Key Points
Cytokine-induced loss of murine as well as human HSPC homing during ex vivo culture can be prevented by addition of BMP4.
In HSPCs, BMP4 directly regulates Integrin-α4 expression through SMAD-independent p38 MAPK-mediated signaling.
Abstract
Although it is well established that BMP4 plays an important role in the development of hematopoietic system, it is less well understood whether BMP4 affects adult hematopoiesis and how. Here, we describe a novel mechanism by which BMP4 regulates homing of murine as well as human hematopoietic stem/progenitor cells (HSPCs). BMP4 treatment of murine BM derived c-kit+Lin−Sca-1+ (KLS) and CD150+CD48−KLS cells for up to 5 days in vitro prevented the culture-induced loss of Integrin-α4 (ITGA4) expression as well as homing. The effect on ITGA4 expression in response to BMP4 is mediated via SMAD-independent phosphorylation of p38 MAPK, which activates microphthalmia-associated transcription factor (MITF), known to induce ITGA4 expression. Elevated ITGA4 expression significantly enhanced HSPC attachment to bone marrow stromal cells, homing and long-term engraftment of the BMP4 treated cells compared with the cells cultured without BMP4. BMP4 also induced expression of ITGA4 on human BM derived Lin−CD34+ cells in culture, which was associated with improved homingpotential. Thus, BMP4 prevents culture-induced loss of ITGA4 expression on HSPCs in a SMAD-independent manner, resulting in improved homing of cultured HSPCs and subsequent hematopoietic reconstitution.
Introduction
BMP4 is a member of the transforming growth factor (TGF) family. After binding of BMP4 to its receptors, the receptor-regulated R-SMADs, SMAD1/5/8, get activated and bind to SMAD4. This complex is translocated into the nucleus to regulate transcription of various target genes.1 In addition to the SMAD-mediated canonical pathway, BMPs can activate several mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38 mitogen activated protein kinase (MAPK).2
The role of BMP4 in mesoderm formation and early hematopoietic development has been well established.3-5 However, studies on the role of BMP4 in adult hematopoiesis have not been conclusive. Bhatia et al, demonstrated that BMP-4 improves maintenance of human umbilical cord blood HSPCs in vitro by acting as a survival factor for stem cell function.6 BMP4 induces development of stress erythroid progenitors by stimulating erythropoietin in spleen of adult mice.7,8 Culture of murine CD34− KLS cells with BMP4 did not affect their proliferation, even though it resulted in phosphorylation of SMAD1/5 in the HSPC-like Lhx2-HPC cell line as well as in Lin− BM cells.9 However, normal hematopoiesis was observed in mice where SMAD5 was conditionally eliminated from HSCs.10 Although SMAD-dependent signaling was shown to be dispensable in adult hematopoiesis, BMP4 hypomorhic (Bmp4S2G/S2G) mice, wherein decreased levels of mature BMP4 ligand are present in the BM niche, showed a significant reduction in HSC number in the BM.11 Thus, although there is evidence that BMP4 plays a role in postnatal hematopoiesis, the mechanisms whereby BMP4 affects the fate of HSPCs in vitro or in vivo remains unknown.
The BM niche provides necessary signals to maintain the stem cell pool,12 as well as signals important for homing and retention of transplanted HSCs.13 Among others, receptor-ligand pairs, such as integrin-α4 (ITGA4)/vascular cell adhesion molecule (VCAM)–1, integrin-β1/osteopontin, and N-cadherin/β-catenin, are involved in anchoring HSPCs to the BM niche.14 It has been well established that ITGA4 plays a major role in HSC homing and retention into the BM.15,16 In addition, there is evidence that ITGA4 is required for HSC maintenance in vivo.17
Regulation of integrin-mediated cell adhesion largely depends on changes in the activation state of integrins, induced by inside-out and later by outside-in signaling events by integrin-ligand interactions.18 However, changes in integrin expression levels have also been implicated in changes in cell adhesion or motility, even though mechanisms underlying such changes in expression levels have been less extensively studied. The murine Itga4 promoter is known to contain binding motifs for Ap1, Ap2, Spi1, and Pu.119 and is directly activated by Runx3 in monocytes.20 There is extensive evidence that TGF-β can enhance expression of integrins.21
As both the loss of BMP4 in the niche11 as well as loss of ITGA4 on HSCs17 create a similar hematopoietic phenotype, with decreased HSC maintenance, we hypothesized that the mechanism via which BMP4 may affect HSCs might be through BMP4-mediated effects on the ITGA4-mediated anchoring of HSPCs to the BM niche. We here demonstrate that BMP4 can act as an external growth factor that regulates ITGA4 expression. This occurs via SMAD independent, p38-MAPK–mediated activation of the transcription factor MITF. As a result, adhesion of HSPCs to stromal cells, in vivo homing, and long-term repopulation is significantly enhanced by BMP4 pretreatment.
Methods
Animals
Six- to 8-week-old C57BL/6J-CD45.2 (Center d'Elevage R. Janvier, Le Genest-St Isle, France), B6.SJL-PTPRCA-CD45.1 (Charles River Laboratories), and Rag2−/−Il2Rγc−/− mice (gift from Prof Chantal Mathieu, Clinical and Experimental Endocrinology, UZ Leuven, Leuven, Belgium) were bred and maintained in the animal facility at KU Leuven. During the experiments, mice were maintained in isolator cages, fed with autoclaved acidified water, and irradiated food ad libitum. All experiments were approved by the university institutional ethics committee.
Human bone marrow samples
Human BM aspirates were obtained from healthy donors by aspiration from the posterior iliac crest. All studies, conducted in accordance with the Declaration of Helsinki, were approved by the Medical Ethics Committee, UZ Leuven, Gasthuisberg.
Hematopoietic progenitor cell isolation
Details of the procedures for murine and human HSPC isolation are provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Cell culture
The in vitro HSPC culture system was adapted from Zhang et al.22 Sorted murine KLS cells (50 cells/well), were cultured in U-bottom 96-well plates (BD Bioscience) in 100 μL of Stemspan (StemCell Technologies) supplemented with 100 ng/mL mTPO and 50 ng/mL mSCF, with or without 100 ng/mL rmBMP-4, 1 μg/mL rmTSG, or 1 μg/mL rmCHD (all from R&D Systems), dorsomorphin (2μM, Sigma-Aldrich) or SB203580 (5μM, Sigma-Aldrich). Recombinant human SCF, TPO, and BMP4 (all from R&D systems) were used to culture hBM-derived Lin−CD34+ (50 000 cells per well in ultra-low attachment 24-well plates). Cells were cultured for 5 days at 37°C with 5% CO2.
Serial transplantation
Freshly isolated or culture progeny of 200 KLS cells from CD45.1 mice were transplanted along with 1 × 106 competitor CD45.2 BM cells into lethally irradiated (10Gy) C57BL/6J-CD45.2 mice. Peripheral blood chimerism analysis was performed every 4 weeks. After 12 weeks, primary recipients were killed, BM harvested, and 1 × 106 cells grafted in 2 secondary lethally irradiated CD45.2 mice. After 3 months, chimerism in secondary recipients was evaluated. Mice with more than 1% multilineage chimerism were considered engrafted.
Flow cytometry
Chimerism and lineage analysis was performed by flow cytometry. Lineage-specific antibodies used were PE-conjugated anti–Mac-1/Gr-1 for myeloid cells, APC-conjugated anti-B220 for B cells, PE-conjugated anti-CD4/CD8 for T cells were used. For chimerism analysis FITC conjugated anti-CD45.1 and PerCPCy5.5-conjugated anti-CD45.2 antibodies were used. All antibodies were procured from BD Pharmingen. ITGA4 expression and phosphorylation status of MAPK-p38 was assayed using anti-h/mITGA4 FITC (BD Pharmingen) and anti-phospho-p38 antibody (Cell Signaling).
In vitro adhesion assays
OP9 cells (5 × 104) were plated per well in 24-well plates. Two × 104 PKH-26 labeled (details in supplemental Methods) KLS progeny were added per well and incubated for 3 hours at 37°C and 5% CO2. Nonadherent cells were removed and adherent cells were harvested along with the feeder layer. Flow cytometry was used to quantify the labeled cells to compare cell attachment.23 Results are represented as percentage of cells adhered. For ITGA4 neutralization experiments, cells were pre-incubated with a blocking anti-ITGA4 antibody (PS/2 against CD49d, Novus Biologicals).24
Quantitative RT-PCR analysis
Quantitative RT-PCR was performed using standard protocols. The details of the procedures, reagent,s and the list of primers used are provided in supplemental Methods.
Immunoblotting
Immunoblotting was performed using standard protocols and reagents. Details of the procedures and antibodies used are provided in supplemental Methods. Band intensities were quantified using ImageJ 1.32 software (National Institutes of Health) after densitometric scanning of the films, and compared with β-actin or histone-H3.
Immunostaining
A protocol adapted from earlier published method25 was used to stain the cultured murine BM derived KLS or CD48−CD150+KLS cells for phospho-SMAD1/5/8, phospho-p38 MAPK and MITF. Details of the procedure and antibodies used are provided in supplemental Methods. Images were captured on a Zeiss AxioImager microscope (Zeiss) fitted with an AxioCam MRc5 digital camera. Images were captured using AxioVision AC Version 4.8 software (Zeiss) and assembled in Adobe Photoshop CS2.
Homing assay
Transduction of Lin− murine BM cells
Scrambled or Mitf specific shRNAs in the miR-30 backbone of the pTRIPZ lentiviral vector were procured from Open Biosystems. Details of the procedures for producing viruses and transduction of the HSPCs are provided in supplemental Methods.
Statistical analysis
Data are shown as mean ± SE. Statistical analysis was performed using a 2-tailed student t test. P values less than .05 were considered statistically significant. For radiation rescue experiments, Kaplan-Meier survival curves were plotted using GraphPad Prism Version 5.0a software.
Results
Culture with BMP4 affects engraftment but not expansion of murine KLS cells
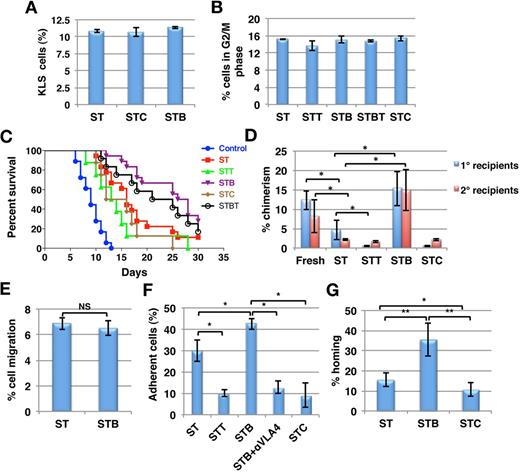
We assessed the effect of BMP4, or its inhibitors chordin (CHD) and twisted gastrulation (TSG) on murine KLS cells cultured in vitro in serum-free medium with stem cell factor (SCF) and thrombopoietin (TPO). Total cell expansion was similar for all culture conditions (supplemental Figure 1A). Likewise the frequency of KLS cells (Figure 1A) as well as colony forming cells (CFCs; supplemental Figure 1B) was similar in all culture conditions, as was the percentage KLS progeny in G0 (supplemental Figure 1C) or G2/M (Figure 1B) phase of the cell cycle. These studies suggested that BMP4 or its inhibitors do not affect expansion of HSPCs cultured in vitro.
Improved radioprotection and chimerism because of culture of KLS cells with BMP4. Murine KLS cells were cultured for 5 days in serum-free medium with SCF+TPO with or without BMP4 and/or TSG or CHD (ST = SCF/TPO; STT = SCF/TPO/TSG; STB = SCF/TPO/BMP4; STBT = SCF/TPO/BMP4 /TSG; STC = SCF/TPO/CHD). (A) Flow cytometry was performed to quantify cells maintaining KLS phenotype after 5 days of culture under different conditions (n = 3). (B) Cell-cycle status was analyzed by propidium iodide staining. The percentage cells in G2/M phase of cell cycle were plotted for the different conditions (n = 3). (C) Survival of mice grafted with the progeny of 200 KLS cells from the different cultures after lethal irradiation. Control denotes mice that did not receive KLS cell progeny. Results were analyzed by log-rank test method for trend and expressed as Kaplan-Meier survival curves (n = 10-18, *P < .01). (D) Donor derived chimerism was determined by FACS in primary recipients, 3 months after the transplantation of either 200 uncultured KLS cells, or of progeny from 200 KLS cells from different culture conditions along with 1 × 106 CD45.2 BM cells. One million total BM cells from primary recipients were transplanted in secondary hosts. Chimerism in secondary recipients was determined after 3 months (n = 8-12; *P < .05). (E) In vitro transwell migration assays was performed to compare the migration potential of the KLS cell progeny (n = 4). (F) PKH-26 labeled KLS cell progeny were allowed to adhere to OP9 cells for 3h. Nonadherent cells were washed and the percentage adherent cells was enumerated and compared for different conditions. In some conditions, a blocking anti-ITGA4 antibody was added in addition to BMP4 (n = 3-4; *P < .05). (G) KLS cell progeny (0.1 × 106) from the different cultures were transplanted intravenously in lethally irradiated mice, and BM harvested after 16 hours. Total BM cells (0.1 × 106) were assayed in methylcellulose colony forming assays and CFU-Cs were enumerated. Percentage homing was determined by comparing total CFU-Cs homed with CFU-Cs injected (n = 6, *P < .05, **P < .01).
Improved radioprotection and chimerism because of culture of KLS cells with BMP4. Murine KLS cells were cultured for 5 days in serum-free medium with SCF+TPO with or without BMP4 and/or TSG or CHD (ST = SCF/TPO; STT = SCF/TPO/TSG; STB = SCF/TPO/BMP4; STBT = SCF/TPO/BMP4 /TSG; STC = SCF/TPO/CHD). (A) Flow cytometry was performed to quantify cells maintaining KLS phenotype after 5 days of culture under different conditions (n = 3). (B) Cell-cycle status was analyzed by propidium iodide staining. The percentage cells in G2/M phase of cell cycle were plotted for the different conditions (n = 3). (C) Survival of mice grafted with the progeny of 200 KLS cells from the different cultures after lethal irradiation. Control denotes mice that did not receive KLS cell progeny. Results were analyzed by log-rank test method for trend and expressed as Kaplan-Meier survival curves (n = 10-18, *P < .01). (D) Donor derived chimerism was determined by FACS in primary recipients, 3 months after the transplantation of either 200 uncultured KLS cells, or of progeny from 200 KLS cells from different culture conditions along with 1 × 106 CD45.2 BM cells. One million total BM cells from primary recipients were transplanted in secondary hosts. Chimerism in secondary recipients was determined after 3 months (n = 8-12; *P < .05). (E) In vitro transwell migration assays was performed to compare the migration potential of the KLS cell progeny (n = 4). (F) PKH-26 labeled KLS cell progeny were allowed to adhere to OP9 cells for 3h. Nonadherent cells were washed and the percentage adherent cells was enumerated and compared for different conditions. In some conditions, a blocking anti-ITGA4 antibody was added in addition to BMP4 (n = 3-4; *P < .05). (G) KLS cell progeny (0.1 × 106) from the different cultures were transplanted intravenously in lethally irradiated mice, and BM harvested after 16 hours. Total BM cells (0.1 × 106) were assayed in methylcellulose colony forming assays and CFU-Cs were enumerated. Percentage homing was determined by comparing total CFU-Cs homed with CFU-Cs injected (n = 6, *P < .05, **P < .01).
Next, we assessed the effect of BMP4 and its antagonists on the ability of KLS cells to provide radioprotection (Figure 1C). Lethally irradiated CD45.2 mice received progeny of 200 KLS cells cultured with SCF+TPO (ST) alone or combined with BMP4 (STB), or the BMP4 inhibitors CHD (STC) or TSG (STT). Irradiated mice without graft died within 2 weeks (median 9 days), whereas the group transplanted with the progeny of ST supplemented cultures survived for a median of 16 days and > 10% of mice survived long-term (> 8 months; Figure 1C). Addition of BMP4 during culture enhanced median (27 days, P = .0001) as well as overall survival (27.78% of mice survived long-term). This effect was partially abolished by addition of BMP4 inhibitor TSG (STBT). The ability of KLS cell-progeny from STC or STT cultures to rescue mice from irradiation was significantly decreased compared with cells from ST cultures (median 14 and 13.5 days, respectively; log rank test method for trend, P < .01, n = 10-18).
We also performed competitive repopulation experiments to examine the effect of BMP4 on the long-term repopulation (LTR)–potential of HSPCs. Freshly isolated or progeny of 200 cultured CD45.1 KLS cells were transplanted along with 1 × 106 competitor CD45.2 BM cells intravenously into lethally irradiated CD45.2 mice. After 3 months, 1 × 106 BM cells from the primary recipients were injected in lethally irradiated secondary recipients. Chimerism in primary recipients that received cells cultured with STB was significantly better than from cells cultured with ST (n = 8, P < .02; Figure 1D). KLS cells cultured in the presence of SCF/TPO showed significantly lower engraftment than freshly isolated cells. BMP4 treatment resulted in chimerism levels in primary as well as secondary recipients comparable with those of freshly isolated cells. By contrast, chimerism from cells treated with STT or STC was significantly lower than from ST treated cells (P < .05). Chimerism in secondary recipients after 3 months was significantly higher for STB compared with ST-treated cells (n = 12, P < .05; Figure 1D). We did not find any differences in the multilineage potential of the transplanted cells in any of the groups (supplemental Figure 2).
BMP4 enhances adhesion of KLS progeny to stroma and their homing in vivo
Initial studies demonstrated thus that although BMP4 or its inhibitors significantly affect the engraftment of short and long-term repopulating HSPCs, this is not associated with effects on KLS cell proliferation in vitro. We therefore examined whether the improved engraftment could be because of changes in HSPC homing. As homing requires attachment of HSPCs to the endothelial lining of BM sinusoids and migration through the endothelium into the BM niche, we performed both adhesion and migration assays with KLS progeny treated with or without BMP4 or its inhibitors. Addition of BMP4 did not affect the migration potential of KLS cell progeny toward OP9 stromal feeders (Figure 1E). However, adhesion of KLS progeny to OP9 stromal cells was significantly increased by BMP4 (n = 3; P = .02), whereas KLS progeny from CHD or TSG containing cultures adhered significantly less to the stromal cells (P < .05; Figure 1F). The effect of BMP4 inhibitors on the adhesion potential of HSPCs was found to be reversible as removal of the inhibitor and culturing the cells in the presence of BMP4 for additional 2 days led to increase in their adhesion potential (supplemental Figure 3A) further indicating an effect on HSPC homing.
To further substantiate this, direct in vivo homing experiments were performed with KLS cell progeny from ST, STB, and STC cultures (Figure 1G). CD45.1 mice derived KLS cells cultured under different conditions were transplanted IV into lethally irradiated animals. After 16 hours, mice were killed and the proportion of transplanted CFCs that homed into the recipient BM was measured by methylcellulose assay. Lethal irradiation depletes the colony forming activity of BM cells; hence, colonies formed from the BM cells after irradiation are derived from the grafted donor cells. The number of homed CFCs compared with the number of CFCs in the graft and homing efficiency was determined.27 Details of the calculations are provided in supplemental Methods. The percentage of homed CFCs was significantly higher for KLS cell progeny from STB cultures than ST control cultures (n = 6; P = .033), whereas progeny from STC cultured cells homed significantly less (Figures 1G; P = .04). These results were also compared with flow cytometric detection of homed donor derived (CD45.1) cells as shown in Figure 3C and supplemental Figure 6A-B.
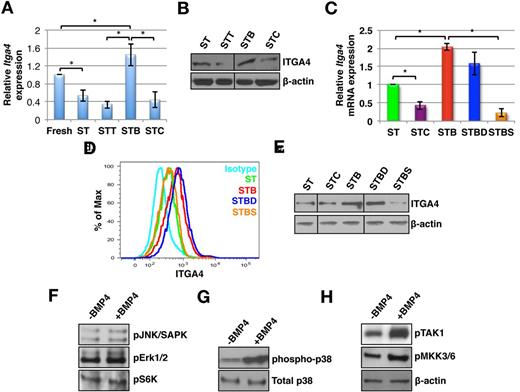
BMP4 improves cell adhesion by SMAD independent induction of ITGA4 expression
We examined transcript levels of various adhesion molecules commonly expressed by HSPCs. No significant change in expression of most of these genes was found (data not shown), except for a significant increase in Itga4 expression (n = 6, P = .045; Figure 2A). Itga4 expression decreased after culture of KLS cells in the presence of SCF and TPO without BMP4, whereas addition of BMP4 prevented this effect. Exposure to CHD/TSG caused further decrease in Itga4 expression (n = 6; P < .02). Consistent with the gene expression data, Western blotting (n = 3; P < .03; Figure 2B, quantitation in supplemental Figure 3B) and flow cytometric analysis (Figure 2D) demonstrated elevated levels of ITGA4 expression in STB treated KLS cell progeny, and decreased levels in STC and STT treated cells (n = 3; P < .05). That the changes in ITGA4 expression levels were responsible for the BMP4–induced increase in cell adhesion was confirmed by incubation of KLS cell progeny with BMP4 and a blocking anti-ITGA4 antibody (P = .01; Figure 1F).
BMP4-induced activation of ITGA4 expression is mediated via SMAD independent p38 MAPK phosphorylation. KLS cells were cultured with SCF+TPO in addition to BMP4 alone or combined with various inhibitors, for 5 days (ST = SCF/TPO; STC = SCF/TPO/CHD; STT = SCF/TPO/TSG; STB = SCF/TPO/BMP4; STBD = SCF/TPO/BMP4/Dorsomorphin; STBS = SCF/TPO/BMP4/SB203580), and analyzed by qRT-PCR, Western blot and flow cytometry. (A) Fold change in Itga4 transcript levels in KLS progeny from cultures stimulated with SCF+TPO and either BMP4, CHD, or TSG compared with KLS progeny from SCF+TPO cultures (n = 6; *P < .05). (B) Immunoblotting with antibodies against ITGA4 and β-actin (n = 3). (C) qRT-PCR analysis for Itga4 using KLS progeny cultured with ST, STC, STB, STBD, and STBS. (n = 3; *P < .05). (D). FACS analysis of KLS progeny cultured with ST, STB, STBD, and STBS using antibodies against ITGA4 (n = 4). (A) Western blot analysis using antibodies against ITGA4 and β-actin in KLS progeny cultured with ST, STC, STB, STBD, and STBS. (E) Western blot analysis of KLS progeny cultured with ST or STB using phospho-specific antibodies against JNK/SAPK, Erk1/2, and S6 kinase. (F) Western blot analysis of KLS progeny cultured with ST, STB using antibodies against phospho-p38 MAPK and total p38 MAPK. (G) Western blot analysis of KLS progeny cultured with ST or STB using antibodies against phospho-TAK1 and phospho-MKK3/6. β-actin was used as internal control (representative for panels D through F). All western blots are representative examples of 3 independent experiments.
BMP4-induced activation of ITGA4 expression is mediated via SMAD independent p38 MAPK phosphorylation. KLS cells were cultured with SCF+TPO in addition to BMP4 alone or combined with various inhibitors, for 5 days (ST = SCF/TPO; STC = SCF/TPO/CHD; STT = SCF/TPO/TSG; STB = SCF/TPO/BMP4; STBD = SCF/TPO/BMP4/Dorsomorphin; STBS = SCF/TPO/BMP4/SB203580), and analyzed by qRT-PCR, Western blot and flow cytometry. (A) Fold change in Itga4 transcript levels in KLS progeny from cultures stimulated with SCF+TPO and either BMP4, CHD, or TSG compared with KLS progeny from SCF+TPO cultures (n = 6; *P < .05). (B) Immunoblotting with antibodies against ITGA4 and β-actin (n = 3). (C) qRT-PCR analysis for Itga4 using KLS progeny cultured with ST, STC, STB, STBD, and STBS. (n = 3; *P < .05). (D). FACS analysis of KLS progeny cultured with ST, STB, STBD, and STBS using antibodies against ITGA4 (n = 4). (A) Western blot analysis using antibodies against ITGA4 and β-actin in KLS progeny cultured with ST, STC, STB, STBD, and STBS. (E) Western blot analysis of KLS progeny cultured with ST or STB using phospho-specific antibodies against JNK/SAPK, Erk1/2, and S6 kinase. (F) Western blot analysis of KLS progeny cultured with ST, STB using antibodies against phospho-p38 MAPK and total p38 MAPK. (G) Western blot analysis of KLS progeny cultured with ST or STB using antibodies against phospho-TAK1 and phospho-MKK3/6. β-actin was used as internal control (representative for panels D through F). All western blots are representative examples of 3 independent experiments.
To determine the signaling pathway involved in regulation of ITGA4 expression by BMP4, immunostaining (supplemental Figure 4A) and immunoblotting (supplemental Figure 4B) was performed to detect SMAD1/5/8 phosphorylation in KLS cells treated with different combinations of growth factors for 5 days. We detected significantly increased SMAD1/5/8 phosphorylation after incubation with BMP4 (n = 3; P = .006), consistent with previously published studies.9 By contrast, the addition of CHD decreased SMAD1/5/8 phosphorylation (supplemental Figure 4A). However, no change in transcript levels of SMAD target genes, such as Id1, Id2, Id3, Runx2, and Gata2 was detected (supplemental Figure 4C).
To further examine whether SMAD1/5/8 was involved in regulating ITGA4 expression, we cultured KLS cells with dorsomorphin, an inhibitor of SMAD1/5/8 phosphorylation (referred to as D)28 along with BMP4, and evaluated transcript (Figure 2C) as well as protein levels of ITGA4 by flow cytometry (Figure 2D) and immunoblotting (Figure 2E, quantitation in supplemental Figure 5A). Although dorsomorphin inhibited SMAD1/5/8 phosphorylation (supplemental Figure 4B), it did not reverse the effects of BMP4 on the levels of ITGA4 expression (n = 3; P < .02; Figure 2C-E). Hence, although BMP4 activated SMAD1/5/8, this signaling pathway was not involved in BMP4-mediated regulation of ITGA4 expression.
BMP4-induced p38 MAPK activation is responsible for the regulation of ITGA4 expression
To further delineate the BMP4 signaling pathway involved in the process, we performed immunoblotting experiments using phospho-specific antibodies against various signal transducers that mediate SMAD-independent signaling.2 No changes were seen in the phosphorylation status of JNK, ERK-MAPK, and S6K on culture with BMP4 (Figure 2F, quantitation in supplemental Figure 5B). However, a significant increase in phosphorylation of p38 MAPK was detected (Figure 2G, quantitation in supplemental Figure 5B). In addition, the upstream regulators of p38 MAPK, TAK1, and MKK3/6, were phosphorylated in response to BMP4 treatment (Figure 2H, quantitation in supplemental Figure 5B).
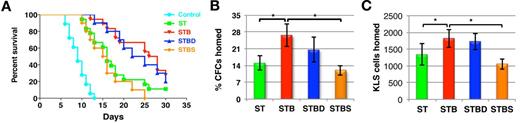
To test whether p38 MAPK phosphorylation was involved in the BMP4-mediated regulation of ITGA4 expression, the p38 MAPK inhibitor, SB203580 (referred to as S), was added to cultures stimulated with BMP4. As expected, SB203580 did not affect BMP4-induced phosphorylation of SMAD1/5/8 although it inhibited phosphorylation of p38 MAPK (supplemental Figure 4B). In contrast to dorsomorphin, addition of SB203580 together with BMP4 reversed the effect of BMP4 on Itga4 transcript and protein expression (n = 3, P < .03; Figure 2C-E). These studies strongly suggest that BMP4 influences ITGA4 expression via SMAD-independent signaling pathways. To extend these findings in vivo, radiation rescue experiments and 16-hour homing assays were performed. As previously shown (Figure 1C), KLS cells cultured in the presence of BMP4 rescued the lethally irradiated mice better than the cells cultured without BMP4 (Figure 3A). We found that this effect was abolished after addition of SB203580 but not by dorsomorphin (P < .0002), suggesting that SMAD-independent p38 MAPK-mediated BMP4 signaling was involved in this effect. To assess homing, we evaluated the number of CFCs, CD45.1, as well as CD45.1-KLS cells in recipient mice 16 hours after infusion of the graft (Figure 3B-C, supplemental Figure 6A-C). In coherence with the previous results, we found a significant increase in total number of CD45.1 KLS cells as well as CD45.1 KLS cells (Figure 3C). SB203580 but not dorsomorphin reversed the BMP4-mediated enhanced in vivo homing (Figure 3B-C; P = .003). The percentage of homed CFCs was significantly higher for KLS cell progeny from STB cultures than ST control cultures (n = 6; P = .033), whereas progeny from STC cultured cells homed significantly less (Figure 1G, P = .04).
Activation of SMAD independent BMP4 signaling leads to better homing potential and radioprotection. KLS cells were cultured for 5 days in SCF+TPO with BMP4 alone or combined with dorsomorphin or SB203580 and radioprotection and homing ability was evaluated. (A) Survival of mice grafted with 0.1 × 106 KLS cell progeny from different culture conditions after lethal irradiation. Results were analyzed by log-rank test method for trend and expressed as Kaplan-Meier survival curves (n = 10-18, P < .002). (B) KLS cell progeny (1 × 105) from the different cultures were transplanted intravenously in lethally irradiated mice, and BM harvested after 16 hours. Total BM cells (0.1 × 106) were assayed in methylcellulose colony forming assays and CFU-Cs were enumerated. Percentage homing was determined by comparing total CFU-Cs homed with CFU-Cs injected (n = 9, P < .01). (C) Aside from analyzing homed CFCs, in homing assays BM of the recipient mice was analyzed by flow cytometry for donor derived CD45.1 cells. Flow cytometric analysis was extended to quantify the KLS cells homed in the 16 hours after transplantation. Total KLS cells homed in each mice was calculated for different conditions (n = 8, P < .02).
Activation of SMAD independent BMP4 signaling leads to better homing potential and radioprotection. KLS cells were cultured for 5 days in SCF+TPO with BMP4 alone or combined with dorsomorphin or SB203580 and radioprotection and homing ability was evaluated. (A) Survival of mice grafted with 0.1 × 106 KLS cell progeny from different culture conditions after lethal irradiation. Results were analyzed by log-rank test method for trend and expressed as Kaplan-Meier survival curves (n = 10-18, P < .002). (B) KLS cell progeny (1 × 105) from the different cultures were transplanted intravenously in lethally irradiated mice, and BM harvested after 16 hours. Total BM cells (0.1 × 106) were assayed in methylcellulose colony forming assays and CFU-Cs were enumerated. Percentage homing was determined by comparing total CFU-Cs homed with CFU-Cs injected (n = 9, P < .01). (C) Aside from analyzing homed CFCs, in homing assays BM of the recipient mice was analyzed by flow cytometry for donor derived CD45.1 cells. Flow cytometric analysis was extended to quantify the KLS cells homed in the 16 hours after transplantation. Total KLS cells homed in each mice was calculated for different conditions (n = 8, P < .02).
BMP4 results in p38 MAPK-mediated activation of MITF
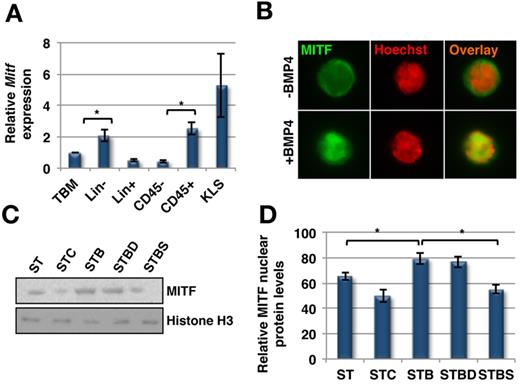
Although little is known regarding the transcription factor(s) that regulate ITGA4 expression in HSPCs, MITF has been shown to bind to a CACTTG motif in the ITGA4 promoter resulting in increased ITGA4 expression in murine mast cells, hence increasing their adhesion potential.29 One of the signaling pathways that regulates the expression and activation of MITF is p38 MAPK.30,31 As there was phosphorylation of p38 MAPK in KLS cells after BMP4 stimulation, we examined whether MITF was activated in response to BMP4 treatment. qRT-PCR (Figure 4A) analysis demonstrated that CD45+ BMCs expressed significantly higher levels of Mitf than CD45− cells (n = 3; P = .034). Mitf was expressed significantly higher in Lin− BM cells compared with Lin+ cells (P = .04), and the highest expression levels were found in KLS cells.
SMAD independent BMP4 signaling leads to activation of MITF. (A) Quantitative RT-PCR for Mitf expression in different subpopulations of mouse BM (n = 3, P < .04). (B-D) KLS cells were cultured with SCF+TPO in addition to BMP4 alone or combined with dorsomorphin or SB203580, for 5 days (ST = SCF/TPO; STC = SCF/TPO/Chd; STB = SCF/TPO/BMP4; STBD = SCF/TPO/BMP4/Dorsomorphin; STBS = SCF/TPO/BMP4/SB203580), and analyzed by Western blot or immunostaining. (B) Immunostaining with an antibody against MITF and Hoechst33342. Representative example of 3 experiments. (C) Western blotting on nuclear extracts of KLS cell progeny with antibodies against MITF and Histone H3 as control. Representative example of 3 experiments. (D) Quantification of nuclear MITF protein levels in KLS cell progeny cultured for 5 days by densitometric analysis of MITF and histone H3 intensities (n = 3; *P < .04).
SMAD independent BMP4 signaling leads to activation of MITF. (A) Quantitative RT-PCR for Mitf expression in different subpopulations of mouse BM (n = 3, P < .04). (B-D) KLS cells were cultured with SCF+TPO in addition to BMP4 alone or combined with dorsomorphin or SB203580, for 5 days (ST = SCF/TPO; STC = SCF/TPO/Chd; STB = SCF/TPO/BMP4; STBD = SCF/TPO/BMP4/Dorsomorphin; STBS = SCF/TPO/BMP4/SB203580), and analyzed by Western blot or immunostaining. (B) Immunostaining with an antibody against MITF and Hoechst33342. Representative example of 3 experiments. (C) Western blotting on nuclear extracts of KLS cell progeny with antibodies against MITF and Histone H3 as control. Representative example of 3 experiments. (D) Quantification of nuclear MITF protein levels in KLS cell progeny cultured for 5 days by densitometric analysis of MITF and histone H3 intensities (n = 3; *P < .04).
As no phospho-specific antibodies against MITF are available, we examined its nuclear translocation after culture of KLS cells in different growth factor combinations as a measure of its activation. Immunostaining for MITF demonstrated its nuclear translocation after BMP4 treatment (Figure 4B). We quantified this effect by immunoblotting nuclear extracts isolated from KLS cells cultured under different conditions (Figure 4C-D). BMP4 treatment significantly increased the nuclear concentration of MITF (n = 3; P < .01) whereas CHD led to a decrease in nuclear localization of MITF (P = .04). In addition, SB203580 but not dorsomorphin inhibited the increase in nuclear levels, and hence the activation of MITF induced by BMP4.
BMP4-dependent activation of MITF is responsible for induction of ITGA4 expression
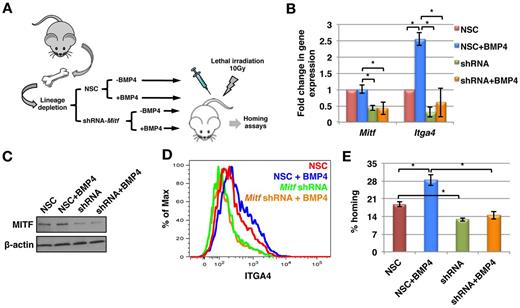
To prove that MITF is the transcription factor responsible for BMP4-mediated regulation of ITGA4 expression, we transduced mouse BM derived Lin− cells with an inducible lentiviral vector encoding either a doxycycline inducible nonsilencing construct (NSC) or a short hairpin RNA (shRNA) targeting Mitf (Mitf-KD; Figure 5A). Transduced cells were selected using hygromycin, and on induction with doxycycline, were identifiable by red fluorescent protein (RFP) expression. Mitf-KD and NSC cells were then cultured with or without BMP4. Before further analyses, down-regulation of Mitf expression at the transcript and protein level was confirmed by qRT-PCR (Figure 5B) and immunoblotting (Figure 5C), respectively. We obtained 65% to 85% knockdown of Mitf in the 3 independent experiments, which was accompanied with a > 75% decrease in Itga4 expression (Figure 5B). As expected, in NSC cells cultured with BMP4, the expression of Itga4 increased significantly. However, knockdown of Mitf inhibited BMP4-mediated induction of Itga4 expression (Figure 5B). Consistent with the gene expression data, MITF protein levels were decreased by 76% ± 3.6% in Mitf-KD cells and this persisted when the cells were cultured with BMP4 (Figure 5C; quantification in supplemental Figure 5C). Cell surface expression of ITGA4 was significantly decreased in cells with decreased expression of MITF (Figure 5D). These results, together with the qRT-PCR results, suggest that MITF might be regulating the basal level of ITGA4 expression in HSPCs. Autocrine regulation of the BMP4 signaling could be one of the determining factors. Further, unlike in NSC cells, BMP4 did not induce increased cell-surface levels of ITGA4 in Mitf-KD cells (Figure 5D).
MITF is essential for BMP4-mediated increase in ITGA4 expression, and HSPC homing. (A) Schematic representation of Mitf knockdown experiments. Lin− cells were transduced with a lentiviral vector containing doxycycline inducible anti–Mitf-shRNA or nonsilencing construct (NSC). Expression of the shRNA was induced with doxycycline and the transduced cells were selected using hygromycin. Expression of the anti–Mitf-shRNA or NSC was detected by RFP expression. Mitf-KD and NSC Lin− progeny were then cultured with or without BMP4 and evaluated by qRT-PCR, Western blotting, FACS and in vivo homing assays. (B) qRT-PCR for Mitf and Itga4 expression (n = 3; P < .02). (C) Western blotting using antibodies against MITF and β-actin. Representative example of 3 independent experiments. (D) FACS analysis with antibodies against ITGA4. Representative example of 3 experiments. (E) KLS cell progeny (0.1 × 106) from the different cultures were transplanted intravenously in lethally irradiated mice, and the BM harvested after 16 hours. Total BM cells (0.1 × 106) were assayed in methylcellulose colony forming assay, Percentage homing was determined by comparing total CFU-Cs homed with CFU-Cs injected (n = 3, P < .02).
MITF is essential for BMP4-mediated increase in ITGA4 expression, and HSPC homing. (A) Schematic representation of Mitf knockdown experiments. Lin− cells were transduced with a lentiviral vector containing doxycycline inducible anti–Mitf-shRNA or nonsilencing construct (NSC). Expression of the shRNA was induced with doxycycline and the transduced cells were selected using hygromycin. Expression of the anti–Mitf-shRNA or NSC was detected by RFP expression. Mitf-KD and NSC Lin− progeny were then cultured with or without BMP4 and evaluated by qRT-PCR, Western blotting, FACS and in vivo homing assays. (B) qRT-PCR for Mitf and Itga4 expression (n = 3; P < .02). (C) Western blotting using antibodies against MITF and β-actin. Representative example of 3 independent experiments. (D) FACS analysis with antibodies against ITGA4. Representative example of 3 experiments. (E) KLS cell progeny (0.1 × 106) from the different cultures were transplanted intravenously in lethally irradiated mice, and the BM harvested after 16 hours. Total BM cells (0.1 × 106) were assayed in methylcellulose colony forming assay, Percentage homing was determined by comparing total CFU-Cs homed with CFU-Cs injected (n = 3, P < .02).
We also performed homing assays using Mitf-KD and NSC cells cultured with or without BMP4. The number of CFCs recovered from recipient BM 16 hours after transplantation of NSC and Mitf-KD cells was compared with the number of CFCs in the graft. Homing of the cells from NSC group was significantly increased after incubation with BMP4 (P = .02, Figure 5E), whereas homing of cells from the Mitf-KD group cells was significantly lower compared with the NSC group. Further, culture of Mitf-KD cells with BMP4 did not result in improved homing potential, confirming that the effect of BMP4 is MITF-mediated (Figure 5E).
BMP4 induces ITGA4 expression in primitive HSCs via p38 MAPK phosphorylation
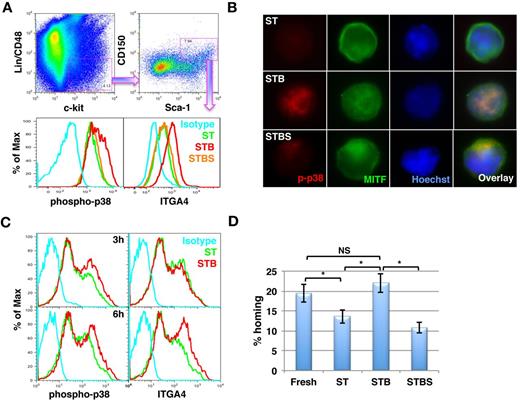
In line with the LTR-engraftment data, we also demonstrated by FACS (Figure 6A) that BMP4 activated the p38 MAPK pathway, which was associated with increased ITGA4 expression in primitive CD48−CD150+KLS cells. Clearly, inhibition of p38 MAPK phosphorylation by SB203580 inhibited ITGA4 expression. In addition, we sorted CD48−CD150+KLS cells and performed immunostaining to analyze activation of p38 MAPK as well as nuclear localization of MITF in response to BMP4 treatment (Figure 6B). We observed that addition of BMP4 resulted in phosphorylation of p38 MAPK, which also resulted in activation of MITF as confirmed by its nuclear localization (middle panel). We also confirmed inhibition of phospho-p38 MAPK by SB203580 (bottom panel), which resulted in reversal of the effect of BMP4 on activation of MITF.
BMP4 enhances ITGA4 expression via p38 phosphorylation in KLS/CD150+CD48− cells within 6 hours. (A-B) Lin− BM cells were cultured in serum free medium in the presence of SCF+TPO in the presence or absence of BMP4 alone or with SB203580 for 5 days. ST = SCF/TPO; STB = SCF/TPO/BMP4; STBS = SCF/TPO/BMP4/SB203580. (A) After 5 days, flow cytometry was performed to analyze p38 MAPK phosphorylation as well as ITGA4 expression. The cells were harvested and stained with antibodies against Lineage positive cells, CD48, CD150, Sca-1, and c-Kit combined with anti–phospho-p38 MAPK or ITGA4 antibodies. CD48−CD150+KLS cells were gated (top panel) and the levels of phosphorylated p38 MAPK (bottom left) and ITGA4 expression (bottom right) were compared among different conditions. Representative example of 5 experiments. (B)The CD48−CD150+KLS subpopulation was sorted from Lin− BM cell progeny cultured under the different conditions. Immunostaining was performed to evaluate p38 MAPK and MITF activation using antibodies against phospho-p38 MAPK and MITF. Representative example of 3 experiments. (C) Fresh KLS cells were cultured in the presence of SCF+TPO with or without BMP4 for 3 hours (top panel) and 6 hours (bottom panel). Flow cytometry was performed to analyze phosphorylation status of MAPK p38 (left) and ITGA4 (right) expression. Representative example of 4 independent experiments. (D) Homing potential of KLS cells cultured for 6 hours in SCF+TPO with or without BMP4 was compared with freshly isolated KLS cells (n = 8; *P = .002).
BMP4 enhances ITGA4 expression via p38 phosphorylation in KLS/CD150+CD48− cells within 6 hours. (A-B) Lin− BM cells were cultured in serum free medium in the presence of SCF+TPO in the presence or absence of BMP4 alone or with SB203580 for 5 days. ST = SCF/TPO; STB = SCF/TPO/BMP4; STBS = SCF/TPO/BMP4/SB203580. (A) After 5 days, flow cytometry was performed to analyze p38 MAPK phosphorylation as well as ITGA4 expression. The cells were harvested and stained with antibodies against Lineage positive cells, CD48, CD150, Sca-1, and c-Kit combined with anti–phospho-p38 MAPK or ITGA4 antibodies. CD48−CD150+KLS cells were gated (top panel) and the levels of phosphorylated p38 MAPK (bottom left) and ITGA4 expression (bottom right) were compared among different conditions. Representative example of 5 experiments. (B)The CD48−CD150+KLS subpopulation was sorted from Lin− BM cell progeny cultured under the different conditions. Immunostaining was performed to evaluate p38 MAPK and MITF activation using antibodies against phospho-p38 MAPK and MITF. Representative example of 3 experiments. (C) Fresh KLS cells were cultured in the presence of SCF+TPO with or without BMP4 for 3 hours (top panel) and 6 hours (bottom panel). Flow cytometry was performed to analyze phosphorylation status of MAPK p38 (left) and ITGA4 (right) expression. Representative example of 4 independent experiments. (D) Homing potential of KLS cells cultured for 6 hours in SCF+TPO with or without BMP4 was compared with freshly isolated KLS cells (n = 8; *P = .002).
To further define the kinetics with which BMP4 affected ITGA4 expression, we cultured freshly isolated KLS cells with ST or STB for 3 hours (top panel) and 6 hours (bottom panel) and evaluated activation of p38 MAPK phosphorylation and subsequent effects on ITGA4 expression (Figure 6C) and in vivo homing into the BM (Figure 6D). BMP4 increased phosphorylation of p38 MAPK within 3 hours (Figure 6C top left) and induced increased expression of ITGA4 after 6 hours of BMP4 treatment (Figure 6C bottom right). Compared with ST treated cells, cells cultured for 6 hours with STB homed significantly better (Figure 6D). After 6 hours of culture with SCF+TPO homing potential of KLS progeny was already decreased compared with uncultured cells, whereas BMP4 prevented this loss. Although not statistically significant, we consistently observed a minor increase in the homing potential of the STB treated cells than the freshly isolated KLS cells (Figure 6D), consistent with the finding that secondary engraftment from SCF+TPO+BMP4 treated cells appears higher than that of the uncultured cells (Figure 1D).
BMP4 also activates p38 MAPK and induces ITGA4 in human BM derived CD34+Lin− cells
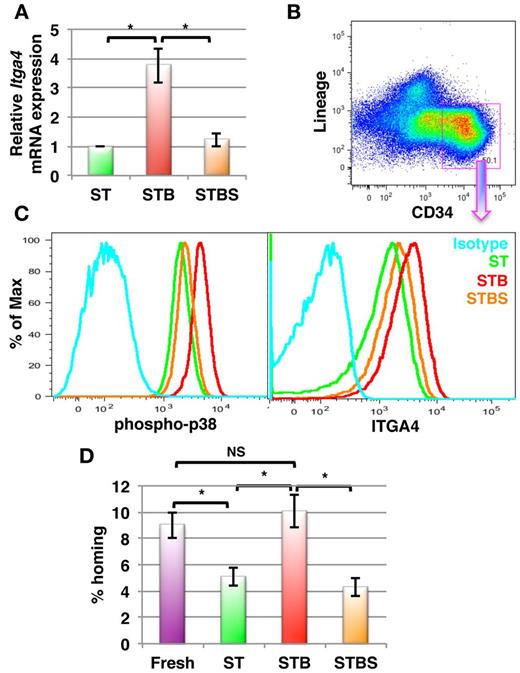
Finally, we tested whether BMP4 has similar effects on human BM derived HSPCs. Lin−CD34+ cells were magnetically sorted from BM mononuclear cells and cultured in the presence of SCF+TPO with or without BMP4. After 5 days, cells were harvested and gene and protein expression analysis was performed by qRT-PCR and flow cytometry, respectively. As in murine BM HSPCs, the expression of ITGA4 mRNA in human Lin−CD34+ cells was significantly increased in response to BMP4 (3.76- ± 0.58-fold, P = .009, n = 5; Figure 7A), which was confirmed by FACS (Figurs 7B-C). We also demonstrated by FACS using a phospho-p38 MAPK specific antibody, that BMP4 activates p38 MAPK (Figure 7C left) in the Lin−CD34+ subpopulation (Figure 7B) of cultured human BM cells. Moreover, BMP4 affected ITGA4 expression in human HSPCs via the p38 MAPK signaling pathway, as addition of SB203580 prevented BMP4-mediated ITGA4 expression (Figure 7C right). To extend these findings in vivo, we performed homing experiments (Figure 7D). Human BM-derived lin−CD34+ cells were cultured in the presence of SCF+TPO with or without BMP4 and/or SB203580. After 5 days, the freshly isolated cells as well as the cultured cells were transplanted in lethally irradiated Rag2−/−γC−/− mice. The number of homed progenitors was calculated by performing colony assay on the injected cells as well as on BM cells harvested 16 hours after infusion of the Lin−CD34+ progeny. As observed in the murine system, Lin−CD34+ progeny from cultures with BMP4 homed significantly better than cells cultured without BMP4 (P = .002, n = 8; Figure 7D). Although we did not find a significant increase in homing potential of the cells cultured with BMP4 over the uncultured cells, BMP4 completely overcame the culture-induced loss of homing potential of human HSPCs. Addition of SB203580 abrogated the effect of BMP4. Hence, BMP4 increases homing and engraftment of both human and murine HSPCs via p38 MAPK-mediated increased ITGA4 expression.
BMP4 increases ITGA4 expression in human BM-derived HSPCs resulting in better homing after transplantation in mice. Human BM derived Lin−CD34+ cells were cultured with SCF+TPO with or without BMP4 alone or with SB203580 for 5 days (ST = SCF/TPO; STB = SCF/TPO/BMP4; STBS = SCF/TPO/BMP4/ SB203580). Levels of ITGA4 mRNA were assessed by qRT-PCR, expression of phospho-p38 MAPK and ITGA4 by FACS, or cells were used for in vivo homing assays. (A) qRT-PCR analysis for ITGA4 in hBM derived Lin−CD34+ progeny from ST, STB and STBS cultures. (n = 5; * P < .01). (B-C) Lin−CD34+ BM progeny were harvested and stained with antibodies against Lineage markers, CD34, phospho-p38 MAPK or ITGA4. Lin−CD34+ cells were gated (B), and phosphorylated p38 MAPK levels (C, left) and ITGA4 levels (C, right) were compared among different conditions by FACS. Representative example of 5 experiments. (D) Freshly isolated or cultured hBM Lin−CD34+ cells (0.1 × 106) were transplanted intravenously in lethally irradiated Rag2−/−Il2Rγc−/− mice, BM was harvested after 16 hours and CFU-Cs enumerated in the recipient BM. Percentage homing was determined by comparing total CFU-Cs homed with CFU-Cs injected (n = 8, P < .005).
BMP4 increases ITGA4 expression in human BM-derived HSPCs resulting in better homing after transplantation in mice. Human BM derived Lin−CD34+ cells were cultured with SCF+TPO with or without BMP4 alone or with SB203580 for 5 days (ST = SCF/TPO; STB = SCF/TPO/BMP4; STBS = SCF/TPO/BMP4/ SB203580). Levels of ITGA4 mRNA were assessed by qRT-PCR, expression of phospho-p38 MAPK and ITGA4 by FACS, or cells were used for in vivo homing assays. (A) qRT-PCR analysis for ITGA4 in hBM derived Lin−CD34+ progeny from ST, STB and STBS cultures. (n = 5; * P < .01). (B-C) Lin−CD34+ BM progeny were harvested and stained with antibodies against Lineage markers, CD34, phospho-p38 MAPK or ITGA4. Lin−CD34+ cells were gated (B), and phosphorylated p38 MAPK levels (C, left) and ITGA4 levels (C, right) were compared among different conditions by FACS. Representative example of 5 experiments. (D) Freshly isolated or cultured hBM Lin−CD34+ cells (0.1 × 106) were transplanted intravenously in lethally irradiated Rag2−/−Il2Rγc−/− mice, BM was harvested after 16 hours and CFU-Cs enumerated in the recipient BM. Percentage homing was determined by comparing total CFU-Cs homed with CFU-Cs injected (n = 8, P < .005).
Discussion
Apart from cell-intrinsic molecular interactions that control HSC function,32 multiple extra-cellular signals play important roles in the regulation of HSPC function.33 BMP4, a member of the TGF-β super-family, is important in the development of the hematopoietic system. Whether it is also important in postnatal hematopoiesis is less clear. Elimination of SMAD5 does not affect adult hematopoiesis in mice,10 suggesting that canonical SMAD-dependent BMP4 signaling might not be important. However, a recent study demonstrated that hypomorphic BMP4 mice have decreased numbers of HSCs,11 suggesting a role of BMP4 in postnatal hematopoiesis. Moreover, Bhatia et al demonstrated that BMP4 maintains, although does not expand, the engraftment ability of human SCID-repopulating cells in culture.6
Here, we describe a novel mechanism by which BMP4 regulates murine and human HSPCs, which provides an explanation for the somewhat contradictory findings described in this paper. Consistent with Utsugisawa et al,9 BMP4 did not affect murine BM-HSPC proliferation. Likewise, consistent with Bhatia et al, we found significantly improved short-term and long-term repopulation from cells cultured with high concentrations of BMP4 compared with cells cultured under the same defined conditions with only SCF+TPO. We demonstrate that the mechanism whereby BMP4 affects repopulation of cultured cells is via increased homing, because of maintained ITGA4 expression during the in vitro culture step, and this via BMP4–mediated SMAD-independent activation of the transcription factor MITF (supplemental Figure 7).
Homing depends both on migration and attachment of HSPCs to the BM niche. Migration of HSPCs is predominantly governed by the CXCL12-CXCR4 axis.34,35 However, we did not observe any change in migration of HSPCs cultured with BMP4 to stromal feeders, strongly suggesting that BMP4 does not affect homing and engraftment of HSPCs by influencing this pathway. Attachment of HSPCs to the BM niche is regulated in large part by ITGA4, and ITGA4 is therefore important for both homing and maintenance of HSCs.16,17,36,37 Here, we demonstrate that cells cultured for as long as 5 days with SCF+TPO in combination with BMP4 home and establish long-term engraftment at least as well as freshly isolated KLS cells. Of note, when KLS cells were treated with either CHD or TSG, 2 extracellular inhibitors of BMP4, an opposite effect was seen, that is, decreased radioprotection and long-term repopulation, along with decreased adhesion and homing, as well as decreased expression of ITGA4. This can be explained by autocrine regulation by BMP4 signaling, as BMP4 expression in HSPCs has been reported.9,38
Although BMP4 caused phosphorylation of SMAD1/5/8, inhibition of this SMAD-dependent pathway by dorsomorphin did not affect ITGA4 expression, the ability of KLS progeny to home into the BM or impart radioprotection. In contrast, when KLS cells were cultured in the presence of the p38 MAPK inhibitor SB203580 along with BMP4, ITGA4 up-regulation was inhibited. This was associated with reversal of BMP4-enhanced homing and radioprotection. Other signaling molecules that can partake in SMAD-independent signaling, such as ERK1/2, JNK, and S6K,2 already active in KLS progeny cultured with SCF+TPO, were not further activated by BMP4 stimulation. The SMAD-independent signaling pathway is initiated by TAK1 phosphorylation,39 which then phosphorylates MKK3/6 or MKK4 to activate p38 MAPK40 or JNK,41 respectively. In KLS cell progeny, BMP4 induced the phosphorylation of TAK1 as well as MKK3/6. Consistent with the finding that BMP4 treatment increased competitive long-term repopulation of the KLS cells we also demonstrated that BMP4 activates p38 MAPK, which causes increased expression of ITGA4 in the primitive CD48−CD150+KLS subpopulation.
Although integrin-mediated adhesion is known to be regulated by changes in the activation state of the receptor,18 changes in integrin transcription also play a role. For instance, it is well established that TGF-β up-regulates expression of αvβ3, αvβ5, αvβ6, and several β1 integrins in several cell types as well as in cancer.21 In general, TGF-β influences integrin expression via SMAD2/3 activation, even though noncanonical pathways also play a role. Among the TFs that regulate ITGA4 expression is MITF.42 MITF binds to a CACTTG motif in the Itga4 promoter.29 It is a member of the basic helix-loop-helix family of TFs and plays an important role in melanoblast biology.42 Mitf expression in melanoblasts is inhibited by TGF-β43 and Notch,44 and positively regulated by Wnt3a43 and p38 MAPK.45 The latter also phosphorylates MITF on Ser307, increasing its ability to activate transcription.31 In the mi/mi mouse, mast cells do not express ITGA4, which is associated with their impaired adhesion to fibroblasts, but is restored on reintroduction of MITF.29,46
These studies led us to test whether MITF is the transcription factor that mediates BMP4-induced increase in ITGA4 expression. On stimulation with BMP4, MITF translocated into the nucleus of KLS cell progeny, consistent with its activation. The same effect was observed in immunocytochemistry experiments in which the primitive CD48−CD150+KLS fraction was sorted from cultured KLS progeny. This effect could be prevented by SB203580 but not by dorsomorphin. When Mitf was knocked down, ITGA4 levels were significantly reduced in HSPCs, and BMP4–mediated induction of ITGA4 was abrogated. Knockdown of Mitf significantly impeded homing and radioprotection of KLS cell progeny, which could no longer be induced by addition of BMP4.
In conclusion, BMP4 treatment improves homing and engraftment of HSPCs, which is mediated by p38 MAPK-induced activation of MITF that up-regulates ITGA4 expression. We also demonstrate that BMP4 increases ITGA4 expression and in vivo homing of human BM lin−CD34+ cells via a similar signaling pathway. As loss of ITGA4 is associated with decreased maintenance of adult HSCs in the BM, our results help to explain the hematopoietic defects in hypomorphic BMP4 mice, which might be because of loss of retention in the BM niche.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Vik Vanduppen for excellent assistance with FACS sorting and analysis, and Rangarajan Sambathkumar and Drs Valerie Roobrouck and Anujith Kumar for critical review for accurateness of the paper.
This work was supported by an FWO grant (1.2.665.11.N.00) to S.K.; FWO funding (G085111N), NIH-PO1-CA-65 493-06, Odysseus funding, CoE and GOA/11/012 funding from KU Leuven, and the Vanwayenberghe fund to C.M.V.
National Institutes of Health
Authorship
Contribution: S.K. designed and performed the experiments, analyzed the data, and wrote the paper; S.B. performed chimerism experiments; S.S. performed FACS analyses and some of the murine homing and engraftment studies; S.E. discussed the experiments and conclusions and reviewed the paper; A.P. discussed the experiments and conclusions and reviewed the paper; M.D. discussed the experiments and conclusions and collected human BM samples; A.Z. discussed the experiments and conclusions and reviewed the paper; and C.V. supervised all the research and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Satish Khurana, Stem Cell Institute, KU Leuven, O&N4, Bus 804, Herestraat 49, 3000 Leuven, Belgium; e-mail: satish.khurana@med.kuleuven.be; or Catherine Verfaillie, Stem Cell Institute, KU Leuven, O&N4, Bus 804, Herestraat 49, 3000 Leuven, Belgium; e-mail: catherine.verfaillie@med.kuleuven.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal