscularly into 25 NSG recipient mice with or without 1 × 106 OP9 stroma cells. After 8 weeks, tumors were harvested and sliced in 10 to 12 smaller pieces in sterile conditions, then incubated for 2 hours at 37°C in a solution of collagenase/dispase (Roche) on a platform agitator. After the incubation, teratomas were mechanically disaggregated and filtered 3 times through 70μM membranes. After this procedure a single-cell suspension of 8 to 10 × 107 cells was produced and then labeled with appropriate antibodies for sorting or FACS analysis. For the experiments analyzing blood development dynamics, teratomas were harvested after 4 and 8 weeks after iPS injection.
qRT-PCR
RNA was isolated by TriReagent (MRC). cDNA synthesis were performed with Random Primers (Invitrogen) with Transcriptor Reverse Transcriptase (Roche Applied Science) according to the manufacturer's recommendation. cDNA was purified with a high pure PCR product purification kit (Roche Applied Science). Sybr green reaction was performed using iQ Sybr Green supermix (Biorad) using the following parameters: 95°C (10 minutes), 40 cycles of 95°C (15 seconds) and 60°C (1 minute). Primers and probe sequences used were described by Aasen et al.19
FACS analysis and sorting
Cells were suspended in Ca++ Mg++-free phosphate-buffered saline (PBS) containing 2% (vol/vol) FBS. HSPCs were recognized by labeling with APC-CD34 or FITC-CD34 (both clone 581), PE-CD45, or Alexa700-CD45 (both clone HI30). Different conjugated fluorochromes were used to ensure specificity. Other mature phenotypes analyzed were represented by Tricolor-CD3 (T cells; clone HIT3a) Cy5.5-CD19 (B cells; clone HIB19), FITC-CD15 (monocytes and granulocytes; clone HI98; all from Biolegend). The murine CD45 antibody used to exclude murine blood cells during the gating was CD45− APC (clone 104; Biolegend). Human glycophorin A+ CD45− erythroid cells were analyzed as previously described.20 To ensure human specificity, murine bone marrow was stained with the same cocktail of human antibodies for all the experiments reported in the study. The background was subtracted by the final values reported.
Nonspecific signals and dead cells were excluded, respectively, by appropriate fluorochrome-conjugated isotype and propidium iodide staining. All the sorting experiments were performed using the FACSAria II (Becton Dickinson). Isotypes IgG were used as negative controls during the experiments and the background subtracted from the final value reported. For mouse depletion, bone marrow was harvested from femurs and tibiae and cells were stained with biotin-conjugated antibodies against murine hematopoietic markers CD45.1 and CD45.2 (ebioscience, clones A20 and 104, respectively) and enriched for the negative fraction using microbead depletion (MACS Miltenyi Biotech). The negative cells were then stained with specific antibodies for human cell detection and sorted using FACSAria II.
Quantification of human cells in teratomas and murine tissues
Based on absolute counts of each tissue type including teratoma, the percentage of viable hCD45 cells were determined as well as that of hCD15, hCD3, hCD19, and hCD34 using a FACSAria II cytometer. The absolute number for each human lineage was calculated as a ratio between the percentage of viable cells and the total number of cells for the specific tissue analyzed.
Colony assay
Two-thousand CD45+CD34+ cells sorted from teratomas and CD45+CD34+ sorted from cord blood were cultured in triplicate in Methocult 4434 (StemCell Technologies) for 15 to 16 days. After this time, the colonies were scored using Eclipse TE300 microscope (Nikon). Images were acquired with the same system.
Transplantation of immunodeficient mice
CD45+ CD34+ progenitors cells isolated from teratoma and from human cord blood were transferred by intraorbital injection into sublethally irradiated (150 cGy) 6-week-old recipient NSG mice. Human CD45+ CD34+ cells were transplanted in a different range of cell numbers: 100, 200, 1000, 2000, 4000, and 10 000 into 3 mice for each group. A total of 36 mice were used for the experiment, whereas 3 additional mice injected with saline solution were used as negative controls. Each transplanted mouse was killed for isolation of bone marrow, spleen, lymph nodes, and peripheral blood at the specified time. For secondary transplantation, one-fourth of the total bone marrow derived from primary transplanted mice was transferred intravenously into sublethally irradiated recipients. Secondary transplantation was performed in triplicate.
Immunization experiments
NSG mice carrying 7-week-old teratomas were injected with 25 micrograms NS5-HCV (NeoBioscience) antigen with 75 μL of Freund Adjuvant complete (Sigma-Aldrich) directly into the teratomas. After 7 days another injection of 25 micrograms NS5-HCV was done. At day 15, teratomas were harvested and CD19+ B cells sorted and cultured for 48 hours in DMEM KO with IL7 10 ng/mL (Peprotech) and LPS (10 ng/mL; Sigma-Aldrich). After 48 hours, the supernatant was evaluated using a Human IgG ELISA kit (Immunology Consultants Laboratory) as recommended by the manufacturer. Human CD19+CD45+ cells were isolated from spleens of NSG mice transplanted with cord blood and teratoma-HSPCs from the spleen after 60 days of transplantation. Cells were cultured for 48 hours in DMEM KO with IL7 10 ng/mL (Peprotech) and LPS (10 ng/mL; Sigma-Aldrich) before the assay. The ELISA plates were analyzed using Victor3 (PerkinElmer).
T cell cytokine assay
Two-thousand CD3+ T cells were isolated from teratoma and peripheral blood and cultured in QBSF-60 medium (Quality Biologic) in presence of IL-2 (10 ng/mL) and anti-CD3 and anti-CD28–coated beads. After 72 hours, the supernatant was harvested and processed with the human Th1/Th2 cytokine kit (BD Bioscience) according to the manufacturer's instructions. LSRII flow cytometer (BD Bioscience) was used for the analysis. Data show average of 3 independent teratomas derived from 3 NSG mice. Human CD3+CD45+ were isolated from NSG mice transplanted with cord blood and teratoma-HSPCs from the spleen after 60 days of transplantation. Cells were cultured in presence of IL-2 (10 ng/mL) and anti-CD3 and anti-CD28–coated beads for 72 hours. Supernatants were analyzed using the Th1/Th2 cytokine kit (BD Bioscience) according to the manufacturer's instructions.
Phagocytosis assay
CD15+ cells from teratomas or peripheral blood were plated with 50 μL of 2.6% solid Fluoresbrite Carboxy YG 0.5-mm latex microspheres (Polysciences). Sixteen hours later, CD15+ cells were isolated side-by-side from the wells for flow cytometry analysis of bead uptake. Twenty thousand cells in 2 technical duplicates were analyzed in 2 biologic replicates.
During analysis untreated cells served as the background to assess fluorescent microsphere uptake.
Statistical analysis
Statistical analysis was performed using ANOVA test with GraphPad Prism Version 6 software for windows. In all the experiments, the error bars represent SD.
Results
Active hematopoiesis occurs during teratoma formation
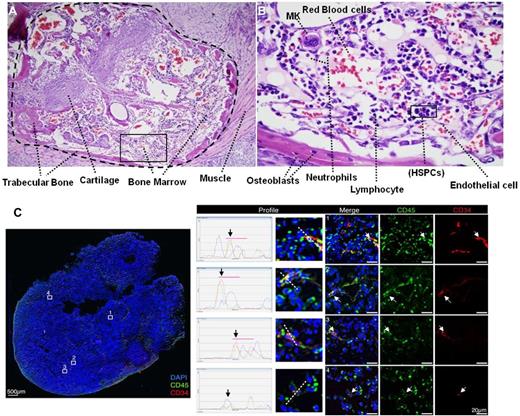
Human keratinocytes were cultured in serum-free and low-calcium medium, which promotes proliferation and maintenance of their undifferentiated state (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Cells were reprogrammed as previously reported,19 and colonies displaying a typical human (h)ES cell–like morphology were picked, plated on MEFs feeder layers, and characterized for pluripotency (supplemental Figure 1B-N). In particular, iPS cells were able to differentiate in vitro into mesoderm, ectoderm, and endoderm derivatives (supplemental Figure 1H-J) and upon injection into NSG mice readily generated teratomas with structures and tissues derived from the 3 embryonic germ layers (supplemental Figure 1K-M). To investigate whether teratomas derived from pluripotent cells could represent a permissive niche for human hematopoiesis, iPS cells derived from human keratinocytes were injected into NSG mice. Histologic analysis on several teratoma sections clearly demonstrated the presence of bone marrow–like structures including trabecular bone and cartilage (Figure 1A). Furthermore, several blood elements including neutrophils, lymphocytes, megakaryocytes (MK), and HSPCs were clearly identified in the bone marrow–like islands (Figure 1B). Indeed, we decided to look for HSPCs inside the teratoma parenchyma by laser scanning cytometry (LSC).21 Human CD45+ blood cells were clearly visualized embedded in the teratomas. In particular, our data showed the presence of a large numbers of CD45+ cells and a more restricted number of CD45+ CD34+ HSPCs (Figure 1C). Human blood cells were widely distributed, suggesting pronounced motility of these cells throughout teratoma structures. Several CD45+ cells and few CD45+CD34+ were found at teratoma borders (supplemental Figure 2A), suggesting that HSPCs may migrate from the bone marrow–like structures in the teratoma to the mouse peripheral blood or lymphatic system.22,23
Active hematopoiesis occurs during teratoma formation. (A) Teratoma section stained with hematoxylin/eosin shown at 10× magnification demonstrating typical teratoma bone marrow–like structures. Trabecular bone, cartilage, and bone marrow are clearly visualized. (B) 60× magnification showing blood elements including neutrophils, lymphocytes, megakaryocytes (MK), and immature blasts (HSPCs) in the bone marrow–like island. (C) LSC showing the presence of human CD45+ blood cells (green) and CD34+CD45+ blood stem/progenitors cells (red and green) indicated by white arrows (right panels, 40× objective, scale bar 20 μm), within the context of an entire teratoma section (left panel, 10× objective, scale bar 500 μm). The profile of intensity in each fluorescent channel along the midline of each cell demonstrates the overlap of the red (CD34) and green (CD45 signals) around DAPI+ (blue) stained nuclei (middle panels).
Active hematopoiesis occurs during teratoma formation. (A) Teratoma section stained with hematoxylin/eosin shown at 10× magnification demonstrating typical teratoma bone marrow–like structures. Trabecular bone, cartilage, and bone marrow are clearly visualized. (B) 60× magnification showing blood elements including neutrophils, lymphocytes, megakaryocytes (MK), and immature blasts (HSPCs) in the bone marrow–like island. (C) LSC showing the presence of human CD45+ blood cells (green) and CD34+CD45+ blood stem/progenitors cells (red and green) indicated by white arrows (right panels, 40× objective, scale bar 20 μm), within the context of an entire teratoma section (left panel, 10× objective, scale bar 500 μm). The profile of intensity in each fluorescent channel along the midline of each cell demonstrates the overlap of the red (CD34) and green (CD45 signals) around DAPI+ (blue) stained nuclei (middle panels).
We investigated the presence of human CD45+ cells in the parenchyma of teratomas at different time points to understand the dynamics of blood development in teratomas. CD45+ cells were detected after 4 weeks after iPS injection, representing 0.75% ± 0.1% of the teratoma cell population. After 8 weeks, CD45+ cells increased to 1.55% ± 0.4% of the total cell number in the teratoma. Absence of the hematopoietic markers CD34 and CD45 on the iPS cells surface excluded the possibility of iPS-expressing hematopoietic markers at a pluripotent stage (supplemental Figure 2B).
OP9 stroma cells increase intra-teratoma hematopoiesis
Our findings supported the hypothesis that teratomas generated from induced pluripotent stem cells represent a permissive niche for human hematopoiesis. Moreover, we asked whether hematopoiesis within the teratomas could be improved or enhanced. It was previously shown that hES cells can differentiate into hematopoietic lineages when co-cultured with OP9 stroma cells. Furthermore, OP9 ectopically expressing Wnt3A (OP9W3a),24 activating the canonical Wnt pathway, augments hematopoiesis25 ; whereas OP9 expressing Delta-like1 (OP9D) specifically supports T-lineage differentiation.26 Therefore, as seen in culture, we hypothesized that co-injection of iPS cells with OP9 stroma cells could improve hematopoietic differentiation within the teratomas through physical interaction or secreted factors.
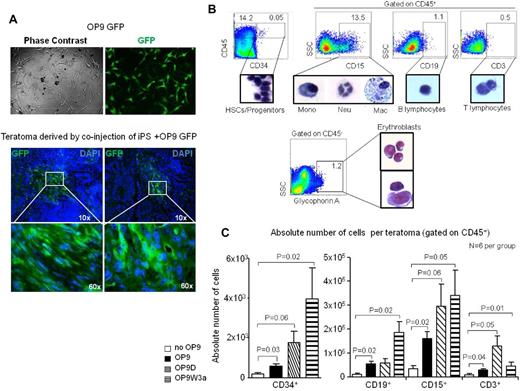
Initially, we evaluated the OP9 stroma cells fate during teratoma formation by taking advantage of OP9 constitutively expressing green fluorescent protein (OP9-GFP; Figure 2A).
OP9 stroma cells increase intra-teratoma hematopoiesis. (A) OP9-GFP+ cells were injected with iPS cells to generate teratomas. After 8 weeks, teratomas showed the presence of GFP+ cells in the parenchyma. (B) FACS analysis reveals the presence of blood progenitor/stem cells, myeloid cells, B, T cells, and glycophorin+ erythroid cells in the teratoma parenchyma. The percentage of each population is indicated for a representative teratoma derived by co-injection of iPS cells with OP9D. The glycophorin+ population shown as the percentage compared with the total teratoma cell number. (C) Quantitative FACS analysis of different blood populations during teratoma formation when iPS were injected in NSG mice or co-injected with OP9 cells or OP9 ectopically expressing Delta-like1 (OP9D) or Wnt3A (OP9W3a). Error bars represent SD.
OP9 stroma cells increase intra-teratoma hematopoiesis. (A) OP9-GFP+ cells were injected with iPS cells to generate teratomas. After 8 weeks, teratomas showed the presence of GFP+ cells in the parenchyma. (B) FACS analysis reveals the presence of blood progenitor/stem cells, myeloid cells, B, T cells, and glycophorin+ erythroid cells in the teratoma parenchyma. The percentage of each population is indicated for a representative teratoma derived by co-injection of iPS cells with OP9D. The glycophorin+ population shown as the percentage compared with the total teratoma cell number. (C) Quantitative FACS analysis of different blood populations during teratoma formation when iPS were injected in NSG mice or co-injected with OP9 cells or OP9 ectopically expressing Delta-like1 (OP9D) or Wnt3A (OP9W3a). Error bars represent SD.
Eight weeks after co-injection of OP9-GFP with iPS cells, teratomas were harvested and analyzed with fluorescent microscopy. Our analysis clearly demonstrated regions in the teratoma parenchyma in which GFP-positive cells were grouped (Figure 2A), suggesting that OP9 stroma cells remain incorporated into the teratoma structures during its formation.
Then, to investigate whether OP9 stroma cells could improve intra-teratoma hematopoiesis, 2 × 106 iPS cells were injected with 106 of each specific OP9 cell line either intramuscularly and subcutaneously. Teratomas generated using co-injection with different OP9 cells did not show significant morphologic differences (supplemental Figure 2C).
Tumors were harvested 8 weeks after iPS injection with or without OP9 stromal cells, disaggregated to acquire single cell suspension, and then analyzed by flow cytometry. Teratomas were simultaneously stained with mouse and human CD45 antibodies27 to specifically separate the murine from human population of CD45+ cells (supplemental Figure 3A).
FACS analysis of representative teratoma obtained by co-injection of iPS cells with OP9D clearly showed the presence of several blood elements, confirming active intra-teratoma hematopoiesis, also in presence of OP9 stroma cells (Figure 2B).
In particular, CD45+ blood cells were detected in 40 of 48 (83%) teratomas, confirming that intra-teratoma hematopoiesis occurs consistently (supplemental Figure 3B). HSPCs CD34+, as well as many myeloid and B and T-lymphoid cells, were isolated by cell sorting and demonstrated morphology typical of those blood elements (Figure 2B). Interestingly, a population of human glycophorin A+ CD45− erythroid cells in different stages of differentiation was also detected (Figure 2B).
Teratomas were subsequently analyzed by flow cytometry to evaluate quantitatively different hematopoietic cell populations in the presence of OP9 stroma cells. We found a significant increased number of CD34+CD45+ cells when iPS were co-injected with different types of OP9 cells (Figure 2C). Recoveries of myeloid CD15+ cells were also strongly increased in the presence of different OP9 cells, and in particular of OP9W3a, which secrete Wnt3a (P = .05). Interestingly, numbers of B and T cells were also significantly augmented when iPS were co-injected with OP9W3a and OP9D cells, respectively (P = .02 and P = .05; Figure 2C). Therefore, our data strongly suggest that is possible to influence specific differentiation through co-injecting pluripotent stem cells with supporting cells. Moreover, our data demonstrate that the presence of OP9W3a stroma cells significantly supports B-cell differentiation from pluripotent stem cells, suggesting a role of the Wnt pathway during B-cell development. In particular, it is of large interest that co-injection of OP9 stroma cells significantly augments intra-teratoma hematopoiesis, suggesting that is possible to direct in vivo tissue specific differentiation.
Human blood cells colonize murine hematopoietic and lymphoid tissues
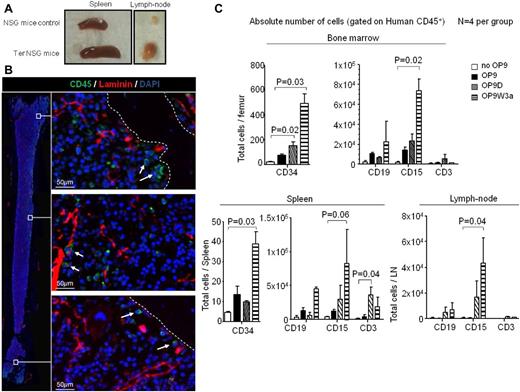
The cellularity of spleen and lymph nodes increased in the majority of NSG mice carrying teratomas compared with NSG controls (Figure 3A). Using LSC technology, we studied femurs of NSG mice carrying teratomas. As shown in Figure 3B, we found rare human CD45+ cells in the murine bone marrow, suggesting some mobilization and/or homing to murine hematopoietic tissues by human blood cells. In particular, a small number of CD45+ cells were found localized in areas enriched for laminin, which stains basement membranes of blood vessels (Figure 3B middle panel). To assess specificity of the human antibodies during the analysis of murine tissues, bone marrow was stained simultaneously with human and murine CD45 antibodies. Our data demonstrate that the human CD45+ population was clearly distinct in bone marrow of mice carrying teratomas (supplemental Figure 3C).
Human blood cells colonize murine hematopoietic and lymphoid tissues. (A) Examples of spleen and lymph nodes of NSG wild-type mouse and NSG carrying 8-week-old teratomas. (B) LSC of femur bone of NSG mice carrying 8-week-old teratoma. The image on the left is the result of merging several images to give the reader a spatial reference of the areas in the femoral cavity from which the high resolution images on the right were obtained. White arrows indicate human CD45+ positive cells (green), whereas mouse laminin is stained red. Femurs of wild-type NSG mice and a secondary IgG were used as negative controls.
Human blood cells colonize murine hematopoietic and lymphoid tissues. (A) Examples of spleen and lymph nodes of NSG wild-type mouse and NSG carrying 8-week-old teratomas. (B) LSC of femur bone of NSG mice carrying 8-week-old teratoma. The image on the left is the result of merging several images to give the reader a spatial reference of the areas in the femoral cavity from which the high resolution images on the right were obtained. White arrows indicate human CD45+ positive cells (green), whereas mouse laminin is stained red. Femurs of wild-type NSG mice and a secondary IgG were used as negative controls.
The finding of only few human blood cells in murine bone marrow confirmed that in the absence of irradiation, engraftment is inefficient.28 As expected, we did not detect significant numbers of human CD34+ cells in the murine bone with this approach. However, FACS analysis demonstrated the presence of human HSPCs, B, T, and myeloid cells in murine bone marrow, and spleen (Figure 3C, supplemental Figure 3D). Human lymphoid and myeloid cells were also found in lymph nodes. Although mice injected with only iPS cells had very low numbers of human CD45+cells in murine bone marrow and no cells in spleen or lymph nodes, the numbers of human cells increased with co-injection of iPS with OP9, OP9W3a, or OP9D cells (Figure 3C). In particular, HSPCs were significantly increased in bone marrow and spleen when iPS cells were co-injected with OP9D and OP9W3a (P = .02 and P = .03, respectively). Furthermore, CD15+ myeloid cells were also strongly augmented in bone marrow, spleen, and lymph nodes in presence of OP9W3a (P = .02, .06, and .04). As expected, co-injection of iPS with OP9D cells improved the T-cell numbers in spleens (Figure 3C, P = .03).
Taken together, these results suggest that co-injection of iPS with OP9 cells improves both intra-teratoma hematopoiesis and extravasation/infiltration to murine organs by human blood cells. We speculate that the increased size of those tissues could be because of the presence of human cells that trigger a host reaction.
In vivo capacity of blood progenitor/stem cells arising in teratomas
To functionally characterize the CD45+CD34+ population of phenotypic hematopoietic stem cells (HSCs) and progenitors,29 these cells were isolated from teratoma obtained by coinjection of iPS and OP9W3a cells and cultured in colony formation unit (CFU) assays as well as transplanted into immunodeficient NSG mice (Figure 4A). CD45+CD34+ cells cultured in methylcellulose gave rise mostly to CFU-GM and CFU-M, demonstrating a CFU frequency of 0.5% ± 0.05% compared with 2.5% ± 0.67% obtained with cord blood cells (Figure 4B, supplemental Figure 4A). To assess engraftment capabilities of the teratoma-derived cells, we performed a transplantation experiment using a range of different cell numbers. In particular, CD34+CD45+ cells isolated from teratomas and cord blood were transplanted in parallel into NSG recipient mice. After 60 days, the animals were killed and analyzed. Our results demonstrate that the engraftment capability of CD34+CD45+ cells derived from teratomas was comparable with that observed using cord blood CD34+CD45+ cells (Figure 4C), suggesting a strong functionality of teratoma-derived HSPCs. The CD34+CD45+ HSPC population in the transplanted animals was also clearly detectable and demonstrated similar percentages comparing teratoma-derived and cord blood donor cells (Figure 4D). Percentages of chimerism for low number of transplanted CD34+CD45+ derived from cord blood were in line with previously reported studies.30
In vivo capacity of blood progenitor/stem cells arising in teratomas. (A) Schematic model for in vitro differentiation and in vivo transplantation of CD45+CD34+ isolated from teratoma. (B) CFU assay revealed the capability of CD45+CD34+ cells to give rise mostly to GM and M colonies. Few E− colonies were also detected. (C) Graph representing human chimerism of CD45+ cells in NSG recipient bone marrow. Three mice per group were transplanted with a different range of human CD45+CD34+ cells isolated from CB and teratoma as indicated. An additional 3 mice were injected with saline solution and used as negative controls. The percentage of human CD45+ cells over total mononuclear cells in bone marrow is shown in the y-axis. (D) Representative FACS analysis showing teratoma and cord blood human CD45+ and human CD34+CD45+ HSPCs populations in NSG recipient bone marrow. (E) Bar-graph showing multi-lineage reconstitution of a NSG bone marrow transplanted with human CD34+CD45+ cells derived from teratomas (Ter) and from cord blood (CB). (F-G) Primary and secondary multi-lineage reconstitution of murine organs by human CD34+CD45+ teratoma cells. For all the transplantation experiments mice injected with saline solution were used as negative control of the experiment. Isotype antibodies were used for gates settings and as additional control. Error bars represent SD. (H) Human-specific PCR of mtDNA shows that human cells are present in engrafted animals. Lane 1: DNA ladder markers. Lanes 2-4: specificity of the assay: human PCR primers efficiently amplify human DNA (lane 4) and do not amplify mouse DNA (lane 2). The latter control is essential as test samples (lanes 5-10) do contain some mouse DNA. Lane 3: validation of the mouse DNA control. The same sample as in lane 2 is successfully amplified with mouse PCR primers to confirm the presence of amplifiable mouse DNA. Lanes 5-7: amplification of FACS-purified human CD45+ cells derived from teratomas isolated from transplanted animals. Lanes 8-9: amplification of FACS-purified human CD34+CD45+ cells derived from teratomas isolated from transplanted animals. Lane 10: amplification of FACS-purified human CD34+CD45+ cells derived from CB isolated from transplanted animals. All the amplifications were performed using human-specific primers which confirm the presence of human cells in the engrafted animals.
In vivo capacity of blood progenitor/stem cells arising in teratomas. (A) Schematic model for in vitro differentiation and in vivo transplantation of CD45+CD34+ isolated from teratoma. (B) CFU assay revealed the capability of CD45+CD34+ cells to give rise mostly to GM and M colonies. Few E− colonies were also detected. (C) Graph representing human chimerism of CD45+ cells in NSG recipient bone marrow. Three mice per group were transplanted with a different range of human CD45+CD34+ cells isolated from CB and teratoma as indicated. An additional 3 mice were injected with saline solution and used as negative controls. The percentage of human CD45+ cells over total mononuclear cells in bone marrow is shown in the y-axis. (D) Representative FACS analysis showing teratoma and cord blood human CD45+ and human CD34+CD45+ HSPCs populations in NSG recipient bone marrow. (E) Bar-graph showing multi-lineage reconstitution of a NSG bone marrow transplanted with human CD34+CD45+ cells derived from teratomas (Ter) and from cord blood (CB). (F-G) Primary and secondary multi-lineage reconstitution of murine organs by human CD34+CD45+ teratoma cells. For all the transplantation experiments mice injected with saline solution were used as negative control of the experiment. Isotype antibodies were used for gates settings and as additional control. Error bars represent SD. (H) Human-specific PCR of mtDNA shows that human cells are present in engrafted animals. Lane 1: DNA ladder markers. Lanes 2-4: specificity of the assay: human PCR primers efficiently amplify human DNA (lane 4) and do not amplify mouse DNA (lane 2). The latter control is essential as test samples (lanes 5-10) do contain some mouse DNA. Lane 3: validation of the mouse DNA control. The same sample as in lane 2 is successfully amplified with mouse PCR primers to confirm the presence of amplifiable mouse DNA. Lanes 5-7: amplification of FACS-purified human CD45+ cells derived from teratomas isolated from transplanted animals. Lanes 8-9: amplification of FACS-purified human CD34+CD45+ cells derived from teratomas isolated from transplanted animals. Lane 10: amplification of FACS-purified human CD34+CD45+ cells derived from CB isolated from transplanted animals. All the amplifications were performed using human-specific primers which confirm the presence of human cells in the engrafted animals.
Furthermore, NSG recipients transplanted with teratoma-HSPCs demonstrated multi-lineage reconstitution with a predominance of human myeloid cells, in contrast to cord blood transplanted mice, which were found to be enriched for B cells (Figure 4E). Whereas CD15+ myeloid cells and CD19+ B cells were strongly enriched in recipient bone marrow, CD3+ T cells were predominantly located in the spleen (Figure 4F). Glycophorin A+ cells were also detected in bone marrow of the transplanted animals, suggesting the potential of teratoma HSPCs to differentiate into erythroid cells after engraftment (supplemental Figure 4B).
The ability to generate CD45+CD34+ hematopoietic progenitors and bone marrow engraftment in primary NSG recipients 2 months after transplantation demonstrates the in vivo functional capacity of cells arising in teratomas. The capacity of reconstitution by teratoma-derived CD45+ cells was limited in secondary recipient NSG mice, 0.04% ± 0.01%, indicating that they do not possess transformed leukemic potential but retain the capability to give rise to both myeloid and lymphoid lineages (Figure 4G). To confirm the presence of human cells in transplanted mice, we performed human-specific PCR amplification of mitochondrial DNA (mtDNA).31 CD45+ and CD34+CD45+ populations were isolated from transplanted mice and analyzed. Both populations produced strong reproducible amplification of the human PCR product, implying clear presence of human cells in transplanted recipients (Figure 4H).
This is the first study showing that human ES/iPS blood progeny differentiated in a physiologic environment in vivo were capable of multi-lineage reconstitution when transplanted into immunodeficient mice.
Functionality of B and T-cell lineages derived from teratoma
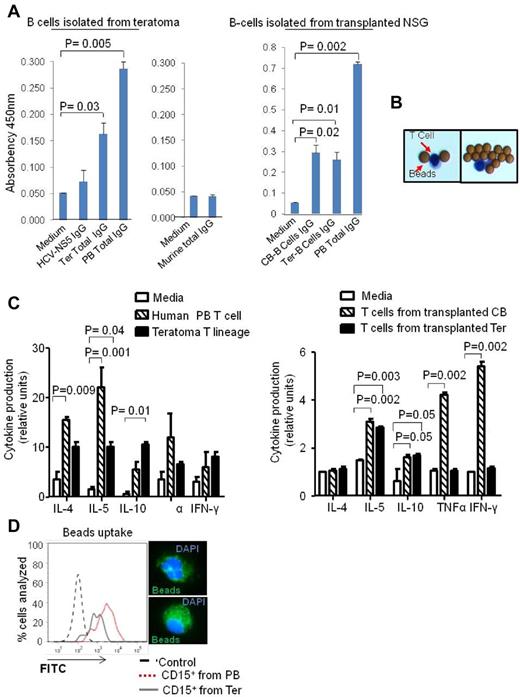
We hypothesized that the teratoma could also be a fruitful environment to produce human antibodies from CD19+ B lineage cells. To test this hypothesis, we injected iPS cells with OP9W3a into NSG mice to allow teratoma development with increased B-cell differentiation. We immunized the mice with NS5 antigen of hepatitis C virus (HCV; supplemental Figure 4C). We performed an ELISA assay to assess the presence of total and specific IgG. The ELISA assay detected significant numbers of IgG producing cells (P = .03) and few cells producing IgG specific for NS5 protein (Figure 5A). Our data support the hypothesis that B cells derived in vivo from iPS cells were able to produce human immunoglobulins. Murine B cells cultured in the same conditions were used as a control. Interestingly, our data demonstrated for the first time that it is possible to generate fully human antibodies from induced pluripotent stem cells, suggesting the potential of the teratoma platform for customized therapies. We also tested the capability of B cells isolated from NSG mice transplanted with teratoma and CB-HSPCs to produce human antibodies. Sixty days after transplantation, human B cells were isolated from murine spleen and cultured for 2 days in vitro. ELISA assays confirmed similar antibody production capabilities between B cells derived from teratomas and CB-HSPCs (Figure 5A right panel).
Functionality of B and T-cell lineages derived from teratoma. (A) ELISA assay showing CD19+ B cells-Ter produce NS5 IgG and total human IgG in amounts comparable with those produced by CD19+ cells isolated from human peripheral blood (left panel). Murine CD19+ B cells were isolated and cultured in the same conditions and used as negative control (middle panel). CD19+ B cells derived from transplanted Ter and CB-CD34+CD45+ produce a similar total human IgGs (right panel). Absorbency at 450 nm is shown in the y-axis. (B) Typical CD3+ T cellsTer morphology after isolation from teratoma parenchyma and culture with IL-2 and anti-CD3 beads. (C) Quantitative cytokine assay showing that T cellsTer produce cytokines when stimulated with IL-2 and anti-CD3 beads. The amounts of cytokines secreted were comparable with those secreted by the same number of CD3+ cells isolated from human peripheral blood (left panel). CD3+ T cells isolated from NSG transplanted with cord blood and teratoma HSPCs were isolated from murine spleens after 60 days of transplantation and evaluated for cytokine production (right panel). Error bars represent SD for both experiments. (D) Fluorescents latex bead-phagocytosis assay for CD15+ cells derived from teratoma (Ter) and of CD15+ derived from human PB. The right panel shows representative CD15+ cells after immunofluorescent bead uptake.
Functionality of B and T-cell lineages derived from teratoma. (A) ELISA assay showing CD19+ B cells-Ter produce NS5 IgG and total human IgG in amounts comparable with those produced by CD19+ cells isolated from human peripheral blood (left panel). Murine CD19+ B cells were isolated and cultured in the same conditions and used as negative control (middle panel). CD19+ B cells derived from transplanted Ter and CB-CD34+CD45+ produce a similar total human IgGs (right panel). Absorbency at 450 nm is shown in the y-axis. (B) Typical CD3+ T cellsTer morphology after isolation from teratoma parenchyma and culture with IL-2 and anti-CD3 beads. (C) Quantitative cytokine assay showing that T cellsTer produce cytokines when stimulated with IL-2 and anti-CD3 beads. The amounts of cytokines secreted were comparable with those secreted by the same number of CD3+ cells isolated from human peripheral blood (left panel). CD3+ T cells isolated from NSG transplanted with cord blood and teratoma HSPCs were isolated from murine spleens after 60 days of transplantation and evaluated for cytokine production (right panel). Error bars represent SD for both experiments. (D) Fluorescents latex bead-phagocytosis assay for CD15+ cells derived from teratoma (Ter) and of CD15+ derived from human PB. The right panel shows representative CD15+ cells after immunofluorescent bead uptake.
FACS and immunofluorescence analysis demonstrated the presence of CD3+ T cells (T cellsTer) in the parenchyma of the teratoma (Figure 2A). To investigate if the activated T cells (Figure 5B) derived from teratoma were functional and had the capability to secrete cytokines, we performed an assay to quantitatively measure several cytokines in single samples. CD3+ T cells derived from teratomas derived from co-injection of iPS and OP9D cells and CD3+ T cells isolated from human peripheral blood (PB) T cells were cultured in presence of IL-2 and anti-CD3/anti-CD28–coated beads. After 72 hours, supernatants were harvested and stained to evaluate the presence of specific cytokines. Our data demonstrated that T cells derived from teratomas were able to secrete cytokines once activated. In particular, the supernatant of both T cellsTer and of PB T cells significantly produced IL-4 and IL-10 (Figure 5C left panel). We conclude that T cellsTer are functionally comparable with human peripheral blood T cells, at least in terms of cytokine response. We tested also the functionality of CD3+ T cells isolated from NSG mice transplanted with teratoma and CB-HSPCs. In particular, after 60 days of transplantation T cells were isolated from the murine spleens and cultured in the presence of IL-2 and anti-CD3/anti-CD28–coated beads. T cells derived originally from teratoma HSPCs produced a significant amount of IL-5 and IL-10 only, whereas T cells derived from cord blood HSPCs produced a significant amount of IL-5, IL-10, TNFα, and IFN-γ (Figure 5C right panel).
Lastly, to test one functional aspect of CD15+ cells derived from teratoma, we cultured them for 24 hours in presence of fluorescent latex beads to allow phagocytosis. Cells were then washed and analyzed by flow cytometry. CD15+ cells derived from teratoma showed a fluorescent intensity because of phagocytosis comparable with the one observed for CD15+ cells isolated from human peripheral blood (Figure 5D). These data demonstrated that myeloid cells derived from teratoma were functional, with levels of phagocytosis similar to those observed for myeloid cells isolated from peripheral blood.
Discussion
The generation of self-renewing multipotent HSPCs from ES or iPS cells continues to be challenging. Previous studies20,32-35 reported derivation of CD34+ HSPCs from pluripotent stem cells showing low engraftment potential of these cells in both primary and secondary transplanted recipients. In particular, a recent report by Ledran and colleagues32 demonstrated that co-culturing human ES cells on primary aorta-gonad-mesonephros (AGM) stroma in presence of cytokines allowed for derivation of hematopoietic progenitors capable of primary hematopoietic engraftment. However, that study failed to achieve a clear multi-lineage reconstitution in the transplanted immunocompromised mice. Furthermore, the CD34+CD45+ population of progenitor cells has never been detected in transplanted animals and their engraftment was evaluated only by the presence of CD45+ population.32 We speculate that the difficulty of producing HSCs capable of long-term reconstitution of irradiated recipients36 might because of the nonphysiologic environment in which the cells were differentiated.37
Here we present a completely novel approach to differentiate human iPS cells into blood progeny. In particular, we demonstrate that human iPS cells can generate HSPCs capable of myeloid and lymphoid differentiation via teratoma formation. This is the first study showing that human ES/iPS blood progeny differentiated in vivo were capable of multi-lineage reconstitution when transplanted into immunodeficient mice. Our findings show that teratomas develop bone marrow–like structures that ultimately allow a physiologic differentiation of HSCs and progenitors from pluripotent stem cells. In all the transplanted animals, the CD34+CD45+ population was clearly detected as well as the differentiated myeloid and lymphoid progeny.
Co-injection of OP9 stroma cells significantly augments the intra-teratoma hematopoiesis suggesting that is possible to promote in vivo tissue specific differentiation. In particular, we found that co-injection of OP9 ectopically expressing WNT3A or Delta-like1 with iPS cells specifically increased the number of HSPCs and T cells, respectively. Although previous studies demonstrated a direct effect of WNT3A and Delta-like1 on human cells during in vitro differentiation, we cannot exclude the possibility of ligands having effects on murine cells which in turn could feedback onto human pluripotent cells.
We believe that this report has critical implications regarding tissue specific differentiation from pluripotent stem cells. In fact, using this approach several specific cell types could be isolated from teratoma and cultured for further studies or also drug screening purposes. This result is of particular relevance for those tissues cell types that lack an in vitro differentiation protocol from pluripotent stem cells. It would be useful in the future to investigate whether donor cell types can influence the differentiation potential of iPS cells in vivo as it does in vitro,38 to improve teratoma differentiation into the tissue of interest.
Furthermore, we report the possibility of generating fully human antibodies outside the human body using the teratoma as the niche. We speculate that this approach will open a new prospective in the generation of therapeutic human antibodies. Lastly, we showed that both human myeloid and T lymphoid cells could be isolated from teratoma parenchyma and might potentially be used in the future for a variety of therapeutic applications or drug screening purposes.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Paul Kincade (OMRF) for providing the OP9, OP9-GFP, OP9W3a, and OP9D cell lines and for critical reading of the paper. The authors thank Riccardo Cortese and Alfredo Nicosia (CEINGE) for sharing protocols and suggestions during immunizations experiments. The authors are grateful to Linda Clayton (Dana-Farber Cancer Institute) for suggestions regarding the T-cell experiments. G.A. was supported by the Collegio Ghislieri fellowship program. R.S.W. was supported by the Jose Carreras fellowship program. C.N.A. was supported by the long-term Human Frontier fellowship.
This work was supported by the Harvard Stem Cell Institute and a grant from the Amelia Peabody Foundation.
Authorship
Contribution: G.A. and R.S.W. designed research, performed experiments, analyzed data, and wrote the paper; C.N.A., A.M.D., A.D.R., A.K.E., Y.K., and M.Y. performed experiments; O.K., D.S.N., K.K., and L.E.S. designed research and analyzed data; D.G.T. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: G.A., R.S.W., and D.G.T. filed a patent for the antibody production method. The remaining authors declare no competing financial intersts.
Correspondence: Daniel Tenen, Harvard Stem Cell Institute, Harvard Medical School, Center for Life Science, 3 Blackfan Circle, Boston, MA 02115; e-mail: dtenen@bidmc.harvard.edu.