In this issue of Blood, Fulzele and colleagues provide exciting new data implicating bone-imbedded osteocytes in the regulation of hematopoiesis.1
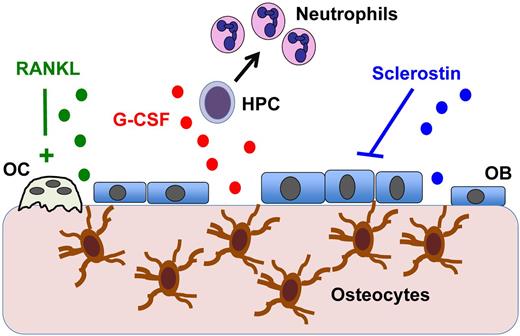
Osteocytes are the most abundant cell type in the bone, comprising more than 90% of all cells within the bone matrix or on bone surfaces. Osteocytes are derived from osteoblasts and are entombed in the bone matrix during bone deposition. Osteocytes were originally thought to be rather inert cells whose function was limited to maintaining bone matrix locally. However, osteocytes are connected to other bone cells, endothelial cells, and hematopoietic cells near endosteal surfaces through an extensive network of canaliculi through which cytoplasmic projections of osteocytes travel. This network allows for the transport of proteins produced by osteocytes to act on cells in the bone marrow cavity. For example, osteocyte production of sclerostin and RANK ligand are key regulators of osteoblast and osteoclast activity, respectively (see figure).2,3 Because osteoblasts have been implicated in hematopoietic stem cell and B-cell maintenance, these observations suggested the hypothesis that signals generated by osteocytes contribute to the regulation of hematopoiesis.
Osteocyte regulation of hematopoiesis. Osteocytes imbedded in bone communicate with cells in the bone marrow through canaliculi. Osteocytes are known to produce RANKL, which activates osteoclasts (OCs), and the Wnt antagonist sclerostin, which suppresses osteoblasts (OBs). The present study by Fulzele et al shows that osteocytes also produce G-CSF (and potentially other myelopoiesis-stimulating factors) that results in hematopoietic progenitor cell (HPC) proliferation and increased neutrophil and monocyte production.1
Osteocyte regulation of hematopoiesis. Osteocytes imbedded in bone communicate with cells in the bone marrow through canaliculi. Osteocytes are known to produce RANKL, which activates osteoclasts (OCs), and the Wnt antagonist sclerostin, which suppresses osteoblasts (OBs). The present study by Fulzele et al shows that osteocytes also produce G-CSF (and potentially other myelopoiesis-stimulating factors) that results in hematopoietic progenitor cell (HPC) proliferation and increased neutrophil and monocyte production.1
To test the hypothesis, Fulzele and colleagues generated mice with a conditional deletion of GαS in osteocytes. GαS is an obligate subunit of several G protein–coupled receptors that are expressed in osteocytes. Surprisingly, deletion of GαS in osteocytes resulted in a myeloproliferative-like syndrome characterized by neutrophilia, thrombocytosis, and splenomegaly. However, unlike human myeloproliferative syndromes, the hematopoietic abnormalities did not result in increased mortality or leukemic transformation. This phenotype was secondary to an altered bone marrow microenvironment, because GαS-osteocytes–deleted mice transplanted with wild-type bone marrow rapidly developed a myeloproliferative phenotype. Indeed, deletion of GαS in osteocytes results in osteocyte expansion, loss of osteoblasts, and osteopenia. The authors show that increased osteocytes expression of sclerostin, a potent Wnt antagonist, is responsible for the loss of osteoblasts. However, sclerostin and osteoblast loss are not required for the myeloproliferative phenotype, because treatment with a sclerostin-neutralizing antibody, while rescuing the osteoblast defect, did not correct the leukocytosis.
The authors next established a novel ex vivo co-culture system using osteocyte-enriched bone explants to look for secreted factors that promote myeloid progenitor proliferation. These studies led to the discovery that osteocytes produce large amounts of granulocyte colony-stimulating factor (G-CSF), the principal cytokine regulating granulopoiesis. Although not tested in vivo, neutralizing antibodies to G-CSF inhibited the ability of osteocyte-conditioned media to stimulate myeloid progenitor growth in vitro, providing direct evidence that G-CSF, at least partially, drives the myeloproliferative phenotype in GαS-osteocytes–deleted mice. Consistent with this conclusion, transgenic mice that constitutively overexpress G-CSF develop a nonfatal myeloproliferative phenotype that is very similar to that observed in GαS-osteocytes–deleted mice, including neutrophilia, splenomegaly, and osteopenia.4
This provocative study raises several important questions. The authors show that impaired parathyroid home/parathyroid hormone-related protein receptor signaling in osteocytes is not responsible for the MPD-like phenotype. Which, then, of the approximately 50 G protein–coupled receptors that are expressed in osteocytes, are responsible for the myeloproliferative-like phenotype? While likely contributing to the neutrophilia, the elevated level of G-CSF is unlikely to cause thrombocytosis, and the factor(s) that are stimulating thrombopoiesis are unclear. Finally, these data raise the possibility that inherited disorders of osteocytes may contribute to the pathogenesis of certain blood disorders.
The important study by Fulzele and colleagues adds osteocytes to the list of bone marrow stromal cells that regulate hematopoiesis. Osteocytes play an essential role in sensing and responding to mechanical stress on bone. Thus, osteocytes may provide a mechanism by which mechanical and other stresses on bone regulate hematopoiesis.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal