Key Points
Notch1/DII4-mediated signals are normally suppressed by LRF, preventing HSCs from premature T-cell differentiation in the bone marrow.
Erythroblastic islands may have the capacity to regulate the fate and function of HSCs.
Abstract
Hematopoietic stem cells (HSCs) are the most primitive cells in the hematopoietic system and are under tight regulation for self-renewal and differentiation. Notch signals are essential for the emergence of definitive hematopoiesis in mouse embryos and are critical regulators of lymphoid lineage fate determination. However, it remains unclear how Notch regulates the balance between HSC self-renewal and differentiation in the adult bone marrow (BM). Here we report a novel mechanism that prevents HSCs from undergoing premature lymphoid differentiation in BM. Using a series of in vivo mouse models and functional HSC assays, we show that leukemia/lymphoma related factor (LRF) is necessary for HSC maintenance by functioning as an erythroid-specific repressor of Delta-like 4 (Dll4) expression. Lrf deletion in erythroblasts promoted up-regulation of Dll4 in erythroblasts, sensitizing HSCs to T-cell instructive signals in the BM. Our study reveals novel cross-talk between HSCs and erythroblasts, and sheds a new light on the regulatory mechanisms regulating the balance between HSC self-renewal and differentiation.
Introduction
For life-long hematopoiesis, most immature hematopoietic stem cells (HSCs), so-called long-term HSCs (LT-HSCs), remain dormant, but in response to hematopoietic stress, they actively cycle to generate multi-lineage blood cells without depleting the HSC pool.1 These fate decisions are governed by intrinsic and extrinsic mechanisms. Relevant to extrinsic regulation, adult HSCs reside in a specialized microenvironment within the bone marrow (BM), the “niche,” which is composed of multiple types of supporting cells that express membrane-bound and secreted factors.2,3 Osteoblasts, endothelial cells, perivascular cells, mesenchymal stem cells, and glial cells have been proposed as components of the BM microenvironment.3 These studies reveal how both self-renewal and quiescence of adult HSCs are maintained; however, how the balance between self-renewal and differentiation is regulated in the niche remains largely unknown.
The highly conserved Notch signaling pathway regulates many cell-fate decisions and homeostasis in various organs.4 In humans its dysregulation is associated with many types of cancer and inherited congenital anomalies.4 There are 4 mammalian homologs of the Notch receptor (Notch 1-4) and 5 ligands: Delta-like-1, 3 and 4, which belong to the Delta ligand family, and Jagged-1 and 2, which belong to the Serrate family.5 After ligand engagement, the intracellular domain of the Notch receptor (ICN) undergoes multiple proteolytic cleavages and translocates to the nucleus, where it binds the recombination signal-binding protein 1 for jκ (RBP-jκ, also known as CSL/CBF1) and mastermind-like coactivators (MAML1-3), and activates downstream targets, such as hairy and enhancer of split homologue-1 (Hes-1).5 Notch is indispensable for the emergence of embryonic hematopoiesis6 ; however, its role in adult HSC function is controversial. In addition, it is not completely understood at which HSC/progenitor stages Notch receptors are expressed and which Notch ligands are expressed in the BM microenvironment.
LRF (for leukemia/lymphoma related factor), also known as Pokemon, ZBTB7a, FBI-1, and OCZF, is a POZ and Krüppel (POK)–type transcription factor with multiple functions in hematopoietic development, oncogenesis, and humoral immunity.7 In mice, Lrf (encoded by the Zbtb7a gene) inactivation in adult HSCs (Zbtb7aFlox/FloxMx1-Cre+) promotes development of CD4/8 double-positive (DP) T cells in the BM at the expense of B-cell development.8 Treatment of Lrf-deleted mice with γ secretase inhibitors, which block Notch signaling, rescues aberrant lymphoid development, suggesting that Lrf antagonizes the Notch pathway at the HSC/progenitor level.8 Furthermore, despite its critical role in the B versus T-lymphoid lineage fate determination, Lrf is dispensable for maintenance of “committed” BM B cells, as early B-cell development in the BM is grossly normal when the Lrf gene is inactivated at the pro-B cell stage.9
In this study, we asked how HSC self-renewal and lymphoid differentiation is balanced in the context of Notch signaling in adult BM. We show that Notch1/Dll4-mediated T-cell instructive signals to LT-HSCs are suppressed by Lrf expression in the BM microenvironment.
Methods
Mice
Lrf conventional (Zbtb7a+/−), Lrf conditional (Zbtb7aFlox/Flox), Notch1 conditional knockout and erythroid-specific Cre mice (ErGFPCre) are described elsewhere.8,10-12 All Mx1-Cre mice were injected with polyinosinic-polycytidylic acid (pIpC; Sigma-Aldrich) 2 or 3 times at 3-day intervals at 4 to 8 weeks of age, unless otherwise indicated. For functional analysis of LT-HSCs, C57BL/6 mice (10-12 weeks old) were obtained from JAX. B6.SJL-PtprcaPepcb/BoyJ mice served as recipients for BM transplant. All mice were housed at the City of Hope (COH) Animal Resources Center or at Animal Resource Children's Hospital Boston (ARCH). All animal experiments were approved by the Institutional Animal Care and Use Committees, according to national, state, and institutional guidelines.
Statistical analysis
P values were determined by Mann-Whitney test using Prism Version 5.0d software (GraphPad).
Results
Lrf knockout mice show significantly reduced numbers of LT-HSCs and Flt3+ lymphoid progenitors
To determine at which HSC/progenitor stage Lrf counteracts Notch signaling, we positively selected c-Kit+ cells and then performed multi-color fluorescence-activated cell sorting (FACS) analysis with antibodies for cell surface markers, including SLAM family proteins, Flt3 and Vcam-1.13,14 LT-HSCs and CLPs were defined as Lin−IL7Rα−Sca1+c-Kit+Flt3−CD150+CD48−Vcam-1dim+ and Lin−IL7Rα+Flt3+CD48+Vcam-1-15, respectively. In Lrf knockout mice, the Flt3-positive population was barely detectable within the LSK fraction (Figure 1A), and Lin−IL7Rα+Sca1+c-Kit+ cells, which we previously proposed to be CLPs (Figure 1A asterisk),8 were Flt3-negative. Instead, early T-cell precursors, which showed high CD25 expression (not shown), accumulated within the LSK fraction (Figure 1A arrowhead, bottom row, second from right). Furthermore, both the proportion and absolute number of LT-HSCs were significantly reduced in Zbtb7aFlox/FloxMx1-Cre+ mice relative to control (Figure 1B). CD34 is a reliable marker of primitive hematopoietic cells,15 few CD34− LT-HSCs were seen in Zbtb7aFlox/FloxMx1-Cre+ mice (Figure 1C). Loss of LT-HSCs in Lrf knockout mice was confirmed by Hoechst 33342–based side population (SP) analysis (Figure 1D). HSC compartments in fetal liver (FL) of Lrf knockout (Zbtb7a−/−) embryos were also examined at 13.5 days postcoitum (dpc). As in adult BM, FL-LT-HSCs numbers were significantly reduced (Figure 1E) with a concomitant increase in the number of T-cell precursors16 at the expense of B-cell development (Figure 1F-G).8 Taken together, these observations indicate that Lrf deficiency significantly reduces the number of LT-HSCs and lymphoid progenitors and is accompanied by enhanced production of T-cell precursors in adults and embryos.
Lrf knockout mice show significantly reduced numbers of LT-HSCs and Flt3+ lymphoid progenitors. (A) Representative HSC/progenitor FACS profiles of control (Zbtb7aFlox/FloxMx1-Cre−) and hematopoietic-specific Lrf knockout mice (Zbtb7aFlox/FloxMx1-Cre+). c-Kit–positive cells were purified from total BM cells using c-Kit microbeads and MACS LS columns and stained for surface markers. Arrowhead (bottom row, second from right) indicates CD48high+ T-cell progenitors within the LSK CD150−Flt3− population. Aberrant Sca1high+ T-cell progenitors within the Lin−Flt3+Il7Rα+ fraction are depicted with an asterisk (second row from bottom, left). LT-HSC: long-term hematopoietic stem cell; CLP: common lymphoid progenitor. (B) Dot graphs of absolute counts of LT-HSCs [LSK (Lin−Sca1+c-Kit+) Il7Rα−Flt3−CD150+CD48−VCAM1dim+] and CLPs (Lin−Il7Rα+Flt3+CD150−CD48+VCAM1−) per 2 legs (2 tibias and 2 femurs). Horizontal black bars: average value; error bars: standard deviation. (C) Proportions of CD34-negative LT-HSCs within the LSK CD150+Flt3− population. Horizontal black bars: average value; error bars: standard deviation. (D) Representative FACS profiles of side population analysis in BM cells from control and Lrf knockout mice. (E) Absolute numbers of LT-HSCs in 13.5 dpc fetal liver (FL) from WT (+/+), Lrf heterozygous (+/−) and Lrf knockout (−/−) mice. Horizontal black bars: average value; error bars: standard deviation. (F) Representative FACS profiles for FL T-cell precursors. (G) Dot graphs show proportions (left) and absolute counts (right) of FL T-cell precursors (TER119−Gr1−c-Kit+PIR+IL7Rα+) in 13.5 dpc FL cells. Horizontal black bars: average value; error bars: standard deviation.
Lrf knockout mice show significantly reduced numbers of LT-HSCs and Flt3+ lymphoid progenitors. (A) Representative HSC/progenitor FACS profiles of control (Zbtb7aFlox/FloxMx1-Cre−) and hematopoietic-specific Lrf knockout mice (Zbtb7aFlox/FloxMx1-Cre+). c-Kit–positive cells were purified from total BM cells using c-Kit microbeads and MACS LS columns and stained for surface markers. Arrowhead (bottom row, second from right) indicates CD48high+ T-cell progenitors within the LSK CD150−Flt3− population. Aberrant Sca1high+ T-cell progenitors within the Lin−Flt3+Il7Rα+ fraction are depicted with an asterisk (second row from bottom, left). LT-HSC: long-term hematopoietic stem cell; CLP: common lymphoid progenitor. (B) Dot graphs of absolute counts of LT-HSCs [LSK (Lin−Sca1+c-Kit+) Il7Rα−Flt3−CD150+CD48−VCAM1dim+] and CLPs (Lin−Il7Rα+Flt3+CD150−CD48+VCAM1−) per 2 legs (2 tibias and 2 femurs). Horizontal black bars: average value; error bars: standard deviation. (C) Proportions of CD34-negative LT-HSCs within the LSK CD150+Flt3− population. Horizontal black bars: average value; error bars: standard deviation. (D) Representative FACS profiles of side population analysis in BM cells from control and Lrf knockout mice. (E) Absolute numbers of LT-HSCs in 13.5 dpc fetal liver (FL) from WT (+/+), Lrf heterozygous (+/−) and Lrf knockout (−/−) mice. Horizontal black bars: average value; error bars: standard deviation. (F) Representative FACS profiles for FL T-cell precursors. (G) Dot graphs show proportions (left) and absolute counts (right) of FL T-cell precursors (TER119−Gr1−c-Kit+PIR+IL7Rα+) in 13.5 dpc FL cells. Horizontal black bars: average value; error bars: standard deviation.
Lrf-deficient LT-HSCs lose their stem cell signature
Next we performed gene expression microarray analysis of FACS-sorted LT-HSCs to assess the effect of Lrf loss on the LT-HSC transcriptome. Nine days after injection of adult mice with pIpC to activate Cre, total RNAs were isolated from double-sorted LT-HSCs from Zbtb7aFlox/+Mx1-Cre+ and Zbtb7aFlox/FloxMx1-Cre+ mice and processed for microarray analysis (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This time point was chosen because: (1) the number of LT-HSCs was temporally increased but equivalent between control and Lrf-deficient mice and (2) to minimize potential effects of pIpC on the HSC cell cycle.17 PIpC-treated Zbtb7aFlox/+Mx1-Cre+ mice served as controls to normalize for potential effects of Cre recombinase expression and pIpC administration. Gene set enrichment analysis (GSEA) was performed using publicly available gene sets representing expression signatures seen in HSC/progenitors.18,19 Although there were no differences in HSC number or expression patterns of HSC markers between control (Zbtb7aFlox/+Mx1-Cre+ pIpC+) and Lrf knockout (Zbtb7aFlox/FloxMx1-Cre+ pIpC+) mice at the time of sample collection, overall gene expression signatures were significantly changed. In Lrf-deficient LT-HSCs, stem cell signatures were substantially lost, whereas genes related to T-cell receptor signaling pathways were enriched (supplemental Figure 1B). Database for annotation, visualization, and integrated discovery (DAVID) analysis revealed 112 probe sets up or down-regulated more than 1.5-fold in Lrf-deficient LT-HSCs relative to controls (supplemental Tables 1 and 2). The most significant and over-represented biologic annotations in those cells were T-cell related (supplemental Figure 1C). Microarray data are available at GEO under accession number GSE41839.
HSC function under stress conditions is impaired in Lrf knockout mice
Cycling HSCs are sensitive to treatment with 5-fluoro-uracil (5-FU), an S phase-specific cytotoxic chemotherapeutic agent.17,20 To examine LT-HSC self-renewal capacity under stress conditions, Zbtb7aFlox/FloxMx1-Cre+ mice were challenged with 5-FU 9 days after the first of 2 (days 1 and 3) pIpC injections, and absolute numbers of LT-HSCs were examined over time. As previously reported, pIpC treatment induced a temporary increase in total LT-HSC number, regardless of genotype.17 These numbers dropped dramatically 1 day after 5-FU treatment (Figure 2A). Although LT-HSC numbers in control mice recovered to pretreatment levels 3 weeks after 5-FU treatment, their levels in knockout mice remained low. Of note, DP T cells, which were not seen before 5-FU treatment in either genotype, developed in the BM of Lrf knockout mice 3 weeks after 5-FU treatment (not shown), suggesting that 5-FU–resistant dormant HSCs differentiate toward the T-cell lineage rather than self-renewing in response to the myeloablative stress (Figure 2B).
In vivo function of Lrf in HSCs. (A) Lrf knockout mice were challenged with 5-FU 9 days after the first pIpC injection and LT-HSC recovery was examined at indicated times. Graph shows absolute numbers of LT-HSCs after 5-FU treatment over time. Horizontal black bars: average value; error bars: standard deviation. (B) In Zbtb7aFlox/FloxMx1-Cre+ mice, 5-FU–resistant dormant HSCs differentiate toward the T-cell lineage rather than self-renewing in response to the myeloablative stress. (C) Schematic representation of bone marrow transplantation (BMT). Fifty double-sorted LT-HSCs were transplanted into lethally irradiated CD45.1 recipient mice. (D) Shown are time-course follow-up of recipients' PB counts [myeloid (Gr1+CD11b+), B (B220+), and T cells (CD4+ or CD8+)]. Error bars: standard deviation. (E) BM hematopoiesis was followed up to 18 months after the last pIpC injection. Representative FACS profiles of hematopoietic development in BM and spleen analyzed 1 year after the last pIpC injection in control (Zbtb7aFlox/FloxMx1-Cre−) and Lrf knockout (Zbtb7aFlox/FloxMx1-Cre+) mice. BM DP T-cell development, B-cell differentiation block, normal myeloid development, and an anemia phenotype in spleen21 were evident in Lrf knockout mice.
In vivo function of Lrf in HSCs. (A) Lrf knockout mice were challenged with 5-FU 9 days after the first pIpC injection and LT-HSC recovery was examined at indicated times. Graph shows absolute numbers of LT-HSCs after 5-FU treatment over time. Horizontal black bars: average value; error bars: standard deviation. (B) In Zbtb7aFlox/FloxMx1-Cre+ mice, 5-FU–resistant dormant HSCs differentiate toward the T-cell lineage rather than self-renewing in response to the myeloablative stress. (C) Schematic representation of bone marrow transplantation (BMT). Fifty double-sorted LT-HSCs were transplanted into lethally irradiated CD45.1 recipient mice. (D) Shown are time-course follow-up of recipients' PB counts [myeloid (Gr1+CD11b+), B (B220+), and T cells (CD4+ or CD8+)]. Error bars: standard deviation. (E) BM hematopoiesis was followed up to 18 months after the last pIpC injection. Representative FACS profiles of hematopoietic development in BM and spleen analyzed 1 year after the last pIpC injection in control (Zbtb7aFlox/FloxMx1-Cre−) and Lrf knockout (Zbtb7aFlox/FloxMx1-Cre+) mice. BM DP T-cell development, B-cell differentiation block, normal myeloid development, and an anemia phenotype in spleen21 were evident in Lrf knockout mice.
We next performed in vivo BM transplantation (BMT)/competitive repopulation experiments. To do so, control or Lrf-deficient LT-HSCs were transferred to lethally irradiated recipient mice and reconstitution of donor-derived cells (CD45.2+) in lymphoid and myeloid lineage cells was determined by peripheral blood (PB) FACS analysis. Lrf inactivation was induced by 2 pIpC injections at 3-day intervals. Then, 50 LT-HSCs (CD45.2+), which had been FACS-sorted twice to ensure purity, together with support BM mononuclear cells (CD45.1+) were transplanted per mouse (Figure 2C). As anticipated,8 we observed significantly fewer donor-derived B-cells in recipients transplanted with Lrf-deficient LT-HSCs. Moreover, despite accumulation of donor-derived DP T cells in the BM (not shown), donor-derived PB T cells were also reduced in recipients transplanted with Lrf-deficient LT-HSCs (Figure 2D). In contrast, reconstitution of myeloid lineage cells was largely unaffected in either genotype. Moreover, Lrf-deficient LT-HSCs produced myeloid and erythroid lineage cells for a long period after Lrf deletion. We followed hematopoiesis in Zbtb7aFlox/FloxMx1-Cre+ mice for up to 18 months after the last pIpC injection (Figure 2E). Although LT-HSC numbers remained low (supplemental Figure 1D), Lrf-deficient LT-HSCs maintained long-term hematopoiesis. DP T cells were evident in the BM of Lrf knockout mice, with a concomitant block of B-cell development. Expansion of immature erythroblasts, which underlies the anemia phenotype seen in Lrf knockout mice,21 and normal development of myeloid lineage cells was observed in the spleen (Figure 2E). Quantitative RT-PCR (qRT-PCR) revealed little or no Lrf mRNA in multiple lineage cells, confirming Lrf-deficient hematopoiesis (not shown).
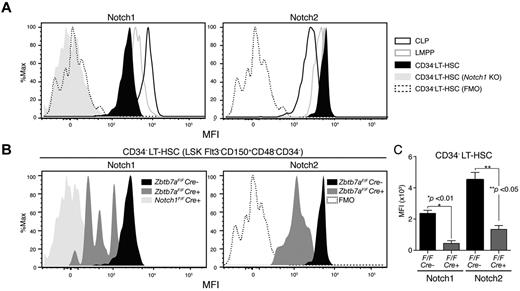
CD34− LT-HSCs expressing high levels of Notch are sensitive to Lrf inactivation
We examined surface Notch expression in HSC/progenitor compartments with various Notch monoclonal antibodies22 using HSCs/progenitors from Notch1 knockout mice (Notch1Flox/FloxMx1-Cre+) as negative controls. For Notch1 FACS, signal intensities of Notch1-deficient cells and those of the FMO control completely overlapped, indicating antibody specificity (Figure 3A). Notch1 protein was expressed at lower levels relative to lymphoid-restricted progenitors in the CD34-negative LT-HSC fraction (LSK CD150+CD48−Flt3−IL7Rα−CD34−: CD34− LT-HSC), which is the most primitive fraction, and Notch1 expression increased as cells differentiated toward lymphoid-restricted progenitors (Figure 3A). By contrast, Notch2 was highly expressed in LT-HSCs and its expression was reduced in CLPs. Notch3 and Notch4 were not detected in any normal HSC/progenitor compartment (not shown), but Notch3 was up-regulated in the Lrf-deficient progenitor fraction, which presumably consists of T-cell precursors (supplemental Figure 2).
CD34− LT-HSCs expressing high levels of Notch are sensitive to Lrf inactivation. (A) Histograms show mean fluorescence intensity (MFI) for Notch1 and Notch2 in indicated HSC/progenitor compartments. Signal levels in Notch1-deficient LT-HSCs (N1 KO: Notch1Flox/FloxMx1-cre+) and fluorescence minus one (FMO) controls are also shown. CLP: Lin−IL7Rα+Flt3+CD48+; LMPP: Lin−IL7Rα−Sca1+c-Kit+Flt3+CD150−CD48+. (B) CD34− LT-HSCs expressing high levels of Notch are lost after Lrf inactivation. Representative FACS profiles for Notch1 and Notch2 in control (Zbtb7aFlox/FloxMx1-Cre−) and Lrf knockout (Zbtb7aFlox/FloxMx1-Cre+) mice. All mice were injected with pIpC 3 times, and Notch expression levels within the CD34− LT-HSC population analyzed 1 month after the last injection. Data for Notch1 KO, FMO, and control are same as those shown in panel A. (C) Graph shows MFI of Notch staining in control (black) and Lrf-deficient (red) CD34− LT-HSCs 1 month after the last injection. Error bars: standard deviation.
CD34− LT-HSCs expressing high levels of Notch are sensitive to Lrf inactivation. (A) Histograms show mean fluorescence intensity (MFI) for Notch1 and Notch2 in indicated HSC/progenitor compartments. Signal levels in Notch1-deficient LT-HSCs (N1 KO: Notch1Flox/FloxMx1-cre+) and fluorescence minus one (FMO) controls are also shown. CLP: Lin−IL7Rα+Flt3+CD48+; LMPP: Lin−IL7Rα−Sca1+c-Kit+Flt3+CD150−CD48+. (B) CD34− LT-HSCs expressing high levels of Notch are lost after Lrf inactivation. Representative FACS profiles for Notch1 and Notch2 in control (Zbtb7aFlox/FloxMx1-Cre−) and Lrf knockout (Zbtb7aFlox/FloxMx1-Cre+) mice. All mice were injected with pIpC 3 times, and Notch expression levels within the CD34− LT-HSC population analyzed 1 month after the last injection. Data for Notch1 KO, FMO, and control are same as those shown in panel A. (C) Graph shows MFI of Notch staining in control (black) and Lrf-deficient (red) CD34− LT-HSCs 1 month after the last injection. Error bars: standard deviation.
We next examined Notch expression levels in Lrf-deficient CD34− LT-HSCs 1 month after the last pIpC injection (Figure 3B). CD34− LT-HSCs expressing high levels of Notch were absent, whereas those expressing low levels remained in the BM (Figure 3B). Mean fluorescence intensitie (MFI) of surface Notch protein in Lrf-deficient CD34− LT-HSCs were significantly lower compared with controls (Figure 3B-C). Thus, CD34− LT-HSCs expressing high levels of Notch proteins are particularly sensitive to Lrf inactivation and differentiate into T cells in the BM.
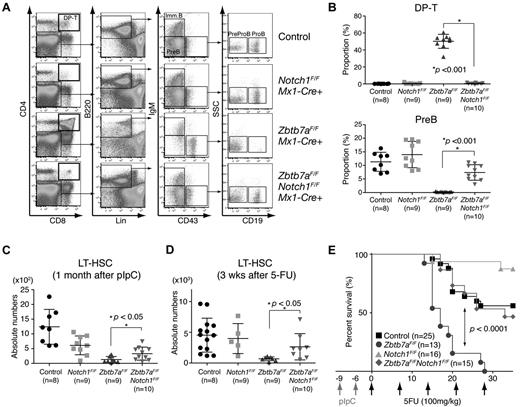
Notch1 loss rescues aberrant lymphoid lineage fate and HSC defects in Lrf knockout mice
Treatment of Zbtb7aFlox/FloxMx1-Cre+ mice with γ-secretase inhibitors rescues normal lymphoid development,8 and Notch1 conditional knockout mice exhibit phenotypes reciprocal to those of Lrf knockout mice.10 Because CD34− LT-HSCs expressing low levels of Notch were largely unaffected by Lrf inactivation, we asked whether loss of Notch receptor(s) in Lrf-deficient LT-HSCs would normalize BM hematopoiesis. To do so, we generated Lrf/Notch1 double-knockout mice (Zbtb7aFlox/FloxNotch1Flox/FloxMx1-Cre+) and analyzed BM hemato/lymphoid development. As expected, DP T cells, which were seen in Lrf knockout mice, were completely absent and the proportions of BM B cells were grossly normal in Lrf/Notch1 double-knockout mice (Figure 4A-B), whereas the anemia phenotype persisted (not shown). Furthermore, absolute numbers of LT-HSCs in Lrf/Notch1 double knockout mice were significantly greater than those seen in Lrf knockout mice (Figure 4C). To determine whether Notch1 deficiency enhances self-renewal of Lrf-deficient HSCs after 5-FU stress, double-knockout mice were treated with 5-FU as described (Figure 2A), and LT-HSC numbers were determined 3 weeks later. LT-HSC numbers in double-knockout mice recovered to pretreatment levels and were equivalent to control and Notch1 single-knockout mice (Figure 4D). We next assessed hematopoietic reconstitution capacity of mutant mice in vivo by monitoring survival after sequential 5-FU injection.20 Control, Lrf knockout, Notch1 knockout and Lrf/Notch1 double-knockout mice were injected with pIpC twice, and 5-FU administered 9 days after the first injection, followed by weekly 5-FU treatment. Lrf knockout mice died significantly earlier than did control mice; however, Lrf/Notch1 double-knockout mice survived as long as controls, suggesting that Notch1 loss restored the number of functional HSCs (Figure 4E). Unexpectedly, lack of Notch1 protected against sequential 5-FU treatment, although mechanisms underlying this observation remain unknown.
Notch1 loss rescued aberrant lymphoid fate determination and HSC defects in Lrf knockout mice. (A) Representative FACS profiles of BM B-cell compartments in control, Lrf knockout (Zbtb7aFlox/FloxMx1-Cre+), Notch1 knockout (Notch1Flox/FloxMx1-Cre+), and Lrf/Notch1 double-knockout (Zbtb7aFlox/FloxNotch1Flox/FloxMx1-Cre+) mice 1 month after the last pIpC injection. (B) Proportions of DP T (top) and pre-B cell compartments (bottom) in BM. Horizontal black bars: average value; error bars: standard deviation. (C) Numbers of LT-HSCs recovered in Lrf knockout mice on Notch1 loss. Dot graph shows absolute numbers of LT-HSCs per 2 legs in indicated mice 1 month after the last pIpC injection. Horizontal black bars: average value; error bars: standard deviation. (D) LT-HSCs numbers recover normally after 5-FU challenge in Lrf/Notch1 double-knockout mice. Dot graph shows absolute numbers of LT-HSCs per 2 legs in indicated mice 3 weeks after 5-FU treatment. Horizontal black bars: average value; error bars: standard deviation. (E) Survival curves after sequential 5-FU treatment. 5-FU (100 mg/kg) was administered weekly. Survival rates in each group were analyzed and P value calculated using a log-rank Mantel-Cox test.
Notch1 loss rescued aberrant lymphoid fate determination and HSC defects in Lrf knockout mice. (A) Representative FACS profiles of BM B-cell compartments in control, Lrf knockout (Zbtb7aFlox/FloxMx1-Cre+), Notch1 knockout (Notch1Flox/FloxMx1-Cre+), and Lrf/Notch1 double-knockout (Zbtb7aFlox/FloxNotch1Flox/FloxMx1-Cre+) mice 1 month after the last pIpC injection. (B) Proportions of DP T (top) and pre-B cell compartments (bottom) in BM. Horizontal black bars: average value; error bars: standard deviation. (C) Numbers of LT-HSCs recovered in Lrf knockout mice on Notch1 loss. Dot graph shows absolute numbers of LT-HSCs per 2 legs in indicated mice 1 month after the last pIpC injection. Horizontal black bars: average value; error bars: standard deviation. (D) LT-HSCs numbers recover normally after 5-FU challenge in Lrf/Notch1 double-knockout mice. Dot graph shows absolute numbers of LT-HSCs per 2 legs in indicated mice 3 weeks after 5-FU treatment. Horizontal black bars: average value; error bars: standard deviation. (E) Survival curves after sequential 5-FU treatment. 5-FU (100 mg/kg) was administered weekly. Survival rates in each group were analyzed and P value calculated using a log-rank Mantel-Cox test.
Lrf represses Dll4 expression in erythroblasts
Our observations suggested that CD34− LT-HSCs expressing high levels of Notch (Figure 3B) are hypersensitive to Notch signaling caused by Lrf deficiency and that the signal is transmitted mainly through Notch1 in the most primitive LT-HSCs (Figure 4). Although microarray analysis (supplemental Figure 1A) had revealed that expression of Notch receptors and ligands was unchanged at the LT-HSC level, the Notch target Hes1 was up-regulated in Lrf knockout cells (supplemental Table 1), a finding confirmed by qRT-PCR of FACS-sorted LT-HSCs harvested at day 9 after the first pIpC injection (supplemental Figure 3A). These data suggest Lrf does not directly regulate transcription of Notch mRNA but rather that Notch transcriptional activity or Notch receptor/ligand efficiency could be modulated. Coimmunoprecipitation (Co-IP) analysis revealed no direct interaction between Lrf and ICN1 or RBP-jκ, whereas ICN1 and RBP-jκ interacted as expected (supplemental Figure 3B). We also asked whether Lrf directly down-regulated transcription of Notch targets. To do so, we used luciferase reporter assays with constructs containing either artificial RBP-jκ/CSL binding sites (4xCSL-Luc) or the mouse Hes1 promoter (mHes1-Luc). Although ICN1 efficiently activated both reporters, no transactivation/repression was seen in the presence of Lrf overexpression (supplemental Figure 3C).
We next asked whether Lrf regulates Notch ligand expression or receptor/ligand efficiency. The fact that Dll4 is indispensable for T-cell development23 and is a nonredundant Notch 1 ligand in thymus24 suggests that Dll4 might be a Notch ligand in the BM. Immunohistochemical (IHC) analysis revealed that Dll4 is expressed on mature vessel endothelium and at lower levels on immature endothelium, and that erythroid, lymphoid, myeloid, and megakaryocyte colonies were Dll4-negative (Figure 5A, supplemental Figure 4). In spleen, Dll4 was seen on arteriolar endothelium of the white pulp and the smaller blood vessels of the red pulp, consistent with a previous report using Dll4-LacZ reporter mice.25 Strikingly, clusters of Dll4-positive cells were evident in the BM of both Lrf knockout and Lrf/Notch1 double-knockout mice, indicating that Lrf deficiency up-regulates Dll4 independent of Notch1 (Figure 5A). FACS analysis further indicated that Dll4 up-regulation was limited to erythroblasts at late stages of differentiation. Specificities of the anti-Dll1 and -Dll4 antibodies were confirmed using a series of OP9 stromal cell lines (supplemental Figure 5A). Ter119+CD71+ cells, which consist primarily of basophilic and polychromatophilic-erythroblasts, expressed Dll4 at high levels, but early erythroid precursors (eg, colony forming unit erythroid [CFU-E]) and terminally differentiated reticulocytes/erythrocytes (Ter119+CD71−FSClow) did not (Figure 5B). Dll4 was not expressed in either Lrf-deficient HSCs/progenitors or nonerythroid cells (Figure 5B-C). Dll4 up-regulation was not because of stress erythropoiesis seen in Zbtb7aFlox/FloxMx1 Cre+ mice,21 as erythroblasts from phenylhydrazine (PHZ)–treated mice, a model of stress hematopoiesis, did not express Dll4 (supplemental Figure 5B). Of note, Dll1 was not expressed in BM erythroblasts regardless of genotype (supplemental Figure 5C). Dll4 up-regulation in Lrf-deficient erythroblasts occurred at the transcriptional level: Dll4 mRNA levels were 80- to 200-fold greater than those seen in PHZ-treated WT mice (Figure 5D). Taken together, Lrf-deficient erythroblasts express Dll4 at high levels, providing T-cell instructive signals to the LT-HSCs in the BM. In agreement with these findings, we occasionally observed competitor-derived (CD45.1+) DP T cells in the BM after BMT. BM mononuclear cells from Zbtb7aFlox/FloxMx1 Cre+ and competitor (CD45.1+) were mixed at 1:1 ratio and transferred to lethally irradiated recipient mice (CD45.1+). Four weeks after BMT, Lrf inactivation was induced with 3 pIpC injections and BM T cells analyzed 1 month after the last pIpC injection. Competitor-derived DP T cells were evident in recipients' BM (supplemental Figure 5D), suggesting that Lrf-deficient erythroblasts provided Dll4-mediated Notch signals to competitor-derived HSCs.
Lrf represses expression of Dll4 protein and mRNA in an erythroblast-specific manner. (A) Immunohistochemstry (IHC) of Dll4 in paraffin-embedded BM sections from indicated mice. Magnification: 200× (top row) and 600× (bottom row). Dll4 expression was evident only in mature endothelium of control mice (top, left; black arrowheads), whereas clusters of Dll4-positive cells (red arrowheads) were observed in Lrf knockout and Lrf/Notch1 double-knockout mice. (B) Dll4 expression levels during erythroid differentiation were examined by FACS in control (gray) and Lrf knockout mice (black) 1 month after pIpC injections. Representative FACS histograms of cell surface Dll4 at each differentiation stage are shown. MkP (megakaryocyte progenitor); PreMegE (pre-megakaryocyte–erythrocyte progenitor); CFU-E: colony-forming units-erythroid and FSC (forward scatter: cell size). (C) Dll4 expression levels in myelo/lymphoid lineage cells. PreGM: pre-granulocyte macrophage progenitor and GMP: granulocyte macrophage progenitor. (D) Dll4 mRNA levels were determined by qRT-PCR in FACS-sorted BM erythroid and myeloid cells from control, PHZ-treated control, and Lrf knockout (Zbtb7aFlox/FloxMx1-Cre+, one month after pIpC) mice. Bar graphs show relative expression levels for each fraction. Error bars: standard deviation.
Lrf represses expression of Dll4 protein and mRNA in an erythroblast-specific manner. (A) Immunohistochemstry (IHC) of Dll4 in paraffin-embedded BM sections from indicated mice. Magnification: 200× (top row) and 600× (bottom row). Dll4 expression was evident only in mature endothelium of control mice (top, left; black arrowheads), whereas clusters of Dll4-positive cells (red arrowheads) were observed in Lrf knockout and Lrf/Notch1 double-knockout mice. (B) Dll4 expression levels during erythroid differentiation were examined by FACS in control (gray) and Lrf knockout mice (black) 1 month after pIpC injections. Representative FACS histograms of cell surface Dll4 at each differentiation stage are shown. MkP (megakaryocyte progenitor); PreMegE (pre-megakaryocyte–erythrocyte progenitor); CFU-E: colony-forming units-erythroid and FSC (forward scatter: cell size). (C) Dll4 expression levels in myelo/lymphoid lineage cells. PreGM: pre-granulocyte macrophage progenitor and GMP: granulocyte macrophage progenitor. (D) Dll4 mRNA levels were determined by qRT-PCR in FACS-sorted BM erythroid and myeloid cells from control, PHZ-treated control, and Lrf knockout (Zbtb7aFlox/FloxMx1-Cre+, one month after pIpC) mice. Bar graphs show relative expression levels for each fraction. Error bars: standard deviation.
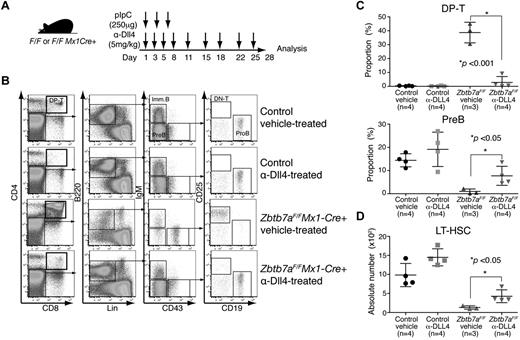
To assess Dll4 loss-of-function, we injected Lrf knockout mice with a blocking anti-Dll4 antibody (5 mg/kg)26 twice a week for 3 weeks (Figure 6A). Dll4 blockade almost completely rescued aberrant lymphoid development in Lrf knockout mice (Figure 6B), whereas injection of blocking anti-Dll1 antibody did not (not shown). B-cell numbers were significantly increased and DP T cells were barely detected in the BM of anti-Dll4–treated Lrf knockout mice (Figure 6C). Furthermore, the numbers of LT-HSCs and Flt3+ lymphoid progenitors were substantially rescued in Lrf knockout mice after anti-Dll4 treatment (Figure 6D, supplemental Figure 6A). Of note, the anemia phenotype seen in Lrf knockout mice21 was not rescued by Dll4 blockade (supplemental Figure 6B), suggesting that it is Dll4-independent.
Dll4 blockade rescues aberrant HSC/lymphoid development in Lrf knockout mice in vivo. (A) Treatment schedule: 9 dosages of anti-Dll4 antibody were administered intraperitoneally over 4 weeks. Mice were treated with pIpC (250 μg/mouse) on days 1, 4, and 7. (B) Hematopoietic development in the BM was analyzed on day 28. Representative FACS profiles for each group are shown. (C) Dot graph shows proportions of DP T (top) and pre-B cell compartments (bottom) in BM. Horizontal black bars: average value; error bars: standard deviation. (D) Dot graph shows numbers of LT-HSCs per 2 legs on day 28. Horizontal black bars: average value; error bars: standard deviation.
Dll4 blockade rescues aberrant HSC/lymphoid development in Lrf knockout mice in vivo. (A) Treatment schedule: 9 dosages of anti-Dll4 antibody were administered intraperitoneally over 4 weeks. Mice were treated with pIpC (250 μg/mouse) on days 1, 4, and 7. (B) Hematopoietic development in the BM was analyzed on day 28. Representative FACS profiles for each group are shown. (C) Dot graph shows proportions of DP T (top) and pre-B cell compartments (bottom) in BM. Horizontal black bars: average value; error bars: standard deviation. (D) Dot graph shows numbers of LT-HSCs per 2 legs on day 28. Horizontal black bars: average value; error bars: standard deviation.
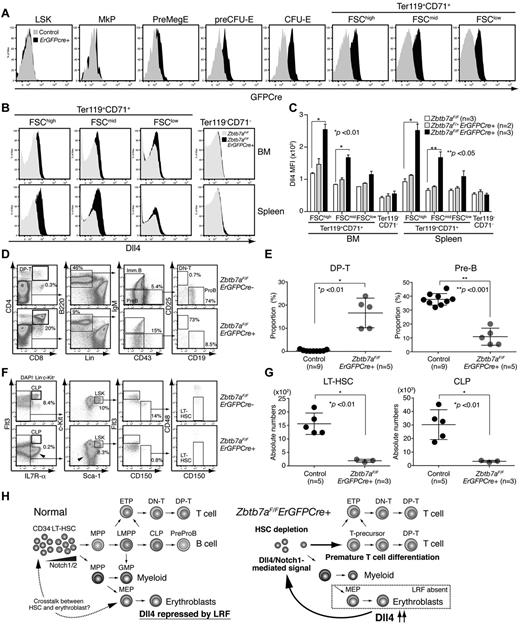
Finally, to test directly whether Lrf-mediated Dll4 repression in erythroblasts is necessary for HSC maintenance, we established Zbtb7aFlox/FloxErGFPCre+ mice in which the GFP/Cre fusion gene is driven by the endogenous EpoR promoter,12 limiting Lrf inactivation to erythroblasts. GFPcre was expressed in erythroid lineage cells largely after the preCFU-E stage (Figure 7A, supplemental Figure 7A). As expected, Dll4 was significantly up-regulated in TER119+CD71+ erythroblasts, whereas its expression was not detected in nonerythroid cells (Figure 7B-C). Dll4 up-regulation occurred at the transcriptional level as observed in Zbtb7aFlox/FloxMx1 Cre+ mice (supplemental Figure 7B). Reduction in the number of Lrf transcripts and up-regulation of Bim, an Lrf target gene in erythroblasts,21 were also confirmed by qRT-PCR (supplemental Figure 7B). Strikingly, Zbtb7aFlox/FloxErGFPCre+ mice phenocopied Zbtb7aFlox/FloxMx1 Cre+ mice, except the anemia phenotype was milder. Aberrant DP T-cell development with a concomitant block in B-cell development was evident in the BM of Zbtb7aFlox/FloxErGFPCre+ mice (Figure 7D-E). Moreover, absolute numbers of LT-HSCs and CLPs were significantly reduced in Zbtb7aFlox/FloxErGFPCre+ mice (Figure 7F-G).
Erythroid-specific deletion of the Lrf gene promotes aberrant lymphoid differentiation and HSC depletion. (A) Expression of the GFPCre fusion protein is limited to erythroid lineage cells. Representative FACS profile of BM cells from control and ErGFPCre+ mice are shown. (B) Dll4 is up-regulated in erythroblasts of Zbtb7aFlox/FloxErGFPCre+ mice. Representative FACS profiles of BM (top) and spleen (bottom) cells are shown. (C) Graph shows MFI scores of Dll4 staining in erythroid (Ter119+CD71+) and nonerythroid (Ter119−CD71−) cells of control (white), Zbtb7aFlox/+ErGFPCre+ (gray), and Zbtb7aFlox/FloxErGFPCre+ (black) mice. Error bars: standard deviation. (D) Hematopoietic development in the BM was analyzed in 1-month-old mice. Representative FACS profiles for each group are shown. (E) Dot graph shows proportions of DP T and pre-B cell compartments in BM. Horizontal black bars: average value. Error bars: standard deviation. (F) Representative HSC/progenitor FACS profiles of control (Zbtb7aFlox/Flox) and erythroid-specific Lrf conditional knockout mice (Zbtb7aFlox/FloxErGFPCre+). Experiments were performed as described in Figure 1A. Aberrant T-cell progenitors within the Lin− fraction are indicated by arrowheads. (G) Dot graphs of absolute counts (per 2 legs) of LT-HSCs and CLPs. Horizontal black bars: average value. Error bars: standard deviation. (H) Notch proteins are expressed in the most primitive CD34− LT-HSCs. No or weak Notch1/Dll4-mediated signals are transmitted to CD34− LT-HSCs under physiologic conditions and no premature T-cell differentiation occurs in the BM (left). In Lrf knockout mice, Dll4 is up-regulated (or de-repressed) in erythroblasts. When CD34− LT-HSCs expressing high levels of Notch are stimulated by the high affinity Notch ligand Dll4, they receive T-instructive signals and differentiate into T cells in the BM, resulting in HSC depletion.
Erythroid-specific deletion of the Lrf gene promotes aberrant lymphoid differentiation and HSC depletion. (A) Expression of the GFPCre fusion protein is limited to erythroid lineage cells. Representative FACS profile of BM cells from control and ErGFPCre+ mice are shown. (B) Dll4 is up-regulated in erythroblasts of Zbtb7aFlox/FloxErGFPCre+ mice. Representative FACS profiles of BM (top) and spleen (bottom) cells are shown. (C) Graph shows MFI scores of Dll4 staining in erythroid (Ter119+CD71+) and nonerythroid (Ter119−CD71−) cells of control (white), Zbtb7aFlox/+ErGFPCre+ (gray), and Zbtb7aFlox/FloxErGFPCre+ (black) mice. Error bars: standard deviation. (D) Hematopoietic development in the BM was analyzed in 1-month-old mice. Representative FACS profiles for each group are shown. (E) Dot graph shows proportions of DP T and pre-B cell compartments in BM. Horizontal black bars: average value. Error bars: standard deviation. (F) Representative HSC/progenitor FACS profiles of control (Zbtb7aFlox/Flox) and erythroid-specific Lrf conditional knockout mice (Zbtb7aFlox/FloxErGFPCre+). Experiments were performed as described in Figure 1A. Aberrant T-cell progenitors within the Lin− fraction are indicated by arrowheads. (G) Dot graphs of absolute counts (per 2 legs) of LT-HSCs and CLPs. Horizontal black bars: average value. Error bars: standard deviation. (H) Notch proteins are expressed in the most primitive CD34− LT-HSCs. No or weak Notch1/Dll4-mediated signals are transmitted to CD34− LT-HSCs under physiologic conditions and no premature T-cell differentiation occurs in the BM (left). In Lrf knockout mice, Dll4 is up-regulated (or de-repressed) in erythroblasts. When CD34− LT-HSCs expressing high levels of Notch are stimulated by the high affinity Notch ligand Dll4, they receive T-instructive signals and differentiate into T cells in the BM, resulting in HSC depletion.
Discussion
In this study, we identified a novel mechanism that prevents LT-HSCs from premature lymphoid differentiation in the BM. Lrf deletion promoted up-regulation of Dll4 in erythroblasts, sensitizing LT-HSCs to T-cell instructive signals via Notch1 (Figure 7H). Our findings are significant as they uncover previously unknown cross-talk between HSC and erythroblasts and shed new light on regulatory mechanisms regulating the balance between HSC self-renewal and differentiation.
To determine the precise HSC/progenitor stage at which Lrf antagonizes the Notch pathway, we performed multi-color FACS analysis using a series of stem cell and lineage markers. Numbers of LT-HSCs, Flt3+ lymphoid biased multi-potential progenitors (LMPPs) and CLPs were severely reduced in Lrf knockout mice. Complete deletion of the Lrf gene in the HSC/progenitor compartment was achieved 1 week after the first pIpC injection, and LT-HSC numbers were reduced in Zbtb7aFlox/FloxMx1-Cre+ as early as 2 weeks after the first pIpC injection (not shown). Although we previously showed that numbers of LSKs and CLPs (defined as Lin−Sca1+c-Kit+IL7Rα+ without Flt3 and Vcam-1 markers) were intact in Lrf knockout mice,8 most of the cells in these populations were T-cell precursors (eg, Flt3−CD150−Vcam1−CD48high+CD25+/− [Figure 1A arrowhead] and Flt3−IL7Rα+Sca-1+c-Kit+CD25+ [Figure 1A asterisk]). In fact, most of these T-cell precursors were positive for Notch3 (supplemental Figure 2), whose expression is normally limited to CD4/8 double-negative T cells in the thymus. Loss of HSCs in Lrf knockout mice was also confirmed by side population assay and gene expression microarray analysis.
When Lrf knockout mice were injected with 5-FU, LT-HSC numbers did not recover to pretreatment levels; instead DP T cells developed in the BM, suggesting that 5-FU–resistant dormant HSCs differentiate toward the T-cell lineage rather than self-renewing in response to the myeloablative stress. In agreement, sequential 5-FU treatment killed Lrf knockout mice much earlier than control mice because of lack of a sufficient HSC pool and subsequent BM failure (Figure 4E). Importantly, this defect was Notch1-dependent, because Notch1 loss rescued HSC defects seen in Lrf knockout mice (Figure 4E). Thus, aberrant Notch signaling accounts for quantitative and qualitative defects in HSC function under stress conditions. Although Lrf deficiency impedes HSC function under myeloablative conditions, Lrf-deficient HSCs supported myeloid reconstitution in a BM transplantation assay (Figure 2D). Furthermore, Lrf-deficient HSCs gave rise to both myeloid and erythroid lineage cells for a long period under nonstress conditions (Figure 2E), suggesting that Lrf is necessary for maintenance of a subset of LT-HSCs, which are dispensable for erythroid and myeloid development under nonstress conditions. Because CD34− LT-HSCs expressing high levels of Notch are particularly sensitive to Lrf deletion and, without that population (as in the case of Lrf knockout) both T and B-lymphoid development is perturbed, it is tempting to speculate that CD34− LT-HSCs expressing high levels of Notch are more “lymphoid-primed” (Figure 7H).
It is unclear whether Notch indeed functions in CD34− LT-HSCs under physiologic conditions rather than being presented on LT-HSCs as a “primed” status for differentiation. Notch function in adult HSCs has been highly debated. Overexpression of ICN27 or treatment of cells with Notch ligands resulted in increased HSC number and reconstitution ability in vitro and in vivo, while suppressing HSC/progenitor differentiation.28,29 However, data from Chiang and colleagues suggests that persistent Notch signaling in murine HSCs promotes T-cell differentiation at the expense of LT-HSC self-renewal,30 results that are consistent with our findings. Notch2, but not Notch1, enhances MPP and HSC repopulation after 5-FU challenge by delaying myeloid differentiation.31 Moreover, culturing human cord blood CD34+ progenitor cells in the presence of fibronectin fragments and immobilized Dll1 ligand significantly reduces the time of myeloid engraftment in patients undergoing myeloablative therapy.32 These data support the idea that Notch positively regulates short-term HSC/progenitor function after myeloablative treatment. However, reports of the role for Notch in adult HSCs under physiologic conditions are more contradictory. Although Duncan et al demonstrated that canonical (RBPjκ/CSL-dependent) Notch pathways were essential for HSC self-renewal via blocking HSC/progenitor differentiation,33 loss-of-function studies of Notch signaling components in mice indicate that the canonical Notch pathway is dispensable for adult HSC function.10,34-36 Our study supports the idea that Notch1 is dispensable for HSC function under stress conditions as reported.31,35,37 Notch1 deletion rescued LT-HSCs numbers in Lrf knockout mice under both nonstress and stress conditions (Figure 4C-D) and prolonged survival of Lrf knockout mice after sequential 5-FU treatment (Figure 4E). Furthermore, Notch1 knockout mice were protected from stress caused by sequential 5-FU treatment (Figure 4E). Although we do not exclude the possibility that Notch2 functions in Lrf-deficient HSCs, our genetic data clearly indicate that Notch1-mediated signals are the primary cause of excessive T-cell differentiation and HSC depletion in Lrf conditional knockout mice.
The function of Notch ligands in the BM microenvironment is not fully understood. Notch ligands are probably present at a functional level in the BM niche, as Notch-mediated signals are necessary for development of niche components, such as osteoblasts38 and endothelial cells.39 Jagged-1 protein is reportedly expressed in primary cultured mouse FL and BM stromal cells.40 Osteoblast-specific activation of the parathyroid hormone receptor in mice leads to expansion of Jagged-1 expressing osteoblastic cells, which support propagation of LSK cells through Notch1/Jagged-1 signals.41 Furthermore, Butler et al recently reported that both Jagged-1 and 2 are expressed in sinusoidal endothelium cells (SECs) in the BM and that Jagged-mediated Notch signals from SECs promote proliferation and prevent exhaustion of LT-HSCs after myeloablation.42 However, inactivation of Jagged-1 in mouse BM HSC/progenitors or BM stromal cells (in which Cre is driven by the Mx1 promoter) had no effect on HSC maintenance.35
Dll4 is constitutively expressed on thymic epithelial cells (TECs)43 and endothelial cells.44 Dll4 also has a critical function in T-cell development23,24 and vascular morphogenesis,39 where it regulates specification of endothelial cells into tip and stalk cells during angiogenic sprouting. However, the role of Dll4 on BM HSC function is not understood. Here, for the first time, we show expression patterns of Dll4 in BM using anti-Dll4 antibody.26 As expected, Dll4 was expressed on mature vessel endothelium and at a lower level on immature endothelium but not in hematopoietic cells (Figure 5A, supplemental Figure 4). FACS analysis confirmed lack of Dll4 expression in hematopoietic cells in the BM, thymus, and spleen (Figure 5B-D and not shown). Unexpectedly, Dll4, but not Dll1, was significantly up-regulated in Lrf-deficient erythroblasts. The up-regulation was cell-type and differentiation-stage specific (Figures 5B-C and 7B) and occurred at the transcriptional level (Figure 5D). It was not a consequence of stress hematopoiesis or PHZ-induced stress erythropoiesis, as no Dll4 was seen in erythroblasts of 5-FU or PHZ-treated WT mice (supplemental Figure 5B and not shown). Furthermore, high Dll4 expression in erythroblasts remained evident in Lrf/Notch1 double-knockout mice, suggesting that Dll4 expression is enhanced in Lrf-deficient erythroblasts in a Notch1-independent fashion. Although Dll4 antibody could also block Dll4 in nonerythroid cells, considering that Dll4 up-regulation is limited to erythroid cells (Figure 5), it is fairly reasonable to expect that anti-Dll4 antibody blocks Dll4 protein in erythroblasts, leading to nearly normal lymphoid development in Lrf knockout mice. Because (1) Dll4 blockade rescued LT-HSC numbers and aberrant lymphoid lineage fate (Figure 6), and (2) erythroid-specific Lrf deletion led to HSC depletion and aberrant lymphoid differentiation (Figure 7G), we conclude that Dll4 repression by Lrf in erythroblasts is essential for HSC maintenance in the BM (Figure 7H).
Vascular endothelial growth factor (VEGF) up-regulates Dll4 transcription in endothelial cells, at least in part, via Fox transcription factors.45,46 However, how Dll4 is regulated in other cell types is entirely unclear. Levels of Dll4 expression must be tightly regulated in hematopoietic cells as well as BM microenvironment, as its aberrant expression could activate Notch-expressing cells, causing disease states such as leukemia. Our study indicates that Notch1/Dll4-mediated signals must be turned off or maintained at low levels in the BM under physiologic conditions to prevent HSCs from differentiating prematurely into T cells in the BM, as seen in Lrf knockout mice. Interestingly, unlike other constitutively active Notch mouse models,47-49 Zbtb7aFlox/FloxMx1-Cre+ mice never develop T-cell leukemia, even after a long period of time, indicating enhanced Notch1/Dll4 signals that Lrf-deficient HSCs receive from erythroblasts are not sufficient for leukemic transformation but rather are similar to signals that thymus-homing progenitors receive from TECs. Although the precise molecular mechanisms by which LRF represses Dll4 in erythroblasts remain elusive, LRF is one of the few factors known to negatively regulate Dll4 expression. LRF may cooperate with an erythroid-specific factor(s) to repress Dll4 transcription, given the ubiquitous expression of LRF in hematopoietic cells.8,21,50
In conclusion, we have identified LRF as a new key factor for adult HSC maintenance. Lrf represses Dll4 in erythroblasts, preventing LT-HSCs from premature differentiation toward T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of COH Animal Resources and ARCH for colony maintenance; Analytical Cytometry Core facility members for assistance with FACS sorting; Biomedical Informatics Core members for help with microarray experiments; Ursula Klingmüller and Stuart Orkin for ErGFPCre mice; Juan Carlos Zúñiga-Pflücker and Michael J. Bevan for OP-9 cells; Hideo Yagita for anti-Dll1 and Jagged-1 antibodies; Keely Walker and Elise Lamar for critical reading of the paper; and Ravi Bhatia, Stephen J. Forman, Ya-Huei Kuo, Mai Suzuki, Christine Chuong, Bonnie Notthoff, and other members in the Division of HSC and Leukemia Research for assistance, advice and helpful discussion.
This work was supported in part by grants to T.M. from STOP Cancer, the V Foundation, the Margaret E. Early Medical Research Trust, the Tim Nesvig Lymphoma Research Fund, the American Society of Hematology, and the National Institutes of Health (R01 AI084905).
National Institutes of Health
Authorship
Contribution: S.U.L. and T.M. designed the project, performed experiments, analyzed the data, and wrote the paper; M.M., Y.I., L.W., N.S., and A.W. performed research and collected data; C.C.C. performed experiments and helped to design HSC transplant experiments; M.L. analyzed microarray data; E.F., A.W., and R.M.D. generated and validated Notch antibodies; F.R. provided Notch1 mice and helped to design experiments; and A.M.J and M.Y. provided anti-Dll4 antibody, performed immunohistochemical analysis, and helped to design experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takahiro Maeda, One Blackfan Circle, Boston, MA 02115; e-mail: tmaeda@partners.org.

![Figure 1. Lrf knockout mice show significantly reduced numbers of LT-HSCs and Flt3+ lymphoid progenitors. (A) Representative HSC/progenitor FACS profiles of control (Zbtb7aFlox/FloxMx1-Cre−) and hematopoietic-specific Lrf knockout mice (Zbtb7aFlox/FloxMx1-Cre+). c-Kit–positive cells were purified from total BM cells using c-Kit microbeads and MACS LS columns and stained for surface markers. Arrowhead (bottom row, second from right) indicates CD48high+ T-cell progenitors within the LSK CD150−Flt3− population. Aberrant Sca1high+ T-cell progenitors within the Lin−Flt3+Il7Rα+ fraction are depicted with an asterisk (second row from bottom, left). LT-HSC: long-term hematopoietic stem cell; CLP: common lymphoid progenitor. (B) Dot graphs of absolute counts of LT-HSCs [LSK (Lin−Sca1+c-Kit+) Il7Rα−Flt3−CD150+CD48−VCAM1dim+] and CLPs (Lin−Il7Rα+Flt3+CD150−CD48+VCAM1−) per 2 legs (2 tibias and 2 femurs). Horizontal black bars: average value; error bars: standard deviation. (C) Proportions of CD34-negative LT-HSCs within the LSK CD150+Flt3− population. Horizontal black bars: average value; error bars: standard deviation. (D) Representative FACS profiles of side population analysis in BM cells from control and Lrf knockout mice. (E) Absolute numbers of LT-HSCs in 13.5 dpc fetal liver (FL) from WT (+/+), Lrf heterozygous (+/−) and Lrf knockout (−/−) mice. Horizontal black bars: average value; error bars: standard deviation. (F) Representative FACS profiles for FL T-cell precursors. (G) Dot graphs show proportions (left) and absolute counts (right) of FL T-cell precursors (TER119−Gr1−c-Kit+PIR+IL7Rα+) in 13.5 dpc FL cells. Horizontal black bars: average value; error bars: standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/6/10.1182_blood-2012-03-418103/4/m_zh89991200690001.jpeg?Expires=1767741397&Signature=ZCJ-Ihf01RDavlFfAhKJh7Hcx2sCf1UztvVgsuBMpuD5rAlKfuL~ADwwiYO6c1UFHbUu79OycR1hwPIP7Atbr0w0Axl4jfKYMbsZdoEg3omP~aWmV-ZNs3At6QTzF~UdBQNII5fSRjRtX7PmgNA1ccKuRo4zBBTmZ70LOP4V7~USX8eamkhvIZI23PKBOSbINGfC1Pb-871P-W~811aalCiWg1K6lcfY70uQayBSL8ix~D-H0OhANTOcGseEHlJQuAbKyWEymqqHM14zzjS6VvgiTb1Gp7PwytoiZbJo-989roh54arjwnoZG2t1BUo9WhYShAFI6lzUZ~T6Wroihg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. In vivo function of Lrf in HSCs. (A) Lrf knockout mice were challenged with 5-FU 9 days after the first pIpC injection and LT-HSC recovery was examined at indicated times. Graph shows absolute numbers of LT-HSCs after 5-FU treatment over time. Horizontal black bars: average value; error bars: standard deviation. (B) In Zbtb7aFlox/FloxMx1-Cre+ mice, 5-FU–resistant dormant HSCs differentiate toward the T-cell lineage rather than self-renewing in response to the myeloablative stress. (C) Schematic representation of bone marrow transplantation (BMT). Fifty double-sorted LT-HSCs were transplanted into lethally irradiated CD45.1 recipient mice. (D) Shown are time-course follow-up of recipients' PB counts [myeloid (Gr1+CD11b+), B (B220+), and T cells (CD4+ or CD8+)]. Error bars: standard deviation. (E) BM hematopoiesis was followed up to 18 months after the last pIpC injection. Representative FACS profiles of hematopoietic development in BM and spleen analyzed 1 year after the last pIpC injection in control (Zbtb7aFlox/FloxMx1-Cre−) and Lrf knockout (Zbtb7aFlox/FloxMx1-Cre+) mice. BM DP T-cell development, B-cell differentiation block, normal myeloid development, and an anemia phenotype in spleen21 were evident in Lrf knockout mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/6/10.1182_blood-2012-03-418103/4/m_zh89991200690002.jpeg?Expires=1767741397&Signature=kMxb57YCchftYivWXXpn3unbtuRDjIrqHIO1bVoOJKQJqoLRLWbKLmmOxwlh9TWps8PbqAjZmt5ePvLF~p3YJS01gEu4jhKF-dSL2LOKOJHTHVgyyuX9lPFIEm9L4tpvjebKUh-bK0l7CTk3EJwnGv6V1emOeb2TGpQnldLWG-y8vLMRRmhYMUs~pTXVdyTQG8jhsQdxgAPPefHEGEP3Qz7AnvORn7PHuar6~98O9IqFxLh~rpxxWyGEMGTXvns1WGkKetVpXKcKcOwo~I8~~UPAKKwgosU7RkHP5zNfAgaek7FpzTGiKBBzoKrPP8Dt4gIXYWJ5gYKHF31PV8xrmA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal