Key Points
Platelets from essential thrombocythemia patients have an intrinsic impairment in the P13kinase/Rap1/integrin αIIbβ3 signaling pathway.
This explains the clinical observation that in vitro platelet aggregation is impaired in patients with essential thrombocythemia.
Abstract
Patients with myeloproliferative disorders (MPDs), such as essential thrombocythemia (ET) have increased risk of thrombosis and bleeding, which are major sources of morbidity and mortality. Most MPD patients have a gain of function mutation in Janus kinase 2 (JAK2V617F), but little is known how JAK2V617F affects platelet function. Here, we demonstrate that platelets from ET patients have impaired SFLLRN-mediated fibrinogen binding and have lost the potentiating effect of thrombopoietin (which couples to JAK2) on this pathway. In contrast, SFLLRN-mediated P-selectin expression, ATP secretion, phosphorylation of the PKC substrate pleckstrin, and Ca2+ mobilization were unaffected in JAK2V617F positive platelets. In addition, thrombopoietin-mediated JAK2 phosphorylation was unchanged, suggesting that signaling pathways activated downstream of JAK2 are impaired. Indeed, we found that platelets from JAK2V617F-positive ET patients have significantly reduced phosphorylation of the PI3 kinase substrate Akt, and have reduced activation of Rap1 in response to thrombopoietin, IGF-1, ADP, SFLLRN, and thrombin. This effect was independent of Giα P2Y12 purinergic receptor function as ADP-mediated inhibition of VASP phosphorylation was unchanged. These results demonstrate that the PI3 kinase/Rap1 pathway is intrinsically impaired in platelets from JAK2V617F-positive ET patients, resulting in diminished thrombin and thrombopoietin-mediated integrin αIIbβ3 activation.
Introduction
The myeloproliferative disorders (MPDs) are a group of clonal hemopoietic disorders characterized by increased proliferation of one or more of the myeloid, erythroid, or megakaryocytic cell lineages. The MPDs include polycythemia vera (PV; excess production of red cells) and essential thrombocythemia (ET; excess production of platelets) and other disorders.1 The clinical course of untreated MPDs is dominated by arterial and venous thrombosis, which are major causes of mortality and morbidity in patients with early stage disease.2-5 Abnormal bleeding occurs in 5% to 10% of patients with ET during the disease course, particularly gastrointestinal, urogenital, and intracranial.5,6 Several independent groups demonstrated in 2005 that the defect in the majority of patients with MPDs is a somatic V617F gain-of-function mutation in the gene encoding Janus kinase 2 (JAK2) in hematopoietic progenitor cells.3 JAK2 V617F is present in peripheral blood cells, including platelets,7,8 in virtually all patients with PV and approximately 60% of patients with ET.8,9 In ET, JAK2 V617F increases the risk of thrombosis by approximately 2-fold10,11 and the risk of thrombosis is even higher in ET patients with > 50% JAK2 V617F allele load.12 The JAK2V617F mutation is also present in some patients presenting with thrombosis who do not meet the diagnostic criteria for MPDs.13-15 Bleeding risk in ET appears not to be associated with the presence of JAK2 V617F. Flow cytometry studies demonstrated that JAK2V617F platelets express higher levels of platelet activation markers, such as P-selectin, compared with platelets from patients without the mutation,16,17 suggesting that increased platelet activation may contribute to the thrombotic phenotype. A recent study also observed that platelet-mediated thrombin production is increased in ET patients.18 In contrast, numerous studies performed over the past 35 years clearly showed that in vitro platelet function, in particular aggregation, is impaired in MPD patients,19-22 a finding difficult to reconcile with the increased thrombotic risk. Although it is generally accepted that platelets contribute to the increased occurrence of thrombosis in patients with MPDs, very little is known how the JAK2V617F mutation changes intracellular platelet signaling pathways, subsequent platelet function and how this contributes to the MPD phenotype.
In this study, we therefore aimed to identify the role of JAK2 in platelet function and how the JAK2V617F mutation affects platelet function in ET patients. We studied agonist-stimulated intracellular signaling pathways and related these findings to integrin αIIbβ3 activation and granule secretion of platelets from healthy control, 20 JAK2 V617F-positive and 6 JAK2 V617F-negative ET patients. Our results demonstrate for the first time that the PI3 kinase/Rap1 pathway and integrin αIIbβ3 activation, but not granule secretion, are intrinsically impaired in platelets from ET patients, independent of their JAK2 V617F status, providing an explanation for the in vitro dysfunction in platelet aggregation observed in the clinic.
Methods
Materials
pSer473 Akt, pThr308 Akt, pSer9 GSK3β, PKC phospho-motif (used for analysis of pleckstrin phosphorylation), pThr202/Tyr204 ERK, pThr180/Tyr182 p38, pTyr694 STAT5α/β, pTyr1007/1008 JAK2, pThr180/182JNK, pSer239 VASP, VASP, and PTEN (D4.3) antibodies were from Cell Signaling Technologies. Akt1(B-1), GAPDH(A-3), Rap1(121), JAK2(C-20), STAT5α/β(N-20), SHIP1 (P1C1) antibodies were from Santa Cruz Biotechnology. Pleckstrin (PLK) antibody was from Abcam. CD62P-PE and cMpl-PE(1.6.1) antibodies were from BD Bioscience. Thrombin-receptor-PE (Clone SPAN12) antibody was from Beckman Coulter. 4G10 antibody was from Millipore. TPO was from R&D Systems. IGF-1 was from IBT systems. Microcystin-LR was from Axxora. JAK2i IV was from Merck Chemicals. AR-C 66 096 and wortmannin were from Tocris. PAR-1 activating peptide (SFLLRN-NH2) was from Bachem. Chronolume reagent was from Labmedics. Fura-2 was from Teflabs. Enhanced chemiluminescent detection reagents were from GE Healthcare. Peroxidase conjugated secondary antibodies were from Jackson Immunoresearch Laboratories. NuPAGE SDS-PAGE sample buffer and CD41 PE antibody were from Invitrogen. All other reagents were sourced from Sigma-Aldrich unless otherwise indicated.
Essential thrombocythemia patients
The study was approved by the National Health Service National Research Ethics Service (REC reference No. 08/H0102/90) and was conducted in accordance with the Helsinki Declaration of 2000. After informed written consent, peripheral blood was obtained from a study group 26 participants with ET defined using modified World Health Organization criteria23 by careful venipuncture into 3.8% sodium citrate. Blood samples were also obtained from 26 age and sex-matched healthy volunteer donors (median age 56.8 years, 11 male/15 female). We detected JAK2V617F mutation in peripheral blood mononuclear cell DNA using a real-time polymerase chain reaction assay24 and allele-specific PCR.25 The clinical and laboratory characteristics of the study group are shown in Table 1.
Characteristics of the 26 study subjects with ET
| Sex (M/F) | 12/14 |
| Median age, y (range) | 60.3 (31-77) |
| Median duration since diagnosis, mo (range) | 34.2 (2-84) |
| Subjects with venous thrombosis | 3/26 (12%) |
| Subjects with arterial thrombosis | 5/26 (19%) |
| Subjects with abnormal bleeding | 2/26 (8%) |
| Median platelet count (range) | 397 × 109/L (198-567) |
| Jak2 V617F (+ve/−ve) | 20/6 |
| No treatment | 2/26 (8%) |
| Aspirin alone | 12/26 (46%) |
| Aspirin + hydroxycarbamide | 12/26 (46%) |
| Sex (M/F) | 12/14 |
| Median age, y (range) | 60.3 (31-77) |
| Median duration since diagnosis, mo (range) | 34.2 (2-84) |
| Subjects with venous thrombosis | 3/26 (12%) |
| Subjects with arterial thrombosis | 5/26 (19%) |
| Subjects with abnormal bleeding | 2/26 (8%) |
| Median platelet count (range) | 397 × 109/L (198-567) |
| Jak2 V617F (+ve/−ve) | 20/6 |
| No treatment | 2/26 (8%) |
| Aspirin alone | 12/26 (46%) |
| Aspirin + hydroxycarbamide | 12/26 (46%) |
Isolation of human platelets
Human platelets were isolated from whole blood as previously described26 and resuspended at 4 × 108/mL in modified HEPES-Tyrode buffer (145mM NaCl, 3mM KCl, 0.5mM Na2HPO4, 1mM MgSO4, 10mM HEPES pH 7.2, 0.1% (wt/vol) D-glucose, 0.02 U/mL apyrase and 10μM indomethacin) until use.
Flow cytometry
Two-color analysis of platelet activation was conducted with FITC-conjugated fibrinogen to assess integrin αIIbβ3 activation and PE-conjugated anti–P-selectin (CD62P) to assess α-granule secretion. Platelets (2 × 107/mL) were treated with vehicle (0.2% DMSO) or the indicated compounds for 15 minutes before stimulation in the presence of 0.2 mg/mL FITC-fibrinogen and PE anti–P-selectin for 10 minutes. Nonspecific binding of FITC–fibrinogen was determined in the presence of 1mM EDTA and for PE anti–P-selectin a matching isotype control was used. Surface receptor expression studies were performed by labeling resting or stimulated platelets (performed as 2-color analysis) with either directly conjugated receptor specific antibodies or matching isotype controls for 10 minutes. For integrin kinetic assays aliquots of washed platelets were mixed with PAR-1 at time “zero.” At different time points, a fixed volume of platelets was removed and incubated with FITC-fibrinogen for 30 seconds before addition of 1% formaldehyde. All samples were fixed with 1% formaldehyde for 30 minutes before analysis by flow cytometry on a BD LSR II (BD Bioscience), using FACSDiva software, and a total of 10 000 platelet events per sample were collected. Data were analyzed using Flowing Version 1.6 software (Turku Centre of Biotechnology, Finland).
Protein extraction
Platelets were treated with vehicle (0.2% DMSO) or compound for 15 minutes, stimulated as indicated and lysed directly in 4 × NuPAGE sample buffer (whole cell lysate). Alternatively, platelets were preincubated with thrombopoietin (TPO) for 5 minutes before stimulation. For immunoprecipitation of JAK2 and STAT5, platelets were extracted with an equal volume of ice-cold (i) RIPA buffer [50mM HEPES pH 7.4, 400mM NaCl, 2mM EDTA, 2% (vol/vol) IGEPAL CA-630, 1% (wt/vol) sodium deoxycholate, 0.2% (wt/vol) SDS, 40mM sodium β-glycerol phosphate, 20mM sodium pyrophosphate, 2mM benzamidine, 2 μM microcystin-LR, 10mM sodium orthovanadate, and 2 μg/mL each of pepstatin, antipain, leupeptin] or (ii) NP-40 buffer [50mM HEPES pH 7.4, 240mM NaCl, 2mM EDTA, 2% (vol/vol) IGEPAL, 40mM sodium β-glycerol phosphate, 20mM sodium pyrophosphate, 2mM benzamidine, 2 μM microcystin-LR, 10mM sodium orthovanadate, and 2 μg/mL each of pepstatin, antipain, leupeptin]. For Rap1 activation assays, platelets were extracted with an equal volume of ice-cold Rap1 activity lysis buffer [50mM HEPES pH 7.4, 400mM NaCl, 5mM MgCl2, 2% (vol/vol) IGEPAL, 20% (vol/vol) glycerol, 2 μM microcystin-LR, 10mM sodium orthovanadate, and 2 μg/mL each of pepstatin, antipain, and leupeptin].
Immunoprecipitation and immunoblotting
JAK2 and STAT5 were immunoprecipitated from RIPA or NP-40 lysates by incubation with 2 μg of anti-JAK2(C-20) or anti-STAT5α/β(N-20) and protein A-sepharose overnight at 4°C. Immune complexes were washed with extraction buffer before elution with 2× NuPAGE sample buffer. Immunoprecipitates (IPs) and whole-cell lysates were analyzed by SDS-PAGE/Western blotting using Bis-Tris gels as previously described.27
Rap1 activation assay
Rap1 activation was performed as described by Lova et al using the GST-tagged Rap binding domain of RapGDS (GST-RalGDS-RBD), which specifically precipitates the active GTP-bound form of Rap1.28 Recombinant purified GST-RalGDS-RBD was coupled to GSH-sepharose (200 μg/100 μL) at 4°C for 2 hours. Platelet lysates were incubated with the immobilized GST-RalGDS-RBD (20 μg) for 1 hour at 4°C. Complexes were washed in extraction buffer and eluted with 2× NuPAGE sample buffer. Active Rap1 was identified by immunoblotting with anti-Rap1(121) in parallel to analysis of total Rap1 in whole-cell lysate.
Platelet aggregation
Washed platelets (2 × 108/mL) were incubated with vehicle (0.2% DMSO) or the indicated compounds for 15 minutes. Agonist-stimulated aggregation was monitored using a Chronolog 490-4D aggregometer at 37°C, with continuous stirring at 1200 rpm. Data were recorded using Aggrolink Version 5.2.3 software (Chronolog).
ATP secretion
The release of ATP from dense granules was measured using a luciferin/luciferase assay (Chronolume). Agonist-stimulated ATP release from washed platelets (2 × 108/mL) was monitored using a Chronolog 590-2A aggregometer at 37°C, under stirring conditions. Data were recorded using Aggrolink software (Chronolog).
Ca2+ mobilization
Platelet intracellular calcium concentration ([Ca2+]i) was determined using the calcium-sensitive ratiometric dye, Fura-2. Platelets were loaded with 3μM Fura-2 acetoxymethyl ester in PRP for 45 minutes at 37°C, pelleted at 650g for 10 minutes and resuspended in modified HEPES-Tyrode at 1 × 108 mL−1. Fluorescence emission at 550 nm was recorded for dual excitation (340/380 nm) using a Hitachi F-4500 spectrofluorimeter (Hitachi Hi-Technologies). Changes in [Ca2+]i were monitored using the 340/380 ratio and calibration. The data are expressed as the increase in [Ca2+]i above basal (Δ[Ca2+]i).
Statistics
Data were analyzed and fitted using GraphPad Prism 4.02 software. All data are presented as the mean ± SEM of at least 3 independent observations. Concentration-response curves were fitted with a 4-parameter logistic equation. Differences in best fit parameters between datasets were determined by F test where the null hypothesis states that both datasets can be fitted using the same parameters. Data presented with statistical analysis were tested using a 1-way ANOVA or 2-way ANOVA as appropriate with Bonferroni multiple comparison posthoc test.
Results
TPO modulates the affinity state and membrane expression of integrin αIIbβ3
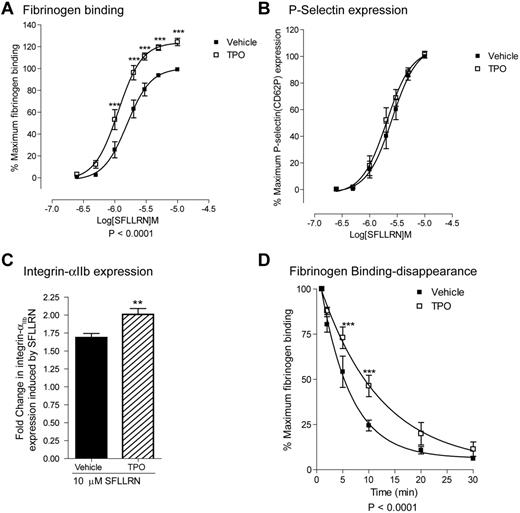
To obtain more insight into the role of JAK2 in platelet function, we examined the effect of thrombopoietin (TPO), a cytokine which activates JAK2 through the c-Mpl receptor, on platelet function. In agreement with previous studies,29,30 we observed that TPO alone was unable to induce platelet activation (data not shown). However TPO enhanced PAR-1–mediated fibrinogen binding (Figure 1A). TPO stimulation resulted in a left shift of the fibrinogen binding curve reducing the EC50 from 1.64μM to 1.16μM (pEC50; 5.78 ± 0.23 to 5.94 ± 0.22). Furthermore maximal fibrinogen binding was increased by 22% ± 3%. This occurred without a concurrent enhancement of PAR-1–mediated P-selectin expression, suggesting that TPO does not affect α-granule secretion (Figure 1B). In contrast, TPO significantly enhanced PAR-1–mediated increases in surface integrin αIIbβ3 expression levels (fold increase from resting; PAR-1 = 1.68 ± 0.06, PAR-1/TPO = 2.00 ± 0.08-fold; Figure 1C). These data suggest that extra integrin αIIbβ3 is derived from the open canalicular system and not α-granules. In agreement with a recent study,31 PAR-1 induces rapid αIIbβ3 activation followed by the disappearance of fibrinogen binding sites (Figure 1D). TPO significantly delayed this disappearance of fibrinogen binding sites on integrin αIIbβ3 (Figure 1D). Together these results indicate that TPO increases PAR-1–mediated fibrinogen binding by (1) decreasing the agonist threshold for integrin αIIbβ3 activation, (2) increasing integrin αIIbβ3 receptor levels on the platelet surface and (3) delaying the closure of fibrinogen binding sites on integrin αIIbβ3.
TPO increases SFLLRN-mediated fibrinogen binding, integrin αIIbβ3 expression levels and delays the disappearance of fibrinogen binding sites. Washed platelets were incubated with 100 ng/mL TPO for 5 minutes before stimulation with the indicated concentrations SFLLRN for 15 minutes in the presence of FITC-fibrinogen (A), PE-anti-P-selectin Ab (B), or PE-anti-integrin αIIb antibody (C). Alternatively, platelets were first stimulated with 10 μM SFLLRN and FITC-fibrinogen was added after the indicated time periods (D) for 15 minutes. Samples were fixed in 1% formaldehyde and analyzed by FACS analysis. Results are expressed as average ± SEM of percentage maximal fibrinogen binding of vehicle treated platelets (A, n = 17), percentage of maximum P-selectin surface expression of vehicle treated platelets (B, n = 9), fold-increase in integrin αIIb integrin expression (C, n = 6), and percentage of maximal fibrinogen binding (D, n = 4).
TPO increases SFLLRN-mediated fibrinogen binding, integrin αIIbβ3 expression levels and delays the disappearance of fibrinogen binding sites. Washed platelets were incubated with 100 ng/mL TPO for 5 minutes before stimulation with the indicated concentrations SFLLRN for 15 minutes in the presence of FITC-fibrinogen (A), PE-anti-P-selectin Ab (B), or PE-anti-integrin αIIb antibody (C). Alternatively, platelets were first stimulated with 10 μM SFLLRN and FITC-fibrinogen was added after the indicated time periods (D) for 15 minutes. Samples were fixed in 1% formaldehyde and analyzed by FACS analysis. Results are expressed as average ± SEM of percentage maximal fibrinogen binding of vehicle treated platelets (A, n = 17), percentage of maximum P-selectin surface expression of vehicle treated platelets (B, n = 9), fold-increase in integrin αIIb integrin expression (C, n = 6), and percentage of maximal fibrinogen binding (D, n = 4).
TPO, but not thrombin, stimulates tyrosine phosphorylation of JAK2
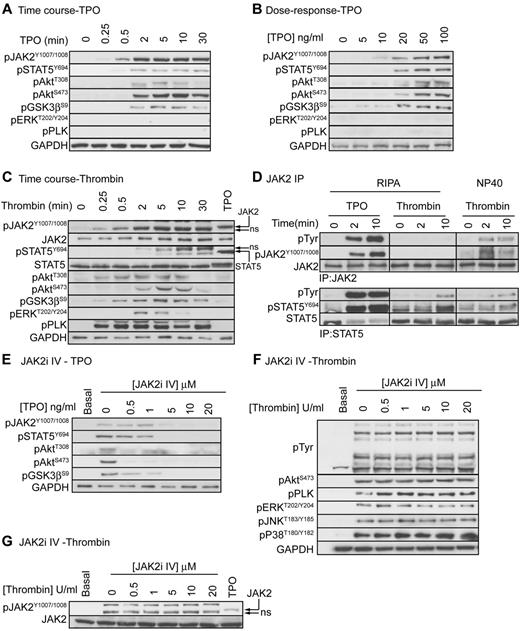
Previous studies reported that, in addition to TPO, thrombin stimulates tyrosine phosphorylation and activation of JAK2 in human platelets. JAK2 may therefore play a role in both TPO and thrombin mediated regulation of platelet function. To investigate whether JAK2 is phosphorylated and activated in response to TPO and thrombin, we used a phosphospecific antibody that recognizes the JAK2 activating tyrosine residues pTyr1007/pTyr1008.32 Figure 2A and B show that TPO stimulates a dose-dependent, rapid and strong phosphorylation of JAK2, which correlates with downstream phosphorylation of STAT5α/β, Akt, and GSK3. No phosphorylation of ERK and pleckstrin was detected demonstrating that the PLC pathway is not activated. As expected, thrombin stimulated rapid and strong phosphorylation of Akt, GSK3, ERK and pleckstrin (Figure 2C). A clear and strong band was also detected by the anti pTyr1007/pTyr1008 JAK2 antibody in the thrombin-stimulated samples, which is in agreement with previous reports.33,34 However, this band had a slightly higher mobility than the band detected with TPO and did not overlap with the JAK2 reprobe. Experiments where JAK2 was immunoprecipitated followed by immunoblotting with the generic pTyr 4G10 and anti pJAK2 antibodies confirmed that JAK2 is phosphorylated in response to TPO, but not thrombin (Figure 2D, RIPA). The previous findings33,34 may therefore be explained by cross-reactivity of 4G10 and JAK2 pTyr1007/pTyr1008 antibodies with proteins other than JAK2, possibly as a result of using a less stringent NP40 based extraction buffer to immunoprecipitate JAK2 (Figure 2D, NP40). In contrast to JAK2, STAT5 phosphorylation was present in total lysate (Figure 2C) and STAT5 immunoprecipitates (Figure 2D, using both RIPA and NP40 extraction buffer) from thrombin-stimulated samples. Thrombin-stimulated STAT5 phosphorylation occurred more slowly than TPO with maximal phosphorylation reached after 10 minutes. Note that TPO stimulated a small STAT5 band shift, which is absent in the thrombin-stimulated samples (Figure 2C). Together, these results demonstrate that thrombin stimulates STAT5-, but not JAK2 phosphorylation.
TPO, but not thrombin, activates the JAK2/PI3 kinase pathway. Platelets were stimulated with 100 ng/mL TPO (A), various concentrations of TPO (B), or 0.2 U/mL thrombin (C) and lysed in 4× NuPage lysis buffer at the indicated time interval (A,C) or after 5 minutes (B). Alternatively, platelets were stimulated with 100 ng/mL TPO or 0.2 U/mL thrombin for 2 or 10 minutes and extracted in the indicated extraction buffer (RIPA or NP40) followed by immunoprecipitation of JAK2 and STAT5 (D). Lastly, platelets were incubated with the indicated concentrations of JAK2i IV for 15 minutes followed by stimulation with 100 ng/mL TPO (E) or 0.2 U/mL thrombin (F-G) for 5 minutes and lysis in 4× NuPage sample buffer. Platelet lysates and JAK2/STAT5 immunoprecipitates were immunoblotted with the indicated antibodies. Results shown are representative of at least 3 independent experiments.
TPO, but not thrombin, activates the JAK2/PI3 kinase pathway. Platelets were stimulated with 100 ng/mL TPO (A), various concentrations of TPO (B), or 0.2 U/mL thrombin (C) and lysed in 4× NuPage lysis buffer at the indicated time interval (A,C) or after 5 minutes (B). Alternatively, platelets were stimulated with 100 ng/mL TPO or 0.2 U/mL thrombin for 2 or 10 minutes and extracted in the indicated extraction buffer (RIPA or NP40) followed by immunoprecipitation of JAK2 and STAT5 (D). Lastly, platelets were incubated with the indicated concentrations of JAK2i IV for 15 minutes followed by stimulation with 100 ng/mL TPO (E) or 0.2 U/mL thrombin (F-G) for 5 minutes and lysis in 4× NuPage sample buffer. Platelet lysates and JAK2/STAT5 immunoprecipitates were immunoblotted with the indicated antibodies. Results shown are representative of at least 3 independent experiments.
JAK2 is essential for TPO but not SFLLRN-mediated regulation of platelet signaling and function
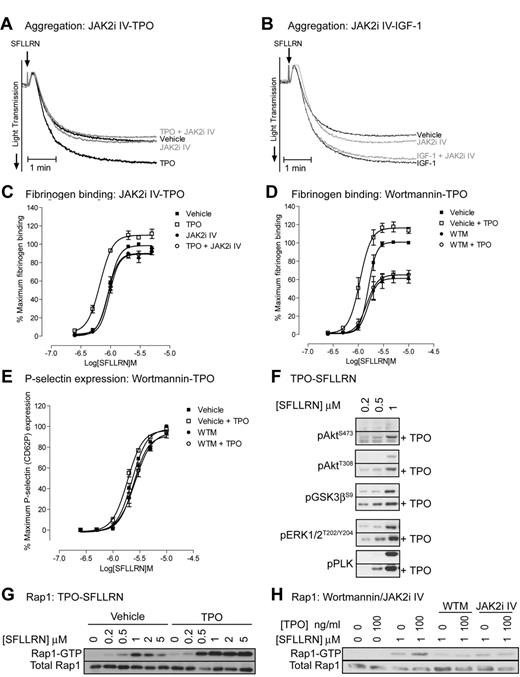
To evaluate the role of JAK2 in platelet function, we used the recently developed JAK2 inhibitor JAK2i IV, which has better efficacy and specificity than the widely used inhibitor AG490.35 JAK2i IV dose-dependently reduced TPO-stimulated phosphorylation of JAK2 and downstream signaling events (Figure 2E), whereas thrombin-stimulated total tyrosine phosphorylation events, which are mainly mediated by the Src kinase family, were unaffected (Figure 2F). The inhibitor had no effect on thrombin-stimulated phosphorylation of Akt, pleckstrin, ERK, JNK, and p38. Note that the signal detected by the pY1007/1008-JAK2 antibody is still present in JAK2i IV treated thrombin-stimulated platelets, further confirming that this is not JAK2 (Figure 2G). In agreement with the findings that JAK2 is not phosphorylated and activated by thrombin, JAK2i IV had no significant effect on PAR-1–mediated platelet aggregation (Figure 3A-B) and fibrinogen binding (Figure 3C). In contrast, JAK2i IV completely prevented TPO-mediated increases in aggregation and fibrinogen binding (Figure 3A,C). This effect was specific for platelet potentiation by TPO as IGF-1–mediated potentiation of aggregation was unaffected (Figure 3B). The PI3 kinase/Akt pathway is activated downstream of JAK2 and wortmannin indeed completely prevented the TPO-mediated increase in fibrinogen binding (Figure 3D). Interestingly, wortmannin did not affect P-selectin expression both in the presence and absence of TPO, suggesting a minor role for PI3 kinase in α-granule secretion (Figure 3E). TPO-stimulated activation of the PI3 kinase pathway resulted in phosphorylation of the downstream substrates Akt and GSK3 (Figure 2A-B) and significantly increased PAR-1–mediated activation of the PI3 kinase/Akt pathway, ERK and pleckstrin phosphorylation (Figure 3F). Moreover, TPO increased PAR-1–mediated Rap1 activation (Figure 3G) in a JAK2/PI3 kinase dependent manner (Figure 3H). Rap1 plays an important role in integrin αIIBβ3 activation and regulation, providing an explanation for the effect of TPO on fibrinogen binding and aggregation (Figure 1).
Thrombopoietin enhances SFLLRN-stimulated aggregation, fibrinogen binding, intracellular signaling, and Rap1 activation in a JAK2/PI3 kinase-dependent manner. Platelets were incubated for 10 minutes with 10μM JAK2i IV (A,C,H) or 100nM wortmannin (D,E,H), followed by 5 minutes incubation with 100 ng/mL TPO (A,C,H) or 100nM IGF-1 (B). Platelets were subsequently stimulated with 0.5μM SFLLRN (A-B) or the indicated concentration of SFLLRN (C-H). Aggregation (A-B) was recorded for 5 minutes and FITC-fibrinogen binding (C-D) and P-selectin expression (E) was measured after 15 minutes stimulation as described in Figure 1A and B. Phosphorylated proteins (F-G) were analyzed by immunoblotting of platelet lysates obtained 5 minutes after stimulation and Rap1 activation (H) by GST-RalGDS pull-down was analyzed from extracts obtained 5 minutes after stimulation. (A,B,F,G,H) Representative results for at least 3 independent experiments. Alternatively, results are expressed as average ± SEM of percentage maximal fibrinogen binding of vehicle treated platelets (C-D, n = 3) or percentage of maximum P-selectin surface expression of vehicle treated platelets (E, n = 3).
Thrombopoietin enhances SFLLRN-stimulated aggregation, fibrinogen binding, intracellular signaling, and Rap1 activation in a JAK2/PI3 kinase-dependent manner. Platelets were incubated for 10 minutes with 10μM JAK2i IV (A,C,H) or 100nM wortmannin (D,E,H), followed by 5 minutes incubation with 100 ng/mL TPO (A,C,H) or 100nM IGF-1 (B). Platelets were subsequently stimulated with 0.5μM SFLLRN (A-B) or the indicated concentration of SFLLRN (C-H). Aggregation (A-B) was recorded for 5 minutes and FITC-fibrinogen binding (C-D) and P-selectin expression (E) was measured after 15 minutes stimulation as described in Figure 1A and B. Phosphorylated proteins (F-G) were analyzed by immunoblotting of platelet lysates obtained 5 minutes after stimulation and Rap1 activation (H) by GST-RalGDS pull-down was analyzed from extracts obtained 5 minutes after stimulation. (A,B,F,G,H) Representative results for at least 3 independent experiments. Alternatively, results are expressed as average ± SEM of percentage maximal fibrinogen binding of vehicle treated platelets (C-D, n = 3) or percentage of maximum P-selectin surface expression of vehicle treated platelets (E, n = 3).
Impaired fibrinogen binding in patients with essential thrombocythemia
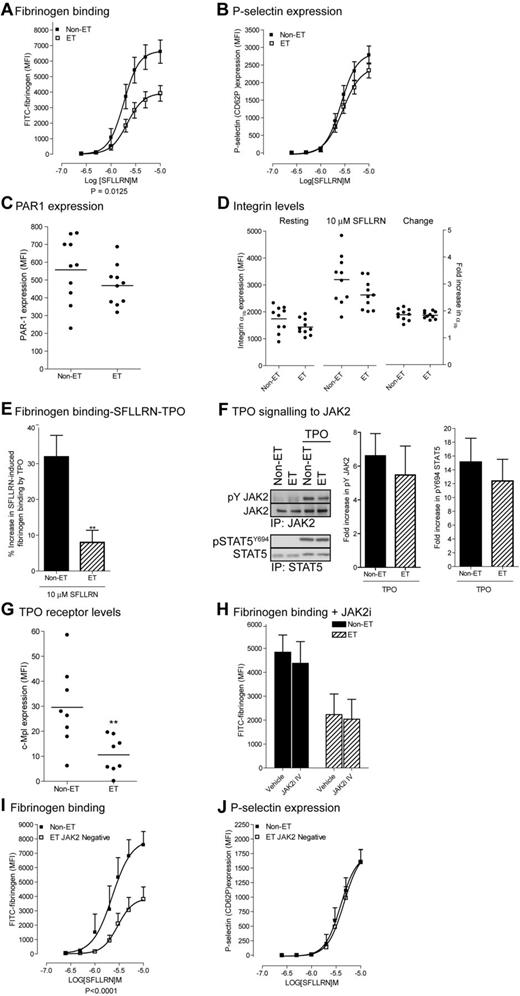
As JAK2 activation increases platelet function, the gain of function mutation JAK2V617F may confer increased platelet function in ET patients. Surprisingly, we found a right shift of the SFLLRN-mediated fibrinogen binding curve (pEC50; 5.71 ± 0.06 to 5.62 ± 0.04) with a 40% decrease in maximal fibrinogen binding (6643 ± 720 MFI to 3867 ± 470 MFI: Figure 4A). P-selectin expression was only slightly reduced with no change in EC50 (Figure 4B), indicating that PAR-1 signaling to α-granule secretion is still largely intact. Impaired fibrinogen binding was not because of a decrease in receptor levels as PAR-1 and integrin αIIbβ3 receptor levels were not significantly different between patients and controls (Figure 4C-D). The TPO-mediated increases in SFLLRN-mediated fibrinogen binding (Figure 4E) were also compromised in ET patients under conditions where phosphorylation of JAK2 and its downstream substrate STAT5 were unchanged (Figure 4F). This was despite a reduction in the platelet TPO receptor c-Mpl levels in ET patients (Figure 4G). Indeed, changes in JAK2 activity were not involved in reduced fibrinogen binding of ET platelets, as the JAK2i IV has no significant effect (Figure 4H). Furthermore, SFLLRN-mediated fibrinogen binding, but not P-selectin expression was reduced to a similar extent in both JAK2V617F-positive and negative platelets (compare Figure 4A-B with I-J). These results suggest that dysfunction of intracellular signaling pathways downstream of PAR-1 and c-Mpl-JAK2 is responsible for impaired fibrinogen binding in JAK2V617F-positive and negative ET platelets.
Platelets from JAK2V617F-positive and negative ET patients demonstrate impaired SFLLRN and TPO-mediated fibrinogen binding. Washed platelets from control subjects, JAK2V617F-positive ET patients (A-H) and JAK2V617F negative ET patients (I-J) were incubated with TPO for 5 minutes (E), JAK2i IV for 15 minutes (H), and stimulated with the indicated concentrations of SFLLRN (A,B,D,E,H,I,J) for 15 minutes in the presence of FITC-fibrinogen (A, n = 14, E, n = 7, H, n = 3, I, n = 6), PE-anti–P-selectin Ab (B, n = 13; J, n = 5), PE-anti-PAR-1 (C, n = 10), PE-anti-integrin αIIb (D, n = 10) or PE-c-Mpl (G, n = 8). Samples were fixed in 1% formaldehyde and analyzed by FACS analysis. Data are expressed in arbitrary units (MFI, average ± SEM). Alternatively, platelets were stimulated with TPO for 5 minutes, and JAK2 and STAT5 immunoprecipitates immunoblotted with the indicated antibodies (F). The bar graphs show the fold increase in phosphorylation on TPO stimulation (average ± SEM, n = 6).
Platelets from JAK2V617F-positive and negative ET patients demonstrate impaired SFLLRN and TPO-mediated fibrinogen binding. Washed platelets from control subjects, JAK2V617F-positive ET patients (A-H) and JAK2V617F negative ET patients (I-J) were incubated with TPO for 5 minutes (E), JAK2i IV for 15 minutes (H), and stimulated with the indicated concentrations of SFLLRN (A,B,D,E,H,I,J) for 15 minutes in the presence of FITC-fibrinogen (A, n = 14, E, n = 7, H, n = 3, I, n = 6), PE-anti–P-selectin Ab (B, n = 13; J, n = 5), PE-anti-PAR-1 (C, n = 10), PE-anti-integrin αIIb (D, n = 10) or PE-c-Mpl (G, n = 8). Samples were fixed in 1% formaldehyde and analyzed by FACS analysis. Data are expressed in arbitrary units (MFI, average ± SEM). Alternatively, platelets were stimulated with TPO for 5 minutes, and JAK2 and STAT5 immunoprecipitates immunoblotted with the indicated antibodies (F). The bar graphs show the fold increase in phosphorylation on TPO stimulation (average ± SEM, n = 6).
Dysfunction of the PI3 kinase/Akt pathway in patients with essential thrombocythemia
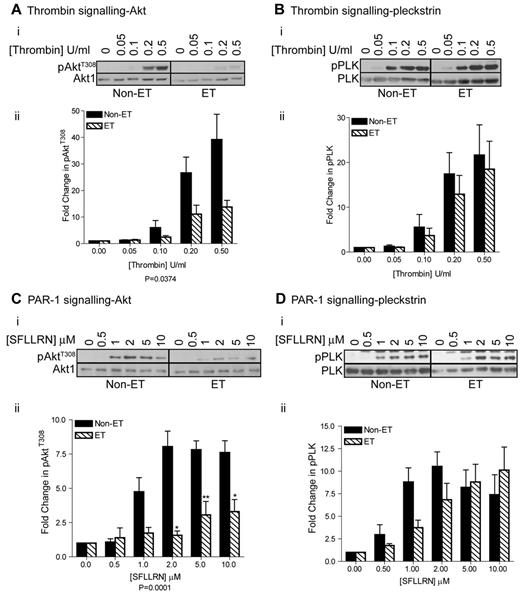
The PI3 kinase/Akt pathway and the PLC pathway leading to Ca2+ mobilization/PKC activation are important in integrin αIIbβ3 regulation and platelet activation. We found that Akt Thr308 phosphorylation in response to thrombin and SFLLRN was significantly impaired in ET patients under all conditions tested (Figure 5A,C). In contrast, thrombin-stimulated pleckstrin phosphorylation, a marker for PKC activation, was unchanged in ET patients (Figure 5B). Furthermore, pleckstrin phosphorylation was not significantly affected at high SFLLRN concentrations although some reduction was seen at lower concentrations (Figure 5D). SFLLRN-stimulated Ca2+ mobilization was also similar between control and ET platelets (Figure 6A), suggesting that the PLC activation is intact. Akt phosphorylation by thrombin is largely dependent on ADP secretion and subsequent activation of P2Y12/PI3kinase.36 The observed impairment in Akt phosphorylation may therefore be caused by reduced ADP secretion and/or impaired P2Y12 signaling. To investigate whether this is the case, we measured (1) ATP secretion (Figure 6B), (2) the effect of a P2Y12 receptor antagonist on fibrinogen binding (Figure 6C), (3) ADP signaling to VASP (Figure 6D), and (4) the effect of ADP, IGF-1, and TPO on Akt phosphorylation (Figure 6E-G). Figure 6B shows that SFLLRN-stimulated ATP secretion in ET patients is not significantly different from control, demonstrating that the differences are not because of reduced ADP secretion. SFLLRN-mediated fibrinogen binding in ET patients was also still largely reliant on P2Y12 signaling (Figure 6C). Furthermore, ADP potently prevented PGE1-mediated VASP Ser 239 phosphorylation, demonstrating that P2Y12 signaling through Gi is intact in ET patients (Figure 6D). In contrast, ADP-stimulated Akt phosphorylation was reduced to a similar degree as for SFLLRN (Figure 6E). Akt phosphorylation in response to IGF-1 and TPO, factors that activate the PI3 kinase/Akt pathway independently of secreted ADP,29,37,38 was also strongly diminished in ET platelets (Figure 6F-G). Taken together, these results clearly demonstrate that the PI3 kinase/Akt pathway is intrinsically impaired in platelets from ET patients. This was not because of up-regulation of expression levels of the lipid phosphatases PTEN and SHIP1 (Figure 6H).
Impaired activation of the PI3 kinase/Akt pathway in platelets from JAK2V617F-ositive ET patients. Washed platelets from control and ET patients were stimulated with the indicated concentrations of thrombin (A, n = 5, B, n = 6) and SFLLRN (C, n = 5, D, n = 10) for 2 minutes before extraction in 4× NuPage lysis buffer and immunoblotting with the indicated antibodies. (i) A representative blot is shown. (ii) The bar graphs (average ± SEM) show the fold-increase in pAktT308 and p-PLK stimulation and are corrected for total Akt and pleckstrin levels.
Impaired activation of the PI3 kinase/Akt pathway in platelets from JAK2V617F-ositive ET patients. Washed platelets from control and ET patients were stimulated with the indicated concentrations of thrombin (A, n = 5, B, n = 6) and SFLLRN (C, n = 5, D, n = 10) for 2 minutes before extraction in 4× NuPage lysis buffer and immunoblotting with the indicated antibodies. (i) A representative blot is shown. (ii) The bar graphs (average ± SEM) show the fold-increase in pAktT308 and p-PLK stimulation and are corrected for total Akt and pleckstrin levels.
Ca2+ mobilization, dense granule release, and P2Y12 function are not affected in platelets from JAK2V617F-positive ET patients. Washed platelets from control and JAK2V617F-positive ET patients were stimulated with the indicated concentrations of SFLLRN (A,B,E,H), SFLLRN (± 1μM AR-C 66096; C), ADP (+100 nM PGE1; D), IGF-1 (F), and TPO (G). Ca2+ mobilization was recorded in Fura-2 labeled platelets (A). ATP secretion was recorded for 5 minutes using a luminometer (B), FITC-fibrinogen binding was measured after 15 minutes stimulation as described in Figure 1A (C), VASP phosphorylation was analyzed by immunoblotting (D), and Akt phosphorylation was analyzed by immunoblotting of platelet lysates obtained 5 minutes after stimulation (E-G). Unstimulated platelet lysates from control subjects and JAK2V617F-positive ET patients were analyzed for PTEN and SHIP1 expression levels by immunoblotting (H). The bar graphs show the average (± SEM) increase in [Ca2+] (A, n = 4), nmol ATP (B, n = 4), percentage reduction in fibrinogen binding (C, n = 3), and Akt phosphorylation in response to ADP (E, n = 3), IGF-1 (F, n = 5), and TPO (G, n = 5) and relative PTEN (n = 14) and SHIP1 (n = 10) expression levels (H).
Ca2+ mobilization, dense granule release, and P2Y12 function are not affected in platelets from JAK2V617F-positive ET patients. Washed platelets from control and JAK2V617F-positive ET patients were stimulated with the indicated concentrations of SFLLRN (A,B,E,H), SFLLRN (± 1μM AR-C 66096; C), ADP (+100 nM PGE1; D), IGF-1 (F), and TPO (G). Ca2+ mobilization was recorded in Fura-2 labeled platelets (A). ATP secretion was recorded for 5 minutes using a luminometer (B), FITC-fibrinogen binding was measured after 15 minutes stimulation as described in Figure 1A (C), VASP phosphorylation was analyzed by immunoblotting (D), and Akt phosphorylation was analyzed by immunoblotting of platelet lysates obtained 5 minutes after stimulation (E-G). Unstimulated platelet lysates from control subjects and JAK2V617F-positive ET patients were analyzed for PTEN and SHIP1 expression levels by immunoblotting (H). The bar graphs show the average (± SEM) increase in [Ca2+] (A, n = 4), nmol ATP (B, n = 4), percentage reduction in fibrinogen binding (C, n = 3), and Akt phosphorylation in response to ADP (E, n = 3), IGF-1 (F, n = 5), and TPO (G, n = 5) and relative PTEN (n = 14) and SHIP1 (n = 10) expression levels (H).
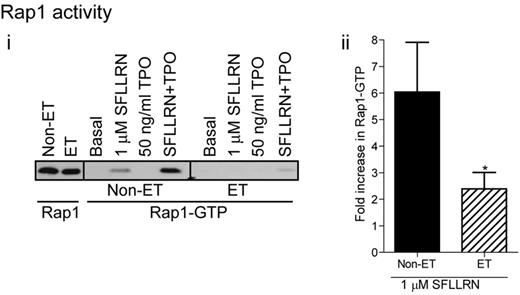
Activation of Rap1 is compromised in patients with essential thrombocythemia
One of the main regulators of integrin αIIbβ3 activation is the small G-protein Rap1, which in its GTP bound state, promotes integrin activation and fibrinogen binding.39-41 Wortmannin significantly reduced SFLLRN and thrombin-stimulated Rap1 activation in control platelets (Figure 3H), demonstrating an important role for PI3 kinase in Rap1 activation. In contrast, TPO did not activate Rap1 by itself, but potently increased SFLLRN-stimulated Rap1 activation in a PI3 kinase dependent manner (Figure 3H). Platelets from ET patients showed significantly reduced levels of Rap1 activation in response to SFLLRN compared with control, both in the absence and presence of TPO (Figure 7). Considering the important role of the PI3kinase/Rap1 pathway in integrin αIIbβ3 activation, dysfunction of this pathway probably underlies impaired integrin activation in patients with ET.
Impaired Rap1 activation in platelets from JAK2V617F-positive ET patients. Washed platelets from control and ET patients were stimulated for 5 minutes with SFLLRN, TPO, or a combination of TPO and SFLLRN. Rap1 activation was subsequently analyzed by GST-RalGDS pull-down and immunoblotting for Rap1 (i). The bar graph shows the average (± SEM) fold-change in Rap1 activation on SFLLRN stimulation (ii, n = 7).
Impaired Rap1 activation in platelets from JAK2V617F-positive ET patients. Washed platelets from control and ET patients were stimulated for 5 minutes with SFLLRN, TPO, or a combination of TPO and SFLLRN. Rap1 activation was subsequently analyzed by GST-RalGDS pull-down and immunoblotting for Rap1 (i). The bar graph shows the average (± SEM) fold-change in Rap1 activation on SFLLRN stimulation (ii, n = 7).
Discussion
In this study, we determined the role of JAK2 in platelet activation using TPO and the specific JAK2 inhibitor JAK2i IV and found that TPO increased platelet function by reducing the threshold of integrin activation, increasing integrin expression levels and delaying the closure of fibrinogen binding sites. Inhibitor studies confirmed that these effects were dependent on the JAK2/PI3 kinase pathway. In contrast, TPO and the JAK2 inhibitor JAK2i-IV had no effect on SFLLRN-stimulated P-selectin expression levels, demonstrating that JAK2 does not signal to α-granule secretion. As the majority of patients with essential thrombocythemia have a gain of function JAK2 V617F mutation, we hypothesized that their platelets may demonstrate increased integrin activation. Surprisingly, we found a significant reduction in SFLLRN-mediated fibrinogen binding in both JAK2 V617F–positive and negative ET patients, whereas P-selectin expression and ATP secretion were left intact. Further biochemical analysis showed a general down regulation of the PI3 kinase/Rap1 pathway in ET platelets, providing an explanation for the observed impaired integrin activation.
Thrombin, in addition to TPO, has been reported to phosphorylate JAK2 in human platelets,33,34 although with much less efficacy then TPO.34 In this study, we directly compared TPO and thrombin-stimulated JAK2 phosphorylation, but were unable to find evidence that thrombin phosphorylates JAK2 in human platelets. Moreover, we found no significant effect of the JAK2i IV on SFLLRN-mediated fibrinogen binding and P-selectin expression. In contrast, JAK2i IV completely prevented TPO-mediated increase in fibrinogen binding. The effect of TPO on platelet function was furthermore blocked by the PI3 kinase inhibitor wortmannin, confirming previous reports.29,30,42 The JAK2/PI3 kinase pathway is thus essential for TPO-mediated increases in fibrinogen binding. Interestingly, we found that platelets from ET patients have impaired SFLLRN-mediated fibrinogen binding and have lost the potentiating effect of TPO on SFLLRN-mediated fibrinogen binding. Phosphorylation of JAK2 and its downstream substrate STAT5 was unchanged in response to TPO under conditions where TPO receptor levels were slightly reduced. These findings are in agreement with a recent study34 and demonstrate that down-regulation of the signaling pathway is at the level of PI3 kinase and not JAK2. Indeed, we found that impaired activation of PI3 kinase/Rap1 was an intrinsic characteristic of ET platelets. Activation of the PI3 kinase pathway leading to phosphorylation of its downstream substrate Akt is largely dependent on Gi-dependent signaling, through the secretion of ADP and subsequent activation of the P2Y12 receptor.36 One possibility is therefore that a reduction in ADP release and/or impaired P2Y12 signaling underlies the ET platelet phenotype. However, we found that ATP (and therefore ADP) release was only mildly affected and that P2Y12 signaling was intact in ET platelets, shown by the ability of ADP to prevent PGE1-mediated VASP phosphorylation. Interestingly, despite normal P2Y12-mediated Gi signaling, ADP-stimulated Akt phosphorylation was significantly reduced, a finding which may partially explain impaired SFLLRN and thrombin-mediated Akt phosphorylation. However, Akt phosphorylation in response to mediators that activate PI3 kinase independently of P2Y12, such as IGF-1 and TPO, was also reduced, demonstrating intrinsic impairment in the PI3kinase/Akt pathway in platelets from ET patients. Many intracellular signaling pathways feed into the PI3kinase/Akt pathway (various PI3kinase isoforms, 3′phosphatase PTEN [converting PI(3,4,5)P3 into PI(4,5)P2), 5′phosphatase SHIP1 (converting P(3,4,5)P3 into PI(3,4)P2), p85 PI3kinase, adaptor molecules, negative feedback pathways, PDK1, mTORC2, PHLPP, PP2A] and therefore changes in expression, activity, phosphorylation, recruitment and/or localization of any these signaling molecules may underlie the decrease in Akt phosphorylation in ET patients. The total 3′ and 5′ lipid phosphatase activity of PTEN and SHIP1 is often regulated by changes in their expression levels; however, this is unlikely to underlie the impairment in the PI3kinase/Akt pathway, as PTEN and SHIP1 expression levels were unchanged in patients with ET.
An additional important pathway that contributes to integrin regulation and platelet activation is the PLC/PKC pathway.43,44 Phosphorylation of the PKC substrate pleckstrin and Ca2+ mobilization in response to thrombin and high concentrations of SFLLRN were unaffected in ET platelets, suggesting the PLC pathway remained functional. Pleckstrin phosphorylation was reduced at lower concentrations of SFLLRN, which may be explained by cross-talk between the PI3 kinase and PLC pathway.45,46 Indeed, we found a similar reduction in pleckstrin phosphorylation in control platelets in the presence of wortmannin (not shown).
Our data demonstrates for the first time that the PI3kinase/Rap1 pathway is intrinsically impaired in platelets from ET patients and that this is probably responsible for significantly reduced fibrinogen binding and platelet function. These results are consistent with specific down-regulation of the PI3 kinase/Rap1 pathway downstream of JAK2, with dysfunction of PI3 kinase regulated fibrinogen binding, but not α and dense granule secretion. Although the majority of ET patients in this study were JAK2V617F positive, similar results were found in 6 JAK2V617F negative patients, showing that impairment of the PI3 kinase pathway and fibrinogen binding is unrelated to JAK2 status. Indeed, the results are unlikely to be because of JAK2 (hyper) activation in platelets themselves as the JAK2 inhibitor JAK2i IV was unable to reverse the phenotype. We also found no difference between patients who did or did not receive hydroxyurea treatment. Our findings provide, for the first time, an explanation for the clinical observation that in vitro platelet aggregation in PRP from ET patients is impaired.28,29 One proposed explanation for this impairment was that pre-activation of platelets in circulation leads to “exhausted” platelets that may have released the majority of their granule contents.22 This is consistent with studies showing that platelets from patients with MPD demonstrate reduced membrane expression receptor levels2,22,47,48 and reduced number of platelet granules/secretion.49,50 However, our study clearly shows that P-selectin expression and ATP secretion are largely unaffected in ET platelets, demonstrating that intracellular signaling pathways leading to granule secretion and the dense granules themselves are still largely intact in ET platelets. Furthermore, membrane expression levels of PAR-1 receptors and integrin αIIbβ3 are in the normal range. Indeed, we obtained no evidence that platelets from ET patients are “preactivated,” a finding that may also be explained by the low aspirin prophylaxis that the majority of ET patients were on.
Although impaired Akt/Rap1/integrin activation may clearly contribute to bleeding in ET patients, it is not directly associated with a bleeding phenotype as this only occurred in 8% of the ET patients. The major complication in ET patients is an increased risk of thrombosis. We therefore do not rule out the possibility that, in addition to a reduction in the Akt/Rap1/integrin pathway, other signaling pathway that contribute to platelet thrombus formation are positively affected in (a subpopulation of) ET patients. Indeed, a recent paper by Randi et al suggested that ET platelets have preactivated Src,34 which is known to play a role in platelet activation and may therefore contribute to thrombus formation in vivo.
Together, this study demonstrates for the first time that the PI3 kinase/Rap1 pathway and integrin αIIbβ3 activation are intrinsically impaired in platelets from ET patients, providing an explanation for the in vitro platelet dysfunction observed in the clinic.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the healthy blood donors within the Medical Sciences building and the essential thrombocythemia patients from the MPD clinic (BRI, Bristol) for their generous blood donations. The authors also thank Dr Roger Evely, University Hospital Bristol for enabling access to the study subjects.
This work was supported by the British Heart Foundation (grants PG/10/100/28658, PG/08/056/25325, and RG/10/006/28299).
Authorship
Contribution: S.F.M. designed and performed research, collected, analyzed, and interpreted data, contributed to discussion, performed statistical analysis, and edited the paper; R.W.H., M.T.H., and J.S.S. performed research, collected data and contributed to discussion; S.S. and S.K.W. contributed reagents; A.W.P. contributed to discussion and edited the paper; A.D.M. designed research, interpreted data, contributed reagents, contributed to discussion, and edited the paper; and I.H. designed and performed research, collected, analyzed, and interpreted data, contributed to discussion, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for R.W.H. is Nestlé Institute of Health Sciences SA, Lausanne, Switzerland.
Correspondence: Ingeborg Hers, School of Physiology and Pharmacology, Medical Sciences Building, University of Bristol, University Walk, Bristol, BS8 1TD, United Kingdom; e-mail: i.hers@bris.ac.uk.

![Figure 6. Ca2+ mobilization, dense granule release, and P2Y12 function are not affected in platelets from JAK2V617F-positive ET patients. Washed platelets from control and JAK2V617F-positive ET patients were stimulated with the indicated concentrations of SFLLRN (A,B,E,H), SFLLRN (± 1μM AR-C 66096; C), ADP (+100 nM PGE1; D), IGF-1 (F), and TPO (G). Ca2+ mobilization was recorded in Fura-2 labeled platelets (A). ATP secretion was recorded for 5 minutes using a luminometer (B), FITC-fibrinogen binding was measured after 15 minutes stimulation as described in Figure 1A (C), VASP phosphorylation was analyzed by immunoblotting (D), and Akt phosphorylation was analyzed by immunoblotting of platelet lysates obtained 5 minutes after stimulation (E-G). Unstimulated platelet lysates from control subjects and JAK2V617F-positive ET patients were analyzed for PTEN and SHIP1 expression levels by immunoblotting (H). The bar graphs show the average (± SEM) increase in [Ca2+] (A, n = 4), nmol ATP (B, n = 4), percentage reduction in fibrinogen binding (C, n = 3), and Akt phosphorylation in response to ADP (E, n = 3), IGF-1 (F, n = 5), and TPO (G, n = 5) and relative PTEN (n = 14) and SHIP1 (n = 10) expression levels (H).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/7/10.1182_blood-2012-05-431288/4/m_zh89991301860006.jpeg?Expires=1769085212&Signature=M~MjtstTQ90VNvSQPHE2oirWbI3dOiTnJnbOyEsEVRDxTFWrgq6F5-PAMm8ok8l~lYQhzgWQpLGi89W-dVJxUZyHgmCO5Ipa1Dv6Y~GZpuzUtwg7Vhyf7ztU~sfRLhb~uhs1gvDFXew6xtN5WKduo6k0var9OUu5~e8vFxwjrglNk9qptCCz5NL-2bS6KKWYV5jJY1kr98IiGer7R~mrG5n9F9NbXW4nxl-jdEFCXk6Xc-r7V8nH45iHxGQFswmarbRpQ0C6YnRzC916BKuGaMRWNFixbbdXo3Wx5mbsgefyT3nAfTFGshcmBmurtUx4xddeK2AQ23gGBGDKfVk8cw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal