Key Points
Deletion of Gsα in osteocytes induces severe osteopenia and a dramatic expansion of cells of the myeloid lineage.
Osteocytes regulate hematopoiesis and specifically contribute to myelopoiesis by secreting proliferative factors such as G-CSF.
Abstract
Hematopoietic progenitors are regulated in their respective niches by cells of the bone marrow microenvironment. The bone marrow microenvironment is composed of a variety of cell types, and the relative contribution of each of these cells for hematopoietic lineage maintenance has remained largely unclear. Osteocytes, the most abundant yet least understood cells in bone, are thought to initiate adaptive bone remodeling responses via osteoblasts and osteoclasts. Here we report that these cells regulate hematopoiesis, constraining myelopoiesis through a Gsα-mediated mechanism that affects G-CSF production. Mice lacking Gsα in osteocytes showed a dramatic increase in myeloid cells in bone marrow, spleen, and peripheral blood. This hematopoietic phenomenon was neither intrinsic to the hematopoietic cells nor dependent on osteoblasts but was a consequence of an altered bone marrow microenvironment imposed by Gsα deficiency in osteocytes. Conditioned media from osteocyte-enriched bone explants significantly increased myeloid colony formation in vitro, which was blocked by G-CSF–neutralizing antibody, indicating a critical role of osteocyte-derived G-CSF in the myeloid expansion.
Introduction
Under normal physiologic conditions, bone marrow (BM) serves as the primary site for hematopoiesis, where the daily replenishment of mature hematopoietic cells is sustained by hematopoietic stem cells (HSCs) residing in the niche. The hematopoietic hierarchy begins with a small population of life-long self-renewing long-term HSCs giving rise to short-term HSCs, which gradually lose certain lineage differentiation potential to become multipotent progenitors. Further lineage restriction of these progenitors gives rise to either common lymphoid progenitors or common myeloid progenitors, which generate the 2 main lineages, lymphoid and myeloid, respectively.1 In addition to cell-intrinsic factors,2 extrinsic factors from cells in the niche regulate the self-renewal, differentiation, and migration of HSCs.3 Primary components of the niche include osteoblasts,4,5 osteoclasts,6 vascular endothelial cells,7 mesenchymal stem cells,8 and cells of the sympathetic nervous system.8,9 The BM harbors specialized niches for lineage-restricted progenitors conducive to the development of specific hematopoietic cell lineages. These include BM stromal cell niches for B-lymphopoiesis through expression of CXCL12 and IL-7,10,11 and BM endothelial cell niches for megakaryocyte progenitors.12 Therefore, the signals from cells of the BM microenvironment appear to be able to direct hematopoietic lineage commitment and also contribute to the pathogenesis of hematopoietic disorders.
Among the cells of the skeletal and BM microenvironment, bone-forming osteoblasts are the most versatile regulators of hematopoiesis. Constitutive activation of the parathyroid hormone (PTH)/PTHrP receptor in osteoblasts increases the numbers of HSCs via increased expression of Notch1 ligand jagged1,4 whereas conditional deletion of Gsα in preosteoblasts leads to decreased B-cell precursors because of decreased IL-7 expression by the osteoblasts.11 Induced osteoblast deficiency results in an early loss of HSCs and subsequent loss of lymphoid, erythroid, and myeloid progenitors in the BM.13
Osteocytes are the most abundant bone cells (95%),14 which are terminally differentiated from osteoblasts and are embedded deep within the bone matrix during bone formation. Compared with the short-lived osteoblasts and osteoclasts, osteocytes can live for years, making them ideal candidates to survey bone quality and initiate a bone-remodeling cycle when necessary. Recent studies demonstrated that these cells coordinate bone remodeling by secreting RANKL, the critical osteoclastogenic factor,15,16 and Sclerostin, a Wnt inhibitor and suppressor of osteoblast proliferation and functions.17 One class of important signaling pathways in osteocytes and osteoblasts are the G protein–coupled receptor (GPCR) signaling pathways that act through heterotrimeric G proteins.18 The best characterized subunit of G-proteins, Gsα, activates adenylyl cyclase that, in turn, catalyzes the production of cAMP and activates protein kinase A, which ultimately regulates gene expression.19 Osteocytes express several Gsα-coupled receptors, including the PTH/PTHrP receptor,20 prostaglandin receptors (EP2 and EP4),21 and other receptors.22 Given the profound influence of osteoblasts and osteoclasts in regulating hematopoiesis, we hypothesized that osteocytes could regulate hematopoiesis through Gsα signaling.
To test this hypothesis, we engineered mice lacking Gsα specifically in osteocytes (OCY-GsαKO). These mice display severe osteopenia because of a decrease in osteoblast numbers and a dramatic expansion of cells of myeloid lineage in BM, spleen, and peripheral blood (PB). Interestingly, the lymphoid cells were not affected in BM and PB. Transplantation of BM from control to OCY-GsαKO mice rapidly recapitulated the myeloproliferation, whereas the converse experiment abolished it, demonstrating that the defect is not intrinsic to the hematopoietic cells but is an effect of an altered BM microenvironment imposed by Gsα-deficient osteocytes. Treatment of these mice with antisclerostin antibody restored the numbers of osteoblasts and normalized bone mineral density; however, the myeloproliferative phenotype persisted, suggesting that osteocytes directly regulate hematopoiesis through a Sclerostin/Wnt signaling independent pathway. In vitro assays revealed that osteocytes secrete several myeloproliferative factors, including G-CSF, capable of regulating myelopoiesis. Our findings show, for the first time, that osteocytes are a major regulator of myelopoiesis, most likely through regulation of myeloid progenitor cell niches. We also show that the osteocyte-myeloid cell interaction is dependent on Gsα-signaling in osteocytes and is partially mediated by osteocyte-derived G-CSF.
Methods
Mice
All procedures involving mice were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital. Mice lacking Gsα in osteocytes were generated by crossing Gsαflox/flox mice23 with mice expressing Cre-recombinase driven by a 10-kb DMP1 promoter.24 Mice lacking PTH-PTHrP receptor (PPR) in osteocytes were generated by mating PPRflox/flox mice25 with DMP1-Cre mice. The genotype of the mice and tissue-specific DNA recombination were performed as described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
For sclerostin neutralization, control and OCY-GsαKO mice were injected subcutaneously with antisclerostin antibody (25 mg/kg, 2 times per week; kindly provided by Michaela Kneissel, Novartis) from 3 days until 4 weeks old.
Complete blood counts and flow cytometry
Complete blood counts from PB were performed by a VetScan HM5 Hematology System (Abaxis). For immunophenotyping of terminally differentiated hematopoietic cells, BM and spleen cells were stained for 15 minutes on ice with fluorescent antibodies to B lymphocytes (B220, IgM, and CD93), T lymphocytes (CD4 and CD8a), granulocytes (CD11b and Gr-1), erythrocytes (Ter119 and CD45), megakaryocytes (CD41 and CD42d), and monocytes/macrophages (F4/80 and CD11b). For hematopoietic progenitor cell analysis, BM and spleen cells were lineage depleted and then stained with fluorescent antibodies to hematopoietic stem cells (LKS: Lin− c-Kit+ Sca-1+ and LKS SLAM: Lin− c-Kit+ Sca-1+ CD150+ CD48−), multipotent progenitors (MPP: Lin− c-kit+ Sca-1+ Thy1.1− Flk-2+), common myeloid progenitors (CMP: IL-7Rα− Lin− c-kit+ Sca-1− FcγRlo CD34+), granulocyte macrophage progenitors (GMP: IL-7Rα− Lin− c-kit+ Sca-1− FcγR+ CD34+), and megakaryocyte erythroid progenitors (MEP: IL-7Rα− Lin− c-kit+ Sca-1− FcγRlo CD34−). All antibodies were obtained from eBioscience. The fluorescently labeled cells were then analyzed on a BD LSRII (BD Biosciences).
BM transplantation
Recipient mice were lethally irradiated with a single dose of 9.5 Gy and then transplanted with lineage depleted mononuclear BM cells from donor mice by tail vein injection. Complete blood cell counts were measured in donor and recipient mice before transplantation and in recipient mice 4 and 9 weeks after transplantation.
OEBE generation and in vitro culture
Osteocyte-enriched bone explants (OEBEs) were generated from long bones of 7-week-old OCY-GsαKO or control mice. After removing the epiphyses and flushing out BM, the explants were digested with collagenase and EDTA to remove endosteal and periosteal osteoblasts and BM cells as described previously.22 For myeloid colony generation, 2 × 104 BM cells from 8- to 10-week-old OCY-GsαKO or control mice were cultured in duplicate in myeloid colony supporting methylcellulose-based complete medium (M3434) containing 3 U/mL erythropoietin, 10 ng/mL recombinant murine IL-3 (rmIL-3), 10 ng/mL rmIL-6, and 50 ng/mL recombinant murine stem cell factor (StemCell Technologies). The cells were cultured in 35-mm culture dishes with a 2-mm grid (174926, Nalge Nunc) and incubated for 10 days at 37°C. Total myeloid colonies per plate were counted by morphologic scoring. For megakaryocyte colony formation, 1 × 105 BM cells from 7-week-old OCY-GsαKO or control mice were cultured in duplicate in MegaCult-C media supplemented with 50 ng/mL thrombopoietin, 10 ng/mL IL-3, and 20 ng/mL IL-6 (StemCell Technologies). The cells were cultured in double-chamber slides and stained for acetylcholinesterase as per the manufacturer's protocol (StemCell Technologies). For the coculture assay, OEBE from 2 tibia and 2 femurs from control and OCY-GsαKO mice were cultured (in IMDM with 10% FBS) on transwells with 2 × 104 BM cells from a control mouse cultured in M3434 methylcellulose medium at the bottom of the 35-mm culture dish. To assess the direct contribution of osteocyte-produced growth factors and cytokines in supporting myeloid or megakaryocytic cell growth, conditioned media was obtained by culturing OEBEs from OCY-GsαKO or control mice for 7 days in 1.5 mL of α-MEM with 10% FBS (Invitrogen). The conditioned media was mixed in a 1:3 proportion with incomplete methylcellulose medium (M3231) or MegaCult-C medium containing FBS but lacking other growth factors. A total of 2 × 104 bone BM cells from a control mouse were cultured in this mixed medium in 12-well low-cell binding plates (145385, Nalge Nunc), incubated at 37°C for 10 days. Colonies were counted at the end.
Bioassays
cAMP was measured in cultured calvarial osteoblasts26 as described previously.20 G-CSF in serum and OEBE conditioned media was measured by ELISA (Raybiotech). SDF1α, TNF-α, and IL-12 levels were measured in OEBE conditioned media by ELISA (Raybiotech). G-CSF and IL-12 in OEBE conditioned media were normalized to OEBE dry weight.
Statistical analysis
All data are presented as mean ± SEM. Statistical significance of differences between groups was determined by Student t test. P values ≤ .05 were accepted as significant.
Additional methods are described in supplemental Methods.
Results
Mice with conditional ablation of Gsα in osteocytes display severe osteopenia
We conditionally ablated the ubiquitously expressed stimulatory subunit of G-proteins, Gsα, in osteocytes (OCY-GsαKO) by mating GsαE1flox/flox mice23 with DMP1-Cre mice24 (Figure 1A). Cre-loxP–mediated DNA recombination was observed in skeletal, but not in hematopoietic tissues of OCY-GsαKO mice (Figure 1B). Skeletal and PB phenotypes were not different between wild-type and DMP1-Cre mice matched by genetic background (supplemental Figure 1A-F). Therefore, littermates lacking the DMP1-Cre transgene but carrying the GsαE1 floxed alleles (GsαE1flox/flox) were used as controls. OCY-GsαKO mice were viable, fertile, and indistinguishable from littermate controls at birth. Gsα mRNA expression in osteoblast- and BM-depleted osteocyte-enriched long bones (supplemental Figure 3A) from OCY-GsαKO mice was decreased by 70% (Figure 1C) but was unchanged in osteoblasts, BM, spleen, monocytes/macrophages, or granulocytes (Figure 1D-F). Moreover, the functional capacity of Gsα to synthesize cAMP was not altered in calvarial osteoblasts from OCY-GsαKO and control mice (Figure 1G), confirming osteocyte-specific disruption of Gsα. OCY-GsαKO mice displayed osteopenia, characterized by a dramatic decrease in trabecular and cortical bone as revealed by von Kossa staining (Figure 1H), bone mineral density (Figure 1I), and bone mineral content (Figure 1J) by dual-energy x-ray absorptiometry. Examination of osteocyte microstructure showed increased osteocyte density (supplemental Figure 4) but a disorganized and reduced lacunar-canalicular network (supplemental Figure 4).
DMP1-Cre–mediated loss of Gsα in osteocytes. (A) Breeding strategy for generating mice with osteocyte-specific disruption of Gsα. (B) Top panel: Schematic representation (not to scale) showing the relative location of loxP sites (triangles), Gsα-exon 1 (rectangle), multiplex PCR primers (F1, R1, R2), and the expected PCR product sizes. Bottom panel: PCR analysis showed Gsα allele recombination in skeletal (tibia and calvaria) tissue but not in hematopoietic (BM, spleen, and liver) tissues from OCY-GsαKO mice (n = 3). (C) Relative expression of Gsα in femur and tibia (n = 4) from control (Con) and OCY-GsαKO (KO) mice. Gsα mRNA expression in (D) osteoblasts, BM, spleen, (E) Gr1+ flow-sorted granulocytes, and (F) F4/80+ CD11b+ flow-sorted monocytes/macrophages (n = 4). (G) cAMP synthesis in calvarial osteoblasts isolated from control and OCY-GsαKO mice in response to PTH and forskolin (FSK; n = 4). (H) von Kossa staining of femurs showing osteopenia in OCY-GsαKO (KO) mice compared with control (Con) mice. (I-J) Whole-body dual-emission x-ray absorptiometry showing (I) bone mineral density (BMD; g/cm2) and (J) bone mineral content (BMC; g) in young (7 weeks old) and adult (21 weeks old) mice (n ≥ 7 mice). Error bars represent mean ± SEM. *P < .05 by t test.
DMP1-Cre–mediated loss of Gsα in osteocytes. (A) Breeding strategy for generating mice with osteocyte-specific disruption of Gsα. (B) Top panel: Schematic representation (not to scale) showing the relative location of loxP sites (triangles), Gsα-exon 1 (rectangle), multiplex PCR primers (F1, R1, R2), and the expected PCR product sizes. Bottom panel: PCR analysis showed Gsα allele recombination in skeletal (tibia and calvaria) tissue but not in hematopoietic (BM, spleen, and liver) tissues from OCY-GsαKO mice (n = 3). (C) Relative expression of Gsα in femur and tibia (n = 4) from control (Con) and OCY-GsαKO (KO) mice. Gsα mRNA expression in (D) osteoblasts, BM, spleen, (E) Gr1+ flow-sorted granulocytes, and (F) F4/80+ CD11b+ flow-sorted monocytes/macrophages (n = 4). (G) cAMP synthesis in calvarial osteoblasts isolated from control and OCY-GsαKO mice in response to PTH and forskolin (FSK; n = 4). (H) von Kossa staining of femurs showing osteopenia in OCY-GsαKO (KO) mice compared with control (Con) mice. (I-J) Whole-body dual-emission x-ray absorptiometry showing (I) bone mineral density (BMD; g/cm2) and (J) bone mineral content (BMC; g) in young (7 weeks old) and adult (21 weeks old) mice (n ≥ 7 mice). Error bars represent mean ± SEM. *P < .05 by t test.
Myelopoiesis, but not lymphopoiesis, is increased in mice lacking Gsα in osteocytes
Examination of hematopoietic organs in OCY-GsαKO mice showed marked splenomegaly (Figure 2A) and focal areas of hypercellularity in the BM (Figure 2B). Normalizing for decreased body weight (Figure 2C), spleen weights were increased 4-fold (Figure 2D) in OCY-GsαKO mice compared with controls. We performed complete blood counts in PB and flow cytometry analysis on BM and spleens in young (7-week-old) and adult (21-week-old) mice. PB from young and adult OCY-GsαKO mice showed a significant increase in leukocytes (Figure 2E) and platelets (Figure 2G) and a 5-fold increase in the percentage of neutrophils (Figure 2F). Lymphocyte numbers were not changed at either age (Figure 2H) in the PB. Hematocrit fraction, red blood cells, and hemoglobin were unchanged at 7 weeks of age in OCY-GsαKO mice compared with controls (HCT, 53.4% ± 5.07% vs 53.9% ± 2.82%; RBC, 10.2 ± 1.13 vs 10.6 ± 0.6 × 106 cells/μL; hemoglobin, 15.0 ± 0.83 vs 16.1 ± 0.59 g/dL; mean ± SEM, n = 10). Immunophenotypic analyses of mature hematopoietic cells in the BM showed a significant increase in the absolute number of Gr1+/CD11b+ granulocytes and F4/80−/CD11b+ monocytes in 7-week-old OCY-GsαKO mice which were further increased in 21-week old OCY-GsαKO mice compared with controls (Figure 2I-J), suggesting that the hematopoietic abnormalities progress with aging. There was a modest but significant decrease in Ter119+/CD45− erythroid cells and an increase in F4/80+/CD11b+ macrophages in the BM of OCY-GsαKO mice. Significant increases in the absolute numbers of granulocytes, monocytes, and macrophages were observed in spleens of OCY-GsαKO mice compared with controls (Figure 2K-L). B220+/IgM+ mature B cells, CD4+ or CD8a+ mature T cells, and CD41+/CD42d+ megakaryocytes were unchanged in the OCY-GsαKO BM and spleen (Figure 2I-L; supplemental Figure 5A). However, the frequency of mature B cells and T cells was modestly, but significantly, decreased in the spleen OCY-GsαKO mice compared with controls (supplemental Figure 2). These findings indicate that osteocyte-specific disruption of Gsα results in an expansion of myeloid cells in the BM, spleen, and PB, suggesting that Gsα signaling in osteocytes is required for normal hematopoiesis.
Osteocyte-specific loss of Gsα results in expansion of myeloid cells in BM and spleen. (A) Splenomegaly in OCY-GsαKO (KO) mice compared with controls (Con). (B) Representative areas of hypercellularity in BM of OCY-GsαKO femurs. (C) Decreased whole-body weight and (D) increased normalized spleen weights in OCY-GsαKO mice (n ≥ 7). (E-H) PB counts showing (E) number of leukocytes, (F) % neutrophils, (G) number of platelets, and (H) number of lymphocytes in 7- and 21-week-old control and OCY-GsαKO female mice (n ≥ 9). (I-L) Immunophenotypic analysis of hematopoietic cells in BM and spleen showing absolute counts for B cells (B220+ IgM+), granulocytes (Gr1+ CD11b+), erythroid cells (Ter119+ CD45−), monocytes (F4/80− CD11b+), macrophages (F4/80+ CD11b+), T cells (CD4+), and cytotoxic T cells (CD8a+) in BM (I-J) and spleens (K-L) of 7-week-old (I,K) and 21-week-old (J,L) control and OCY-GsαKO female mice (n ≥ 6). Error bars represent mean ± SEM. *P < .05 by t test.
Osteocyte-specific loss of Gsα results in expansion of myeloid cells in BM and spleen. (A) Splenomegaly in OCY-GsαKO (KO) mice compared with controls (Con). (B) Representative areas of hypercellularity in BM of OCY-GsαKO femurs. (C) Decreased whole-body weight and (D) increased normalized spleen weights in OCY-GsαKO mice (n ≥ 7). (E-H) PB counts showing (E) number of leukocytes, (F) % neutrophils, (G) number of platelets, and (H) number of lymphocytes in 7- and 21-week-old control and OCY-GsαKO female mice (n ≥ 9). (I-L) Immunophenotypic analysis of hematopoietic cells in BM and spleen showing absolute counts for B cells (B220+ IgM+), granulocytes (Gr1+ CD11b+), erythroid cells (Ter119+ CD45−), monocytes (F4/80− CD11b+), macrophages (F4/80+ CD11b+), T cells (CD4+), and cytotoxic T cells (CD8a+) in BM (I-J) and spleens (K-L) of 7-week-old (I,K) and 21-week-old (J,L) control and OCY-GsαKO female mice (n ≥ 6). Error bars represent mean ± SEM. *P < .05 by t test.
Alteration of the BM microenvironment by Gsα-deficient osteocytes is necessary to induce the myeloproliferation
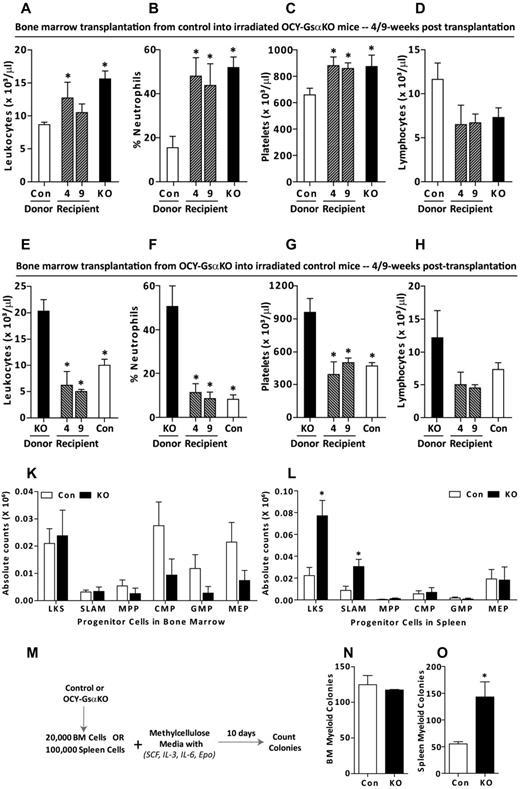
To determine whether the hematologic abnormalities present in the OCY-GsαKO mice were intrinsic to the hematopoietic cells or imposed by an osteocyte-dependent altered BM microenvironment, we performed BM transplantation experiments. BM transplantation from control donor mice into lethally irradiated OCY-GsαKO recipient mice rapidly induced leukocytosis, neutrophilia, and thromobocytosis in recipient mice beginning 4 weeks after transplantation and continuing at 9 weeks after transplantation (Figure 3A-D). Reciprocal transplantation of BM from OCY-GsαKO donor mice with severe myeloproliferation into lethally irradiated control recipients completely normalized the hematopoietic profile already at 4 weeks after transplantation (Figure 3E-H). These results indicate that the altered BM microenvironment of Gsα-deficient osteocytes is required for initiation and maintenance of the myeloproliferation and that the hematopoietic abnormality is not intrinsic to the hematopoietic cells. To identify the defect in progenitor cells that may lead to myeloid proliferation, we enumerated HSCs (LKS and LKS SLAM) and myeloid progenitor cells (multipotent progenitor cell [MPP], common myeloid progenitor cell [CMP], granulocyte macrophage progenitor cell [GMP], and megakaryocyte erythroid progenitor cell [MEP])1 in BM and spleen of these animals. None of the progenitors was significantly different in BM of control and OCY-GsαKO mice (Figure 3K). In spleens from OCY-GsαKO mice, LKS and LKS-SLAM HSCs were significantly increased, although there was no difference in myeloid progenitors (Figure 3L). Similarly, in vitro methylcellulose colony assays (Figure 3M) showed a 3-fold increase in the number of myeloid colonies generated by splenocytes from OCY-GsαKO mice over controls (Figure 3O), indicating an increase in HSCs in OCY-GsαKO spleens. There was no difference in the number of myeloid or megakaryocytic colonies formed by BM cells from control and OCY-GsαKO mice (Figure 3N; supplemental Figure 5B), demonstrating the requirement of an altered BM microenvironment to induce myeloproliferation in OCY-GsαKO mice.
Alteration of the BM microenvironment by Gsα-deficient osteocytes is necessary to induce myeloproliferation. (A) BM transplantation schema from control to lethally irradiated OCY-GsαKO mice or (F) vice versa. (B-E) PB cell counts of 7-week-old control donor and OCY-GsαKO recipient mice before transplantation and recipient mice 4 and 9 weeks after transplantation showing (B) number of leukocytes, (C) % neutrophils, (D) number of platelets, and (E) number of lymphocytes. (G-J) PB cell counts from 7-week-old OCY-GsαKO donor and control recipient mice before transplantation and recipient mice 4 and 9 weeks after transplantation showing (G) number of leukocytes, (H) % neutrophils, (I) number of platelets, and (J) number of lymphocytes (n = 3 for donors and n = 8 for recipients). (K-L) LKS (Lin− c-Kit+ Sca-1+), LKS SLAM (Lin− c-Kit+ Sca-1+ CD150+ CD48−), MPPs (Lin− c-kit+ Sca-1+ Thy1.1− Flk-2+), CMPs (IL-7Rα− Lin− c-kit+ Sca-1− FcγRlo CD34+), GMPs (IL-7Rα− Lin− c-kit+ Sca-1− FcγR+ CD34+), and MEPs (IL-7Rα− Lin− c-kit+ Sca-1− FcγRlo CD34−) in (K) BM and (L) spleen from control and OCY-GsαKO female mice (n = 6). (M) Strategy for methylcellulose colony formation assay. (N-O) Myeloid colonies from (N) 2 × 104 BM cells and (O) 1 × 105 spleen cells from 8- to 10-week-old control and OCY-GsαKO mice (n = 4). Error bars represent mean ± SEM. *P < .05 by t test compared with donor.
Alteration of the BM microenvironment by Gsα-deficient osteocytes is necessary to induce myeloproliferation. (A) BM transplantation schema from control to lethally irradiated OCY-GsαKO mice or (F) vice versa. (B-E) PB cell counts of 7-week-old control donor and OCY-GsαKO recipient mice before transplantation and recipient mice 4 and 9 weeks after transplantation showing (B) number of leukocytes, (C) % neutrophils, (D) number of platelets, and (E) number of lymphocytes. (G-J) PB cell counts from 7-week-old OCY-GsαKO donor and control recipient mice before transplantation and recipient mice 4 and 9 weeks after transplantation showing (G) number of leukocytes, (H) % neutrophils, (I) number of platelets, and (J) number of lymphocytes (n = 3 for donors and n = 8 for recipients). (K-L) LKS (Lin− c-Kit+ Sca-1+), LKS SLAM (Lin− c-Kit+ Sca-1+ CD150+ CD48−), MPPs (Lin− c-kit+ Sca-1+ Thy1.1− Flk-2+), CMPs (IL-7Rα− Lin− c-kit+ Sca-1− FcγRlo CD34+), GMPs (IL-7Rα− Lin− c-kit+ Sca-1− FcγR+ CD34+), and MEPs (IL-7Rα− Lin− c-kit+ Sca-1− FcγRlo CD34−) in (K) BM and (L) spleen from control and OCY-GsαKO female mice (n = 6). (M) Strategy for methylcellulose colony formation assay. (N-O) Myeloid colonies from (N) 2 × 104 BM cells and (O) 1 × 105 spleen cells from 8- to 10-week-old control and OCY-GsαKO mice (n = 4). Error bars represent mean ± SEM. *P < .05 by t test compared with donor.
Myeloproliferation in Gsα-deficient osteocytes is independent of osteoblasts and the Wnt/β-catenin signaling pathway
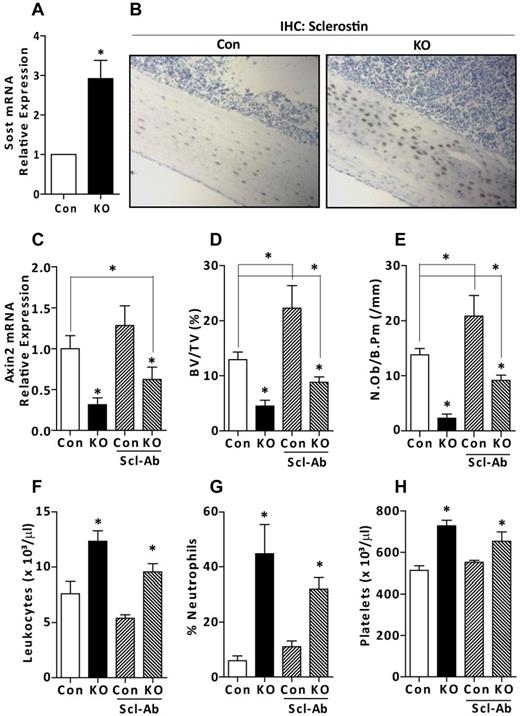
Osteocytes may influence hematopoiesis directly by secreting cytokines or chemokines or indirectly by acting via intermediate cells, such as osteoblasts. The dramatic decrease in the numbers of osteoblasts in OCY-GsαKO mice (Figure 4E), could, per se, alter the BM microenvironment and cause the myeloproliferation. Mature osteocytes are the primary source of sclerostin, a potent suppressor of Wnt/β-catenin signaling, osteoblast numbers, and activity.27 Expression of SOST, the gene encoding sclerostin, was increased 3-fold in mRNA from osteoblast- and BM-depleted osteocyte-enriched long bones from OCY-GsαKO mice compared with controls (Figure 4A). Sclerostin protein was also increased in tibia sections from OCY-GsαKO mice (Figure 4B) as assessed by immunohistochemistry. Moreover, mRNA expression of Axin2, downstream target of Wnt signaling, was significantly decreased in the CD11b+ FACS-sorted myeloid cells from OCY-GsαKO mice (Figure 4C), indicating suppressed Wnt/β-catenin signaling. To investigate whether the myeloproliferation seen in the OCY-GsαKO mice was a consequence of reduced osteoblast number (Figure 4B) and/or suppressed Wnt/β-catenin signaling (Figure 4C), we treated control and OCY-GsαKO mice with a sclerostin-neutralizing antibody. We used a 25 mg/kg body weight dose of antisclerostin antibody, which has previously been shown to effectively neutralize circulating sclerostin.28 Antisclerostin antibody treatment partially restored the suppressed Wnt signaling in OCY-GsαKO mice, as shown by a significant increase in Axin2 gene expression in CD11b+ myeloid cells (Figure 4C). As expected, and as previously reported,28 treatment with antisclerostin antibody significantly increased trabecular bone fraction and osteoblast numbers both in control and OCY-GsαKO mice (Figure 4D-E). However, the bone volume fraction and osteoblast numbers were still significantly decreased in antisclerostin treated OCY-GsαKO mice compared with nontreated controls, suggesting that additional factors, besides sclerostin might be responsible for the decreased osteoblast numbers and osteopenia in these mice. Despite the significant increase in osteoblast numbers, antisclerostin-treated OCY-GsαKO mice continued to exhibit leukocytosis, neutrophilia, and thromobocytosis (Figure 4F-H), indicating that the myeloproliferation is not dependent on reduced osteoblast numbers or suppression of Wnt/β-catenin signaling.
Antisclerostin antibody normalizes osteoblast numbers but does not normalize myeloid cell expansion. (A) Relative expression of sclerostin (Sost) mRNA from femur and tibia from 7-week-old control (Con) and OCY-GsαKO (KO) mice (n = 4). (B) Immunohistochemical staining for sclerostin on tibia sections from 7-week-old control and OCY-GsαKO mice (n = 3). (C) mRNA expression of Axin2 in the CD11b+ FACS-sorted myeloid cells of 7-week-old control (Con) and OCY-GsαKO (KO) mice (n = 4) in saline or antisclerostin antibody-treated mice. (D) Trabecular bone fraction, BV/TV (%), and (E) numbers of osteoblasts per bone perimeter, N.Ob/B.Pm (/mm) in antisclerostin antibody-treated control (Con) and OCY-GsαKO (KO) mice (n = 4). (F-H) PB counts showing (F) number of leukocytes, (G) % neutrophils, and (H) number of platelets in antisclerostin antibody-treated control (Con) and OCY-GsαKO (KO) mice (n = 6). Error bars represent mean ± SEM. *P < .05 by t test.
Antisclerostin antibody normalizes osteoblast numbers but does not normalize myeloid cell expansion. (A) Relative expression of sclerostin (Sost) mRNA from femur and tibia from 7-week-old control (Con) and OCY-GsαKO (KO) mice (n = 4). (B) Immunohistochemical staining for sclerostin on tibia sections from 7-week-old control and OCY-GsαKO mice (n = 3). (C) mRNA expression of Axin2 in the CD11b+ FACS-sorted myeloid cells of 7-week-old control (Con) and OCY-GsαKO (KO) mice (n = 4) in saline or antisclerostin antibody-treated mice. (D) Trabecular bone fraction, BV/TV (%), and (E) numbers of osteoblasts per bone perimeter, N.Ob/B.Pm (/mm) in antisclerostin antibody-treated control (Con) and OCY-GsαKO (KO) mice (n = 4). (F-H) PB counts showing (F) number of leukocytes, (G) % neutrophils, and (H) number of platelets in antisclerostin antibody-treated control (Con) and OCY-GsαKO (KO) mice (n = 6). Error bars represent mean ± SEM. *P < .05 by t test.
Myeloid expansion in OCY-GsαKO mice is not driven by the PPR
Numerous GPCRs signal through the common downstream effector Gsα. Osteocytes express hundreds of GPCRs, as recently reported.22 Among these receptors, the PPR, which signals primarily through Gsα, has been well studied, and its effect on hematopoiesis has been previously reported. Constitutive activation of PTH/PTHrP receptor in osteoblasts increases the numbers of HSCs via increased expression of Notch1 ligand jagged1.4 To test whether the PPR signaling is responsible for the osteocyte-mediated myeloid cell expansion, we engineered mice lacking PPR in osteocytes (OCY-PPRKO) by mating PPR-floxed mice with DMP1-Cre mice. Successful PPR gene ablation in the OCY-PPRKO mice was demonstrated by a significantly reduced PPR mRNA expression in osteocyte-enriched long bones from OCY-PPRKO compared with littermate controls (Figure 5A). In contrast to the complete blood count profile of PB from OCY-GsαKO mice (Figure 2E-H), OCY-PPRKO mice showed no changes in the numbers of leukocytes, neutrophils, platelets, and lymphocytes compared with controls (Figure 5B-E). Immunophenotypic analysis of mature hematopoietic cells in BM and spleens of control and OCY-PPRKO mice showed no difference in the percentage of granulocytes (Gr1+/CD11b+), monocytes (F4/80−/CD11b+), macrophages (F4/80+/CD11b+), erythrocytes (Ter119+/CD45−), B cells (B220+/IgM+), and T cells (CD4+ or CD8a+; Figure 5F-G). Taken together, these results demonstrate that PPR signaling in osteocytes is not driving the myeloproliferation present in the OCY-GsαKO mice.
Disruption of PPR in osteocytes shows no hematologic abnormalities. (A) Relative expression of PPR mRNA in femur and tibia (n = 4) from control (Con) and OCY-PPR (KO) mice. (B-D) PB counts showing (B) number of leukocytes, (C) % neutrophils, (D) number of platelets, and (E) number of lymphocytes in OCY-PPRKO and littermate controls (n ≥ 16). (F-G) Immunophenotypic analysis of hematopoietic cells in BM and spleen showing B cells (B220+ IgM+), granulocytes (Gr1+ CD11b+), erythroid cells (Ter119+ CD45−), monocytes (F4/80− CD11b+), macrophages (F4/80+ CD11b+), T cells (CD4+), and cytotoxic T cells (CD8a+) in (F) BM and (G) spleens of 12-week-old OCY-PPRKO and littermate controls (n ≥ 16). Error bars represent mean ± SEM. *P < .05 by t test.
Disruption of PPR in osteocytes shows no hematologic abnormalities. (A) Relative expression of PPR mRNA in femur and tibia (n = 4) from control (Con) and OCY-PPR (KO) mice. (B-D) PB counts showing (B) number of leukocytes, (C) % neutrophils, (D) number of platelets, and (E) number of lymphocytes in OCY-PPRKO and littermate controls (n ≥ 16). (F-G) Immunophenotypic analysis of hematopoietic cells in BM and spleen showing B cells (B220+ IgM+), granulocytes (Gr1+ CD11b+), erythroid cells (Ter119+ CD45−), monocytes (F4/80− CD11b+), macrophages (F4/80+ CD11b+), T cells (CD4+), and cytotoxic T cells (CD8a+) in (F) BM and (G) spleens of 12-week-old OCY-PPRKO and littermate controls (n ≥ 16). Error bars represent mean ± SEM. *P < .05 by t test.
Osteocytes secrete myeloproliferative factors and G-CSF that partially drives the myeloproliferation.
To investigate whether osteocytes influence hematopoiesis directly by secreting myeloproliferative factors, we developed an ex vivo coculture method in which osteoblast- and BM-depleted OEBEs from control or OCY-GsαKO mice are cocultured with BM cells from control mice in standard growth factor-supplemented methylcellulose media (M3434, StemCell Technologies; Figure 6A). After 10 days of coculture, the number of myeloid colonies was significantly increased in OEBE from control mice and was further significantly increased in the presence of OEBEs derived from OCY-GsαKO mice (Figure 6B). We then used conditioned media collected from control and OCY-GsαKO OEBE cultures (7-day cultures) to generate myeloid colonies from control BM cultured in growth factor-deficient methylcellulose media (M3231). As shown in Figure 6C, conditioned medium induced colony formation, indicating that osteocytes secrete factor(s) that promote myeloproliferation. These factors were further increased in Gsα-deficient osteocytes as the number of myeloid colonies was significantly increased in the presence of conditioned media from OCY-GsαKO OEBE compared with control (Figure 6C). Conditioned media from both control and OCY-GsαKO OEBE also modestly increased the numbers of megakaryocytic colonies in growth factor-deficient MegaCult-C media (supplemental Figure 5C).
Osteocyte-dependent in vitro myeloproliferation. (A) Schematic representation of cocultures of OEBEs or conditioned media from OEBEs with BM cells in methylcellulose media. (B) Number of myeloid colonies in growth factor-supplemented methylcellulose media (M3434) from coculture of BM cells from control mice with OEBEs from control or OCY-GsαKO mice (n = 4). (C) Number of colonies in growth factor-deficient methylcellulose media (M3231) after coculture of BM cells from control mice with conditioned media from OEBEs from control or OCY-GsαKO mice (n = 4). (D) G-CSF mRNA expression in OEBEs (n = 5). G-CSF in (E) serum (n ≥ 7) and (F) OEBE-conditioned media (n = 6) from control and OCY-GsαKO mice. (G) Number of colonies in growth factor-deficient methylcellulose media (M3231) formed after coculture of BM cells from control mice with conditioned media from OEBEs from control or OCY-GsαKO mice and in the presence or absence of a G-CSF neutralizing antibody (n = 4). Error bars represent mean ± SEM. *P < .05 by t test.
Osteocyte-dependent in vitro myeloproliferation. (A) Schematic representation of cocultures of OEBEs or conditioned media from OEBEs with BM cells in methylcellulose media. (B) Number of myeloid colonies in growth factor-supplemented methylcellulose media (M3434) from coculture of BM cells from control mice with OEBEs from control or OCY-GsαKO mice (n = 4). (C) Number of colonies in growth factor-deficient methylcellulose media (M3231) after coculture of BM cells from control mice with conditioned media from OEBEs from control or OCY-GsαKO mice (n = 4). (D) G-CSF mRNA expression in OEBEs (n = 5). G-CSF in (E) serum (n ≥ 7) and (F) OEBE-conditioned media (n = 6) from control and OCY-GsαKO mice. (G) Number of colonies in growth factor-deficient methylcellulose media (M3231) formed after coculture of BM cells from control mice with conditioned media from OEBEs from control or OCY-GsαKO mice and in the presence or absence of a G-CSF neutralizing antibody (n = 4). Error bars represent mean ± SEM. *P < .05 by t test.
To identify the putative factor(s) secreted by control and Gsα-deficient osteocytes, we examined the expression of several cytokines known to promote granulopoiesis and thrombopoiesis.29,30 mRNA expression for IL-4, IL-6, GM-CSF, TNF-α, and thrombopoietin was not different between OEBEs from control and OCY-GsαKO mice (supplemental Figure 3C). RNA expression of SDF-1 and IL-12 was significantly increased in OEBE from OCY-GsαKO mice compared with controls (supplemental Figure 3C), although we could not detect SDF-1 in conditioned media from either OEBE. In accordance with the increased IL-12 gene expression, IL-12 secretion into conditioned media by OCY-GsαKO OEBE was also significantly increased over controls (supplemental Figure 3D). Interestingly, mRNA expression for G-CSF, a potent promoter of production, maturation, function, and mobilization of granulocytes both in vitro and in vivo,30 was increased 2-fold in OEBE from OCY-GsαKO compared with controls (Figure 6D). G-CSF was also increased in serum of OCY-GsαKO mice (Figure 6E) and 7-day conditioned media from GsαKO OEBE (Figure 6F). Pretreatment of 7-day conditioned media from control and OCY-GsαKO OEBE with a G-CSF neutralizing antibody significantly decreased the numbers of methylcellulose colonies compared with isotype-matched IgG-treated conditioned media (Figure 6G), indicating that increased G-CSF production by Gsα-deficient osteocytes partially drives the myeloproliferation in these mice. G-CSF–neutralizing antibody treatment also decreased megakaryocytic colonies; however, the changes were not statistically different from the corresponding groups (supplemental Figure 5C). To investigate whether Gsα-signaling could directly regulate G-CSF expression in bone, we treated OEBE with forskolin and measured G-CSF mRNA expression after 4 hours. Interestingly, forskolin significantly increased G-CSF mRNA expression, rather than suppressing it (supplemental Figure 6), suggesting that the increase in G-CSF present in the OCY-GsαKO mice is not a direct but rather a mediated mechanism.
Discussion
Here we report, for the first time, that disruption of Gsα-signaling specifically in osteocytes results in a dramatic expansion of cells of myeloid lineage in BM, spleen, and PB. The hematopoietic defect is not intrinsic to the hematopoietic cells or dependent on osteoblasts but is dependent on a BM microenvironment altered by Gsα deficiency in osteocytes. The direct regulation of hematopoiesis by osteocytes is supported by several evidences. In our mouse model, Gsα expression and activity is normal in hematopoietic cells and osteoblasts, thereby excluding the possibility of an osteoblastic- or hematopoietic-cell autonomous defect resulting from off-target Cre-recombinase activity. Moreover, transplantation experiments with control BM into lethally irradiated OCY-GsαKO mice fully recapitulate the hematopoietic abnormalities, demonstrating a dependence of the myeloproliferation on a defective BM environment. OCY-GsαKO mice have a dramatic reduction in the number of osteoblasts, in part as a consequence of the elevated sclerostin expression. To investigate whether the hematopoietic defect was dependent on the reduced osteoblast number, we treated OCY-GsαKO and control with antisclerostin antibodies. Antibody treatment increased the numbers of osteoblasts in control and OCY-GsαKO mice but did not abolish the myeloproliferation in the OCY-GsαKO mice. Interestingly, there was a 2-fold increase in the percentage of neutrophils in sclerostin antibody-treated control mice, suggesting that sclerostin per se might be a tonic inhibitor of neutrophils. Therefore, in OCY-GsαKO mice, the decrease in osteoblasts or the suppression of Wnt-βcatenin signaling in BM is not sufficient to drive the myeloproliferation. To investigate whether the myeloid expansion was associated with stem cell dysregulation, we analyzed HSC and myeloid progenitors both in BM and spleen of these OCY mice. Interestingly, there was a significant increase in HSCs in spleens from OCY-GsαKO mice, whereas there was no difference in myeloid progenitors.
Several GPCRs are expressed on osteocytes, including the PPR, EP2, and EP4 prostaglandin receptors, and many more, as recently reported.22 Interestingly, ablation of the PPR in osteocytes (OCY-PPRKO) did not induce hematopoietic abnormalities, indicating that other GPCRs might contribute to the myeloproliferation observed in OCY-GsαKO mice. Alternatively, the OCY-GsαKO phenotype might be the result of lack of signaling from multiple Gsα-coupled receptors, and single receptors deletion in osteocytes might not fully recapitulate the phenotype of the OCY-GsαKO mice.
In this study, we have developed an in vitro model to coculture osteoblast- and BM-depleted OEBE with BM cells that allowed us to explore the relative contribution of osteocytes on the differentiation and function of hematopoietic cells. As shown in Figure 6B, osteocytes directly affected the number of myeloid cells formed in this coculture system. Conditioned media from either control or OCY-GsαKO OEBEs induced a significant increase in myeloid colonies, demonstrating, for the first time, that osteocytes secrete myeloproliferative factors through Gsα-dependent and -independent (CM from control OEBEs) mechanisms. One such factor is G-CSF, whose expression and secretion are significantly increased in Gsα-deficient osteocytes. Osteoblasts have been shown to produce G-CSF through which they can support hematopoiesis.31 Here we show that osteocytes also produce G-CSF and that its increased production by Gsα-deficient osteocytes partially drives the myeloproliferation, as demonstrated by our neutralizing antibody experiments.
A previous study found that an increase in TNF-α in the BM microenvironment led to MPS in RARγ-deficient mice32 and TNF-α promoted granulopoiesis by suppressing the expression of SDF-1.33 However, TNF-α or SDF-1 proteins could not be detected in conditioned media from either control or OCY-GsαKO OEBE.
In conclusion, we have demonstrated that osteocytes can regulate myelopoiesis through the secretion of several factors, including G-CSF, via a Gsα-dependent mechanisms. Using antisclerostin antibodies, we demonstrated that the myeloproliferation is independent of osteoblasts or the Wnt-βcatenin pathway. Lastly, phenotypical characterization of the OCY-PPRKO mice revealed that PPR receptor signaling is not involved in the myeloid proliferation, suggesting that other Gsα-dependent receptors might be involved in osteocytic regulation of hematopoiesis.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Michaela Kneissel and Novartis for providing the antisclerostin antibody and David Dombkowski for assistance with FACS.
This work was supported by the National Institutes of Health (grants AR060221 and DK079161, P.D.P.; grant K08CA138916, D.S.K.), the Intramural Research Program of National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (L.S.W.), and the Harvard Stem Cell Institute (J.Y.W.).
National Institutes of Health
Authorship
Contribution: P.D.P., K.F., and D.S.K designed and interpreted the experiments and wrote the manuscript; K.F., D.S.K., P.D.P., V.S., C.P., and L.B. performed experiments; K.J.B. and X.L. provided technical help; S.L. and R.B. performed histomorphometric analysis; M.C., L.S.W., and J.Q.F. provided material; and J.Y.W., H.M.K., and D.T.S. provided scientific input and critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paola Divieti Pajevic, Endocrine Unit, Massachusetts General Hospital, 50 Blossom St, Thier 1101, Boston, MA 02114; e-mail: divieti@helix.mgh.harvard.edu.
References
Author notes
K.F. and D.S.K. contributed equally to this study.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal