Abstract
Patients with chronic immune thrombocytopenia may have bleeding resulting from low platelet counts. Eltrombopag increases and maintains hemostatic platelet counts; however, to date, outcome has been reported only for treatment lasting ≤ 6 months. This interim analysis of the ongoing open-label EXTEND (Eltrombopag eXTENded Dosing) study evaluates the safety and efficacy of eltrombopag in 299 patients treated up to 3 years. Splenectomized and nonsplenectomized patients achieved platelets ≥ 50 000/μL at least once (80% and 88%, respectively). Platelets ≥ 50 000/μL and 2 × baseline were maintained for a median of 73 of 104 and 109 of 156 cumulative study weeks, respectively. Bleeding symptoms (World Health Organization Grades 1-4) decreased from 56% of patients at baseline to 20% at 2 years and 11% at 3 years. One hundred (33%) patients were receiving concomitant treatments at study entry, 69 of whom attempted to reduce them; 65% (45 of 69) had a sustained reduction or permanently stopped ≥ 1 concomitant treatment. Thirty-eight patients (13%) experienced ≥ 1 adverse events leading to study withdrawal, including patients meeting protocol-defined withdrawal criteria (11 [4%] thromboembolic events, 5 [2%] exceeding liver enzyme thresholds). No new or increased incidence of safety issues was identified. Long-term treatment with eltrombopag was generally safe, well tolerated, and effective in maintaining platelet counts in the desired range. This study is registered at www.clinicaltrials.gov as NCT00351468.
Key Points
The ongoing open-label EXTEND study is investigating the long-term safety and efficacy of eltrombopag in patients with chronic ITP.
This interim analysis shows that treatment with eltrombopag for up to 3 years was effective in increasing and maintaining platelet counts.
Introduction
Chronic immune thrombocytopenia (ITP) is characterized by increased destruction and impaired production of platelets caused by autoantibodies directed against the platelets and megakaryocytes. Patients with ITP have an increased risk of bleeding ranging from minor to life-threatening events and a diminished health-related quality of life (HRQoL).1,2
The treatment goal in chronic ITP is to increase and then maintain platelets in a safe range to prevent bleeding; in addition, improving HRQoL is an important goal for the majority of patients. American Society of Hematology guidelines suggest that platelet counts of 30 000-50 000/μL in patients without other risk factors are required to preclude the most serious complications of ITP (intracerebral or major gastrointestinal hemorrhage)3 and platelets ≥ 50 000/μL are considered “safe” even for the most invasive procedures.4,5
Guidelines issued by different groups3,5,6 recommend corticosteroids as first-line therapy and splenectomy as one of several second-line therapies. Different options exist for patients who do not undergo or do not respond to splenectomy including IVIg, IV anti-D, rituximab, danazol, azathioprine, vinca alkaloids, and cyclophosphamide. Recent consensus statements and guidelines recommend thrombopoietin-receptor (TPO-R) agonists as second- and third-line treatments.5,7
Eltrombopag is the first oral, nonpeptide TPO-R agonist approved for the treatment of chronic ITP in patients with insufficient response to at least one other therapy. Eltrombopag increases platelet production by binding to the transmembrane domain of the TPO-R; it does not compete with endogenous TPO in vitro and it induces proliferation and differentiation of BM progenitor cells in the megakaryocyte lineage.8 Studies have shown that as a result of eltrombopag treatment for up to 6 months, platelets increase to ≥ 50 000/μL in up to 80% of patients.9-11 However, because patients may require treatment for years, it is important to study the efficacy and safety of ITP treatments beyond 6 months.
In this analysis of the ongoing open-label EXTEND (Eltrombopag eXTENded Dosing) study, the safety, efficacy, and tolerability of treatment with eltrombopag for up to 3 years were assessed in 299 patients with ITP of > 6 months' duration enrolled between June 2006 and February 2010.
Methods
EXTEND is an ongoing, global, multicenter, open-label extension study. Patients with chronic ITP (> 6 months' duration) who completed a prior eltrombopag study with the corresponding off-treatment follow-up period (ie, the TRA100773A,9 TRA100773B,10 RAISE,11 and REPEAT12 studies) were eligible for EXTEND. To qualify for the prior studies, patients must have had thrombocytopenia and insufficient response to ≥ 1 previous ITP treatment and platelets < 30 000/μL (20 000-50 000/μL in REPEAT). There were no platelet count criteria for entry in EXTEND. Patients must not have experienced any eltrombopag-related serious adverse event (SAE) during the prior eltrombopag study and were allowed to continue on EXTEND until there was commercial availability of eltrombopag in their country. Patients who were on study for at least 2 years and who withdrew from EXTEND because of commercial availability of treatment were considered to have completed the study whether they continued treatment with commercial eltrombopag or not. This trial is registered at www.clinicaltrials.gov as NCT00351468. The study protocol, any amendments, informed consent according to the Declaration of Helsinki, and other information that required preapproval were reviewed and approved by relevant national, regional, and/or investigational center ethics committees or institutional review boards.
Treatment
Eltrombopag treatment was initiated at 50 mg once daily in all patients. Dosing instructions were based on platelet counts and use of concomitant ITP medications at baseline; dose and schedule were individualized with the goal of achieving and maintaining platelets ≥ 50 000/μL and < 200 000/μL (supplemental Tables 1 and 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Study design
The study design is shown in supplemental Figure 1. In Stage 1, eltrombopag was initiated at 50 mg once daily and adjusted to ≥ 100 000/μL to support dose reduction of concomitant ITP medications. Stage 2 was designed to reduce concomitant ITP medications while maintaining platelets ≥ 50 000/μL. In Stage 3, the dose of eltrombopag was adjusted between 75 and 25 mg once daily or less frequently to identify the minimal dose of eltrombopag necessary to maintain platelets ≥ 50 000/μL in conjunction with the minimal dose of any concomitant ITP medication. In Stage 4, the long-term dosing was evaluated in conjunction with the minimal dose of any concomitant ITP medication. The safety and efficacy of eltrombopag were assessed throughout the study. Patients who did not achieve platelets ≥ 50 000/μL but experienced benefit from eltrombopag treatment (as determined with their physician) were permitted to remain on treatment in the study.
Laboratory safety assessments were performed at each on-treatment visit and at the weeks 1, 2, and 4 follow-up visits. Ocular and renal assessments were performed periodically during treatment and at the 3- and 6-month follow-up visits.
Study end points
The primary end points of the study were safety and tolerability parameters, including clinical laboratory tests, ocular examinations, and AEs. Assessments of efficacy were prespecified secondary end points and included the proportion of patients achieving platelets ≥ 50 000/μL at least once during treatment, individual maximum duration of platelet elevation ≥ 50 000/μL during treatment, reduction in use of ITP medications taken at baseline, use of rescue medication, HRQoL, and World Health Organization (WHO) bleeding grades.13
Analyses
Safety and efficacy analyses were conducted for all patients who received eltrombopag. Safety and tolerability were evaluated using AE reporting (CTCAE 3.0), laboratory data, ocular examinations, electrocardiograms, and BM biopsies. Ocular examinations were scheduled regularly throughout the study; cataract-related findings were reported as SAEs.
Hepatobiliary laboratory abnormalities (HBLAs) were reported following the US Food and Drug Administration (FDA) guidance for assessing potential drug-induced liver injury (DILI)14 (supplemental Table 3). HBLA resolution was defined as a return to baseline values. HBLA-related protocol-defined stopping criteria were alanine aminotransferase (ALT) ≥ 3 × the upper limit of normal (ULN) and bilirubin ≥ 1.5 × ULN (> 35% direct); ALT ≥ 5 × ULN; or ALT ≥ 3 × ULN if progressive or associated with hepatitis symptoms or rash.
BM reticulin was to be quantified by local pathologists using the modified European consensus myelofibrosis (MF) scale.15 Baseline BM biopsy data were collected retrospectively when available. BM examinations were performed: (1) annually per study protocol amendment, (2) at any time for patients with immature/dysplastic cells in the WBC count differential or peripheral blood smear, and (3) at the investigator's discretion.
Thromboembolic events (TEEs) were reported as AEs or SAEs. Incidence and severity of bleeding symptoms were both prospectively reported as AEs and graded using the WHO Bleeding Scale: Grade 0 = no bleeding, Grade 1 = petechiae, Grade 2 = mild blood loss, Grade 3 = gross blood loss, and Grade 4 = debilitating blood loss.
Platelet elevations and their duration were the main efficacy end points. A platelet increase to ≥ 50 000/μL in the absence of any new or increase in other ITP therapy was considered a response; a platelet count was not deemed a response after an on-study splenectomy, within 7 days after a platelet transfusion, or during treatment with or within 6 weeks after the end of a new or increased ITP medication. Depending on platelet increases, the frequency of study assessments ranged from weekly to every other month throughout the study. Because there were no minimum platelet requirements for entering the EXTEND study, a post hoc analysis defined response as platelets ≥ 50 000/μL and at least twice the baseline value without requiring any new or increases in concomitant ITP treatment. For each patient, cumulative and continuous responses were assessed, defined as the number of total and continuous weeks, respectively, that platelets were ≥ 50 000/μL and at least twice the baseline value in the absence of any new or increased ITP treatment. An additional post hoc analysis assessed prolonged response, defined as platelets ≥ 50 000/μL for ≥ 12 weeks after the last dose of eltrombopag without the need for additional or increased ITP treatment. All platelet counts were used for efficacy analyses.
Results
The EXTEND study started in June 2006 and is ongoing in countries where eltrombopag is not yet commercially available. Data are reported herein for 299 patients up to February 2010. The median duration of eltrombopag treatment in EXTEND was 100 weeks (698.5 days; range, 2-1267); 249 patients were exposed to eltrombopag for ≥ 26 weeks, 210 for ≥ 52 weeks, 188 for ≥ 78 weeks, and 138 for ≥ 104 weeks (2 years).
Patient demographics and dispositions
The median age of patients was 50.0 years, 66% were women, and 79% were white (Table 1). At baseline, 33% were receiving concomitant ITP medications and 38% had undergone splenectomy. Before study entry, 78% of patients had received ≥ 2 and 37% of patients had received ≥ 4 prior ITP treatments (including splenectomy). The most common prior ITP medications were corticosteroids, IVIg, and rituximab (23% were unresponsive to or had relapsed after rituximab). Seventy percent of patients had baseline platelets < 30 000/μL, 17% had baseline platelets from 30 000-50 000/μL, and 13% had baseline platelets > 50 000/μL (Table 1).
Summary of demographics and baseline characteristics
| Characteristic . | N = 299 . |
|---|---|
| Median age, y (min-max) | 50.0 (18-86) |
| Sex, n (%) | |
| Women | 198 (66) |
| Ethnicity, n (%) | |
| White | 237 (79) |
| Asian | 45 (15) |
| Other | 17 (6) |
| Baseline platelets, n (%) | |
| ≤ 15 000/μL | 128 (43) |
| > 15 000/μL to < 30 000/μL | 80 (27) |
| 30 000/μL to 50 000/μL | 52 (17) |
| > 50 000/μL | 39 (13) |
| Prior therapies, n (%)* | |
| 1 | 67 (22) |
| 2 | 73 (24) |
| 3 | 47 (16) |
| ≥ 4 | 112 (37) |
| Splenectomy, n (%) | |
| Yes | 115 (38) |
| Concomitant ITP medication, n (%) | |
| Yes | 100 (33) |
| Characteristic . | N = 299 . |
|---|---|
| Median age, y (min-max) | 50.0 (18-86) |
| Sex, n (%) | |
| Women | 198 (66) |
| Ethnicity, n (%) | |
| White | 237 (79) |
| Asian | 45 (15) |
| Other | 17 (6) |
| Baseline platelets, n (%) | |
| ≤ 15 000/μL | 128 (43) |
| > 15 000/μL to < 30 000/μL | 80 (27) |
| 30 000/μL to 50 000/μL | 52 (17) |
| > 50 000/μL | 39 (13) |
| Prior therapies, n (%)* | |
| 1 | 67 (22) |
| 2 | 73 (24) |
| 3 | 47 (16) |
| ≥ 4 | 112 (37) |
| Splenectomy, n (%) | |
| Yes | 115 (38) |
| Concomitant ITP medication, n (%) | |
| Yes | 100 (33) |
ITP indicates immune thrombocytopenia.
Including splenectomy.
Of the 299 enrolled patients, 154 (52%) were still on study at the time of this writing (all for ≥ 64 weeks); 23 (8%) patients completed the study (they were on study for at least 104 weeks and withdrew because of commercial availability of eltrombopag in their country), and 122 (41%) withdrew (Figure 1). The most common reasons for withdrawal were AEs (13%) and patient decision (12%).
Summary of patient disposition in EXTEND (intent-to-treat population).aPatients were considered to have completed this ongoing study if they were on study for at least 2 years and withdrew due to commercial availability of eltrombopag. bPatients may have had > 1 adverse event leading to withdrawal. cAccording to protocol specification. dOther adverse events leading to withdrawal were experienced by 1 patient each (Table 2). eOne of these patients, in the eltrombopag group and a responder in a prior study, withdrew due to other reason of “lack of response to drug.” Of these 30 patients, only 1 patient achieved platelets > 50 000/μL and > 2 × baseline without receiving rescue medication (1 platelet count of > 50 000/μL [54 000/μL], with baseline platelets of 15 000/μL). fOperation on cataract/lost to follow-up/protocol violation (n=1); lost effect of eltrombopag (n=1); stable platelets (n=1); Evan syndrome (n=1); pregnancy (n=1); and hemoglobin over the limit (n=1).
Summary of patient disposition in EXTEND (intent-to-treat population).aPatients were considered to have completed this ongoing study if they were on study for at least 2 years and withdrew due to commercial availability of eltrombopag. bPatients may have had > 1 adverse event leading to withdrawal. cAccording to protocol specification. dOther adverse events leading to withdrawal were experienced by 1 patient each (Table 2). eOne of these patients, in the eltrombopag group and a responder in a prior study, withdrew due to other reason of “lack of response to drug.” Of these 30 patients, only 1 patient achieved platelets > 50 000/μL and > 2 × baseline without receiving rescue medication (1 platelet count of > 50 000/μL [54 000/μL], with baseline platelets of 15 000/μL). fOperation on cataract/lost to follow-up/protocol violation (n=1); lost effect of eltrombopag (n=1); stable platelets (n=1); Evan syndrome (n=1); pregnancy (n=1); and hemoglobin over the limit (n=1).
Efficacy
Platelet response.
In the following analyses, patients with baseline platelet counts > 50 000/μL were included unless stated otherwise. Table 2 summarizes platelet changes during EXTEND. A total of 85% of patients (254 of 299) achieved platelets ≥ 50 000/μL at least once on treatment in the absence of any new or increase in other ITP therapy. This applied to: (1) 86% (69 of 80) who received placebo during their prior eltrombopag study; (2) patients with baseline platelet counts ≤ 50 000/μL, of whom 84% (218 of 260) achieved platelets ≥ 50 000/μL; (3) 80% of patients with baseline platelets < 30 000/μL; (4) 98% with baseline platelets 30 000-50 000/μL; and (5) 66% (49 of 74) of the patients who were nonresponders to eltrombopag in the prior study. Forty-one of the 45 patients who did not achieve a response had baseline platelets < 30 000/μL, and 18 of these 41 (44%) achieved platelets ≥ 30 000/μL at least once during the study.
Response and duration of response
| Efficacy end points . | Result . |
|---|---|
| Platelet elevation, n/N (%) | |
| Platelet count ≥ 50 000/μL at least once | 254/299 (85)* |
| Platelet elevation by stratification variables, n/N (%) | |
| Splenectomized | 92/115 (80) |
| Nonsplenectomized | 162/184 (88) |
| Baseline concomitant ITP medications | |
| Yes | 86/100 (86) |
| No | 168/199 (84) |
| Baseline platelets | |
| < 30 000/μL | 167/208 (80) |
| 30 000/μL to 50 000/μL | 51/52 (98) |
| > 50 000/μL | 36/39 (92) |
| Median no. of cumulative wk with platelets ≥ 50 000/μL and 2 × baseline | |
| All patients (n = 298)† | 44‡ |
| Patients on study for 26 wk (n = 253) | 17 |
| Patients on study for 52 wk (n = 218) | 35 |
| Patients on study for 104 wk (n = 147) | 73 |
| Patients on study for 156 wk (n = 32) | 109 |
| Efficacy end points . | Result . |
|---|---|
| Platelet elevation, n/N (%) | |
| Platelet count ≥ 50 000/μL at least once | 254/299 (85)* |
| Platelet elevation by stratification variables, n/N (%) | |
| Splenectomized | 92/115 (80) |
| Nonsplenectomized | 162/184 (88) |
| Baseline concomitant ITP medications | |
| Yes | 86/100 (86) |
| No | 168/199 (84) |
| Baseline platelets | |
| < 30 000/μL | 167/208 (80) |
| 30 000/μL to 50 000/μL | 51/52 (98) |
| > 50 000/μL | 36/39 (92) |
| Median no. of cumulative wk with platelets ≥ 50 000/μL and 2 × baseline | |
| All patients (n = 298)† | 44‡ |
| Patients on study for 26 wk (n = 253) | 17 |
| Patients on study for 52 wk (n = 218) | 35 |
| Patients on study for 104 wk (n = 147) | 73 |
| Patients on study for 156 wk (n = 32) | 109 |
If patients with platelet counts > 50 000/μL at study entry (n = 39) are removed, 84% of patients are responders (218/260).
End date for treatment missing for 1 patient.
Range of response: 0-164 wk. Overall median treatment duration: 100 wk.
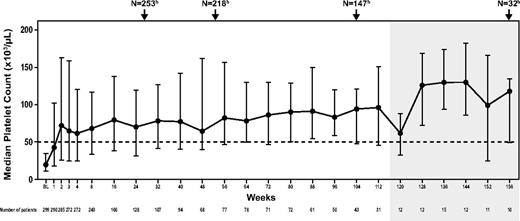
Median platelets increased to > 50 000/μL by week 2 and remained consistently above ≥ 50 000/μL through week 164 (Figure 2). Eighty-5 patients (28%) required additional ITP therapy at least once while on study. The proportion of patients achieving platelets ≥ 50 000/μL at any point during the study without requiring other new or increased ITP treatments was similar regardless of splenectomy status (80% and 88% for splenectomized and nonsplenectomized patients, respectively) or use of concomitant ITP medication at baseline (86% and 84% for patients with and without concomitant baseline ITP medication use; Table 2). Of the 70 patients who relapsed after response to or were refractory to rituximab, 52 (74%) achieved platelets ≥ 50 000/μL at least once in the absence of any new or increased ITP treatments.
Median platelets during EXTEND. Shaded area indicates assessments of ≤ 15 patients. Assessments include 39 patients with platelets ≥ 50 000/μL at baseline. Vertical bars indicate interquartile range (Q1 to Q3). BL indicates baseline. aData shown do not include all assessments; the time points included here illustrate median platelets for the first several weeks of treatment and then at regular intervals thereafter. Patients enrolled in EXTEND at different time points and consequently have differing assessment schedules; therefore, not all patients on study were assessed during each assessment shown. bTotal patients on study at weeks 26, 52, 104, and 156.
Median platelets during EXTEND. Shaded area indicates assessments of ≤ 15 patients. Assessments include 39 patients with platelets ≥ 50 000/μL at baseline. Vertical bars indicate interquartile range (Q1 to Q3). BL indicates baseline. aData shown do not include all assessments; the time points included here illustrate median platelets for the first several weeks of treatment and then at regular intervals thereafter. Patients enrolled in EXTEND at different time points and consequently have differing assessment schedules; therefore, not all patients on study were assessed during each assessment shown. bTotal patients on study at weeks 26, 52, 104, and 156.
Of the 299 patients enrolled, 62% achieved platelets ≥ 50 000/μL without new or increased ITP treatments in > 50% of study assessments. Among patients who had previously received ≥ 4 therapies for ITP, 52% (58 of 112) had platelets ≥ 50 000/μL for > 50% of weeks on treatment without new or increased ITP treatment. For the majority of weeks, from week 6-156 (143 of 151 weeks), at least 75% of patients on eltrombopag had platelets ≥ 30 000/μL.
The cumulative and continuous weeks that platelets were ≥ 50 000/μL and at least twice baseline were assessed. Patients on treatment for at least 104 weeks (n = 147) and 156 weeks (n = 32) had a median cumulative response for 73 and 109 weeks, respectively (Table 2). Continuous response was demonstrated by 47% of patients (119 of 254) for at least 25 weeks and 26% of patients (56 of 218) for at least 52 weeks.
A prolonged response (platelets ≥ 50 000/μL sustained for ≥ 12 weeks after the last dose of eltrombopag without rescue treatment) was seen in 13 patients (4%). Two of these patients were receiving corticosteroids at study entry and in both cases they were interrupted before or during the prolonged response period. The median time since diagnosis of ITP in these patients was 26 months (range, 9-128). Five had been splenectomized before enrollment in EXTEND. The median treatment duration with eltrombopag in EXTEND before the prolonged response was 160 days (range, 14-1107).
Dosing.
The median daily dose of eltrombopag was 51.5 mg, which remained stable throughout the study. Of the 299 patients, 50% received 25 mg or less and 61% required 75 mg at some time during the study.
Bleeding symptoms.
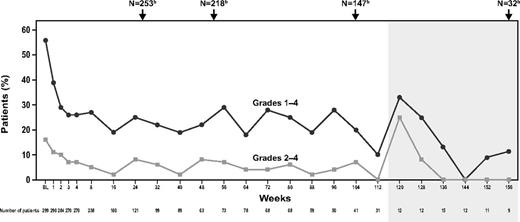
At baseline, 56% of patients (168 of 299) reported bleeding symptoms (WHO Grades 1-4) compared with 27% (33 of 123), 16% (12 of 77), 20% (8 of 41), and 11% (1 of 9) of patients at 26, 52, 104, and 156 weeks, respectively (Figure 3). Similarly, 16% of patients (47 of 299) reported clinically significant bleeding (Grades 2-4) at baseline compared with 5% (6 of 123), 3% (2 of 77), 7% (3 of 41), and 0% (0 of 9) of patients at 26, 52, 104, and 156 weeks, respectively (Figure 3). For the majority of weeks, the proportion of patients with any bleeding or clinically significant bleeding was reduced by approximately half from baseline.
Bleeding during EXTEND. Shaded area indicates assessments of ≤ 15 patients. Bleeding was measured using the WHO scale. BL indicates baseline. aData shown do not include all assessments; the time points included here illustrate median bleeding scores for the first several weeks of treatment and then at regular intervals thereafter. Patients enrolled in EXTEND at different time points and consequently have differing assessment schedules; therefore, not all patients on study were assessed during each assessment shown. bTotal patients on study at weeks 26, 52, 104, and 156.
Bleeding during EXTEND. Shaded area indicates assessments of ≤ 15 patients. Bleeding was measured using the WHO scale. BL indicates baseline. aData shown do not include all assessments; the time points included here illustrate median bleeding scores for the first several weeks of treatment and then at regular intervals thereafter. Patients enrolled in EXTEND at different time points and consequently have differing assessment schedules; therefore, not all patients on study were assessed during each assessment shown. bTotal patients on study at weeks 26, 52, 104, and 156.
Concomitant ITP medication use.
At baseline, 100 patients (33%) reported the use of concomitant ITP medications. Sixty-nine of these patients attempted to reduce or discontinue these medications while on EXTEND. Forty-five of these 69 patients were able to reduce or discontinue these medications without requiring subsequent rescue treatment and 43 of 45 (96%) maintained this discontinuation or reduction for ≥ 24 weeks. Thirty-seven of 69 patients (54%) completely discontinued at least 1 baseline ITP medication and 34 of 69 (49%) permanently discontinued all baseline ITP medications in the absence of any rescue medications. Corticosteroids were the most frequently discontinued or reduced concomitant ITP medication (42 of 45, 93%), followed by danazol (8 of 45, 18%), azathioprine (4 of 45, 9%), and IVIgs and cyclosporine (1 of 45 each, 2%).
Patients with platelets > 50 000/μL at baseline.
Of the 299 patients, 39 (13%) had baseline platelets > 50 000/μL; separate analyses were performed to assess response (defined for this group as platelets ≥ 50 000/μL and 2 × baseline), bleeding, and concomitant medication use for these patients. Fifty-four percent of patients (21 of 39) achieved a response at least once without receiving rescue therapy. Three patients were withdrawn before week 23. At baseline, 38% of patients (15 of 39) reported bleeding symptoms (WHO Grades 1-4). At 6 months (weeks 23-29), 7 of these patients did not report any WHO bleeding symptoms and 5 reported Grades 1-2 bleeding. At baseline, 44% of these patients (17 of 39) reported use of concomitant ITP medications, 59% of whom (10 of 17) had a sustained reduction of a baseline concomitant medication for at least 4 weeks without receiving on-treatment rescue therapy.
Safety
AEs were reported in 262 patients (88%) while on therapy; 59% of patients reported AEs only of Grades 1-2. The most common were headache, nasopharyngitis, upper respiratory tract infection, and fatigue (Table 3). These common events were mostly mild and led to withdrawal in 2 patients with headache (< 1%) and in 1 with fatigue (< 1%; supplemental Table 4). The most common Grade ≥ 3 AEs were thrombocytopenia, increased ALT, and fatigue (Table 4). On-therapy AEs considered by the investigator to be related to study medication were reported in 42% of patients (125 of 299). The AEs most frequently considered to be treatment related were headache (10%, all grade 1 or 2) and hyperbilirubinemia (6%, 1 patient with grade 3). Cataracts (n = 15, 5%) were the most frequently reported on-therapy SAE (the study protocol required that cataracts be reported as SAEs), followed by ALT increases (n = 5, 2%), deep vein thrombosis (DVT, n = 5, 2%), and pneumonia (n = 5, 2%). Fifteen patients reported cataract SAEs; 1 had a trauma-induced cataract, 4 developed new cataracts, and 10 had progression of preexisting cataracts. Chronic prior use of corticosteroids was reported in 13 of the 15 patients.
Adverse events in ≥ 10% of patients
| . | N = 299 . |
|---|---|
| Any AE, n (%) | 262 (88) |
| Headache | 77 (26) |
| Nasopharyngitis | 68 (23) |
| Upper respiratory tract infection | 62 (21) |
| Fatigue | 45 (15) |
| Arthralgia | 41 (14) |
| Diarrhea | 41 (14) |
| Nausea | 33 (11) |
| Back pain | 31 (10) |
| Urinary tract infection | 30 (10) |
| . | N = 299 . |
|---|---|
| Any AE, n (%) | 262 (88) |
| Headache | 77 (26) |
| Nasopharyngitis | 68 (23) |
| Upper respiratory tract infection | 62 (21) |
| Fatigue | 45 (15) |
| Arthralgia | 41 (14) |
| Diarrhea | 41 (14) |
| Nausea | 33 (11) |
| Back pain | 31 (10) |
| Urinary tract infection | 30 (10) |
Grade ≥ 3 adverse events in ≥ 2 patients
| Preferred term . | N = 299 (%) . |
|---|---|
| Any AE of grade ≥ 3 severity, n (%) | 83 (28) |
| Thrombocytopenia | 7 (2) |
| ALT increased | 6 (2) |
| Fatigue | 6 (2) |
| Pain in extremity | 5 (2) |
| Anemia | 4 (1) |
| Aspartate aminotransferase increased | 4 (1) |
| Back pain | 4 (1) |
| Hypertension | 4 (1) |
| Myocardial infarction | 4 (1) |
| Abdominal pain | 3 (1) |
| Blood bilirubin increased | 3 (1) |
| Cataract | 3 (1) |
| Dyspnea | 3 (1) |
| Menorrhagia | 3 (1) |
| Pneumonia | 3 (1) |
| Arthritis | 2 (< 1) |
| Chest pain | 2 (< 1) |
| Deep vein thrombosis | 2 (< 1) |
| Diarrhea | 2 (< 1) |
| Dizziness | 2 (< 1) |
| Gastrointestinal hemorrhage | 2 (< 1) |
| Headache | 2 (< 1) |
| Insomnia | 2 (< 1) |
| Migraine | 2 (< 1) |
| Renal failure | 2 (< 1) |
| Preferred term . | N = 299 (%) . |
|---|---|
| Any AE of grade ≥ 3 severity, n (%) | 83 (28) |
| Thrombocytopenia | 7 (2) |
| ALT increased | 6 (2) |
| Fatigue | 6 (2) |
| Pain in extremity | 5 (2) |
| Anemia | 4 (1) |
| Aspartate aminotransferase increased | 4 (1) |
| Back pain | 4 (1) |
| Hypertension | 4 (1) |
| Myocardial infarction | 4 (1) |
| Abdominal pain | 3 (1) |
| Blood bilirubin increased | 3 (1) |
| Cataract | 3 (1) |
| Dyspnea | 3 (1) |
| Menorrhagia | 3 (1) |
| Pneumonia | 3 (1) |
| Arthritis | 2 (< 1) |
| Chest pain | 2 (< 1) |
| Deep vein thrombosis | 2 (< 1) |
| Diarrhea | 2 (< 1) |
| Dizziness | 2 (< 1) |
| Gastrointestinal hemorrhage | 2 (< 1) |
| Headache | 2 (< 1) |
| Insomnia | 2 (< 1) |
| Migraine | 2 (< 1) |
| Renal failure | 2 (< 1) |
AE indicates adverse event; ALT, alanine aminotransferase; and AST, aspartate aminotransferase.
Thirty-eight patients (13%) experienced AEs leading to withdrawal (Figure 1 and supplemental Table 4), with 17 (45%) meeting protocol-defined stopping criteria: 11 (4%) were TEEs and 6 (2%) were liver enzyme thresholds.
Thirty patients (10%) withdrew because of lack of efficacy; of these, 21 had received eltrombopag in their prior study and 67% (14 of 21) had been nonresponders. The remaining 9 patients received placebo in their prior eltrombopag study. Forty-two patients withdrew for nonmedical reasons: 5 because of noncompliance, 2 because of protocol violation, and 35 because of patient decision.
Twenty-three percent of patients had ≥ 1 on-therapy bleeding AE (70 of 299 patients, 210 events); the most common were epistaxis, contusion, petechiae, ecchymosis, and oral hemorrhage. Most bleeding events were CTCAE Grade 1 (145 of 210 events); 10 patients had 11 on-therapy Grades 3-4 bleeding AEs: menorrhagia (4 events in 3 patients); gastrointestinal hemorrhage (3 patients); and contusion, oral hemorrhage, epistaxis, and hematemesis (1 each). Eight patients had 10 posttherapy bleeding AEs; 7 events occurred between 2 and 30 days after interruption of eltrombopag treatment (2 concurrent with reoccurrence of thrombocytopenia) and 3 occurred > 30 days after treatment. Platelet counts were < 100 000/μL and < 50 000/μL in 82% and 61% of the bleeding AEs, respectively.
Sixteen (8%) of the 210 patients who temporarily or permanently interrupted treatment experienced reoccurrence of thrombocytopenia (defined as off-therapy platelets < 10 000/μL and at least 10 000/μL less than baseline within 4 weeks after interruption or discontinuation16 ); 6 of 16 required an increased dose or a new ITP therapy. Two patients had bleeding AEs (grade 3 menorrhagia and grade 2 metrorrhagia) associated with reoccurrence of thrombocytopenia.
Five deaths occurred; according to investigators, all were unrelated to the study drug. Two deaths occurred on therapy: 1 patient died in a motor vehicle accident and one died at home with the cause of death undetermined. Three deaths, all in nonresponders, occurred more than 30 days after therapy. Two patients, both with platelets < 10 000/μL at their last visit, died of bleeding events (hypovolemic shock secondary to gastrointestinal hemorrhage and intracranial hemorrhage). The third patient, a nonsplenectomized 77-year-old female, died because of multiorgan failure secondary to sepsis of pulmonary origin 42 days after stopping eltrombopag.
Hepatobiliary laboratory abnormalities
Ten percent of patients (29 of 299) had 1 or more HBLAs that met ≥ 1 FDA-defined screening criteria for potential DILI. Six patients discontinued treatment because of HBLAs (2 because of increased ALT and 4 because of increased ALT and bilirubin); of these, 5 were withdrawn because of protocol-defined stopping criteria and 1 was withdrawn at the investigator's discretion because of an ALT of > 4 × ULN. All bilirubin elevations were because of indirect bilirubin, which is not indicative of DILI. There was no pattern in the time to onset of HBLAs (median, 103 days; range, 1-980). For the majority of patients (19 of 29), the HBLA resolved (16 despite continued treatment and 3 after interruption or discontinuation of treatment). Of the remaining 10 patients, 5 had HBLAs that were resolving, 4 had elevated hepatobiliary values at baseline and intermittent elevations throughout the study (for 1 patient, the HBLA resolved while on treatment), and in 1 patient (withdrawn from the study because of a DVT) the HBLA had not resolved at the time of the last available follow-up.
Eighteen eltrombopag-treated patients who experienced an HBLA meeting screening criteria for possible DILI in previous eltrombopag studies were rechallenged with eltrombopag in EXTEND. Of these 18, 7 experienced an HBLA that met DILI screening criteria in EXTEND and 11 did not. In most of the 7 patients who again experienced HBLAs meeting DILI screening criteria, the severity of the elevation(s) in hepatobiliary laboratory values was similar to or less than those observed in the previous study (supplemental Table 5). There was no apparent pattern in the time to onset (median, 105 days; range, 1-164). Of these 7 patients, 4 had HBLAs at baseline and intermittently during the study, 2 had HBLAs that resolved (1 while on treatment and 1 after discontinuation of treatment), and the remaining patient was diagnosed with cholangitis and withdrawn because of HBLAs meeting protocol-defined liver stopping criteria.
TEEs
Of the 299 patients, 9 (3%) had a history of TEEs before enrollment in the preceding eltrombopag study. During EXTEND, 5% of patients (16 of 299) experienced 20 confirmed TEEs and 1 suspected TEE (Table 5); 4 patients experienced ≥ 2 events each. Observed TEEs were DVT (n = 9), CNS ischemic events (n = 5, including 1 suspected case of prolonged reversible ischemic neurologic deficit [PRIND] identified by the sponsor's medical review of symptoms reported as AEs but not as a TEE), myocardial infarction (n = 4), and pulmonary embolism (n = 3). This corresponds to an incidence rate of 3.17 of 100 patient-years (95% confidence interval, 1.81-5.15), which has remained stable since a 2009 interim analysis (4.5 of 100 patient-years, 95% confidence interval, 2.39-7.69) despite a 1.7-fold increase in exposure (data not published). Two of the 16 patients (13%) did not achieve platelets ≥ 50 000 of μL, although both had increases in their platelets. Fourteen of 16 patients (88%) experienced TEEs at platelet levels lower than their maximum levels achieved during eltrombopag treatment and 50% experienced TEEs with platelets below the normal range. Platelets proximal to the TEEs ranged from 14 000-482 000/μL. Of the 13 patients who provided samples for genetic evaluation, 2 were identified as being heterozygous for Factor V Leiden (R506Q). Although all patients who experienced a TEE had at least 1 risk factor (eg, hypertension, smoking, obesity, family history, or genetic predisposition), no single common etiologic factor was identified.17
Thromboembolic events
| Patient no. . | Event . | Baseline platelet count* . | Platelet count prior to/day of the event . | Maximum platelet count† . | Days to onset . | Outcome . |
|---|---|---|---|---|---|---|
| 1 | TIA | 13 | 27 | 301 | 59 | Resolved |
| 2 | PE | 15 | 407 | 407 | 58 | Resolved |
| 3 | DVT | 23 | 248 | 577 | 387 | Resolved |
| 4 | CNS ischemia | 25 | 60 | 122 | 971 | Not resolved |
| Subclavian/brachial vein thrombosis | Unknown | 981 | Not resolved | |||
| 5 | DVT | 26 | 220 | 482 | 134 | Resolved‡ |
| MI | 420 | 362 | Resolved | |||
| DVT | 482 | 387 | Resolved | |||
| 6 | MI | 21 | 197 | 364 | 476 | Resolved |
| 7 | PE | 9 | 246 | 246 | 143 | Resolved‡ |
| 8 | DVT | 15 | 61 | 108 | 114 | Resolved |
| 9 | DVT | 13 | 40 | 55 | 279 | Not resolved |
| 10 | MI | 23 | 146 | 208 | 761 | Resolved |
| 11 | Cerebral infarction | 7 | 143 | 324 | 300 | Resolved‡ |
| 12 | Cerebral infarction | 25 | 219 | 476 | 244 | Resolved‡ |
| 13 | PE | 29 | 94 | 304 | 215 | Resolved |
| DVT | 228 | 304 | 229 | Resolved | ||
| 14 | Balance disorder, speech disorder, dizziness (suspected PRIND) | 14 | 14 | 44 | 1 | Resolved |
| 15 | DVT (8 d posttherapy) | 6 | 28 | 40 | 45 | Resolving |
| 16 | DVT (7 d posttherapy) | 23 | 214 | 465 | 57 | Resolved |
| Patient no. . | Event . | Baseline platelet count* . | Platelet count prior to/day of the event . | Maximum platelet count† . | Days to onset . | Outcome . |
|---|---|---|---|---|---|---|
| 1 | TIA | 13 | 27 | 301 | 59 | Resolved |
| 2 | PE | 15 | 407 | 407 | 58 | Resolved |
| 3 | DVT | 23 | 248 | 577 | 387 | Resolved |
| 4 | CNS ischemia | 25 | 60 | 122 | 971 | Not resolved |
| Subclavian/brachial vein thrombosis | Unknown | 981 | Not resolved | |||
| 5 | DVT | 26 | 220 | 482 | 134 | Resolved‡ |
| MI | 420 | 362 | Resolved | |||
| DVT | 482 | 387 | Resolved | |||
| 6 | MI | 21 | 197 | 364 | 476 | Resolved |
| 7 | PE | 9 | 246 | 246 | 143 | Resolved‡ |
| 8 | DVT | 15 | 61 | 108 | 114 | Resolved |
| 9 | DVT | 13 | 40 | 55 | 279 | Not resolved |
| 10 | MI | 23 | 146 | 208 | 761 | Resolved |
| 11 | Cerebral infarction | 7 | 143 | 324 | 300 | Resolved‡ |
| 12 | Cerebral infarction | 25 | 219 | 476 | 244 | Resolved‡ |
| 13 | PE | 29 | 94 | 304 | 215 | Resolved |
| DVT | 228 | 304 | 229 | Resolved | ||
| 14 | Balance disorder, speech disorder, dizziness (suspected PRIND) | 14 | 14 | 44 | 1 | Resolved |
| 15 | DVT (8 d posttherapy) | 6 | 28 | 40 | 45 | Resolving |
| 16 | DVT (7 d posttherapy) | 23 | 214 | 465 | 57 | Resolved |
CNS indicates central nervous system; DVT, deep vein thrombosis; MI, myocardial infarction; PE, pulmonary embolism; PRIND, prolonged reversible ischemic neurologic deficit; and TIA, transient ischemic event.
Baseline platelet count of the study in which the patient was first exposed to eltrombopag.
Maximum platelet count includes platelet counts across studies while exposed to eltrombopag (ie, prior study and EXTEND study).
Events reported as resolved with sequelae.
BM abnormalities
There were 147 on-treatment BM biopsies locally evaluated for reticulin/collagen once the study protocol was amended to include routine yearly BM evaluations. BM biopsies were collected from 135 patients who had received eltrombopag for a median of 12 months (range, 1-32).
No BM biopsies were prompted by abnormal peripheral blood smears or clinical symptoms. BM biopsies were analyzed for BM fiber deposition. Of the 147 samples evaluated locally by the participating institutions for reticulin/collagen, 88 had MF grade 0, 48 had MF grade 1 (1 patient with reported collagen), and 11 (8%) had MF grade 2 (2 had collagen reported). No sample was graded as MF grade 3. No patient had symptoms related to BM dysfunction. Eleven patients had a second biopsy after being treated for ≥ 2 years. Compared with the first biopsy, 8 of 11 patients had no change in reticulin grade, 1 of 11 experienced an increase of MF grade 1 to grade 2, and 2 of 11 experienced a decrease in reticulin grade (MF grade 2 to grade 0 and grade 1 to grade 0).
None of the 80 patients with review of a BM smear showed an increased BM blast count > 3% or any BM abnormality other than those compatible with ITP. In addition, none of 19 samples tested for cytogenetics had an abnormal karyotype.
Hematologic malignancies
Two patients were diagnosed with lymphoma (none with leukemia) during the 491 patient-years of exposure to eltrombopag during EXTEND. One patient was diagnosed with diffuse large B-cell lymphoma after 64 days on treatment in EXTEND and 122 days on treatment in a prior eltrombopag study (REPEAT). A second patient, who had been off treatment for more than 18 months because of persistently normal platelets, was diagnosed with Hodgkin lymphoma 3 years after the first dose of eltrombopag in EXTEND.
Discussion
The effect of TPO-R agonists is transient, and platelet counts usually return to baseline within 2 weeks unless treatment is continued. Many patients with chronic ITP require long-term maintenance treatment and data on the continued use of eltrombopag for longer than 6 months are very limited.18-21
The EXTEND study provides comprehensive data on long-term treatment of chronic ITP with eltrombopag. Sustained platelet elevations and reduction in bleeding and use of concomitant ITP medications were observed—in many patients for prolonged periods of time (months), confirming extension of the response to eltrombopag observed in the pivotal 6-week and 6-month trials.9-11 Overall, treatment with eltrombopag was well tolerated. The long-term study of the subcutaneous TPO-R agonist romiplostim demonstrated similar findings regarding the ability of TPO-R agonists to maintain patients for long periods of time on treatment and reduction in ITP concomitant and rescue medications.18
Failure to respond to previous treatments did not prevent response to eltrombopag. Seventy percent or more of patients who failed to respond to or relapsed after either rituximab or splenectomy had platelets ≥ 50 000/μL after eltrombopag treatment. Approximately half the patients who had been treated with ≥ 4 prior ITP treatments had platelets ≥ 50 000/μL for more than 50% of study visits. The response to eltrombopag over time did not systematically diminish, as reflected by stable median dose and platelets throughout the treatment period.
A posthoc analysis showed that a prolonged response of ≥ 12 weeks after the last dose of eltrombopag without rescue treatment was seen in 5% of patients. It is not clear if these patients experienced remission of chronic ITP, because > 12 weeks of follow-up is not available for most patients. Cases of prolonged response have also been reported with romiplostim treatment.18,22 Induction of CD4+ regulatory T cells is being investigated as a potential mechanism for lasting platelet responses.23
As platelets increased, an approximately 50% reduction in bleeding symptoms, including clinically significant bleeding (WHO Grades 2-4), was observed throughout the 3 years of data reported, reflecting the inverse relationship between platelets and bleeding severity in patients with chronic ITP.5
Six safety events (liver laboratory abnormalities, BM fibrosis, reoccurrence of thrombocytopenia after discontinuation, thrombosis, hematologic malignancy, and cataracts) have been of interest because of preclinical signals, data from clinical trials, or theoretical concerns.
Treatment with eltrombopag has been reported to be associated with HBLA.9-11 In EXTEND, after eltrombopag treatment for up to 3 years, only 6 patients withdrew because of liver enzyme elevations. Furthermore, 11 of 18 patients who had experienced HBLAs that met DILI screening criteria during a prior study were treated with eltrombopag during EXTEND without recurrence of HBLA that met DILI screening criteria. Overall, treatment with eltrombopag is infrequently associated with HBLAs that may appear at any time during treatment but are mostly mild, reversible, and not accompanied by clinically significant symptoms indicative of impaired liver function. Nonetheless, monthly monitoring is needed and, if HBLAs occur, treatment may need to be at least temporarily discontinued.
EXTEND provides a large database of BM biopsies in patients with chronic ITP treated with eltrombopag for > 1 year. None of the ITP patients, including the few with MF-2 or collagen findings, showed clinical signs, symptoms, or laboratory abnormalities that would indicate BM dysfunction. In 11 patients with a second BM biopsy after an additional year of treatment with eltrombopag, there was no evidence of progression of fibrosis. Theoretically, prolonged stimulation of megakaryocytes with TPO-R agonists might increase the risk of BM fibrosis.24,25 Because BM biopsies are often not performed in patients with chronic ITP, there are limited BM data available in these patients before starting eltrombopag. The BM data from EXTEND reported herein arise from local review of the samples. A centralized pathology examination is in progress to ensure a standardized review and will be reported separately.26 In addition, a 2-year, longitudinal BM study (NCT01098487) is ongoing, which includes baseline and repeated BM examinations to further address this safety question. BM is also being evaluated in other ongoing clinical trials of TPO-R agonists. The incidence of BM fibrosis as a result of prolonged treatment with TPO-R agonists is apparently low, although neither the exact mechanism nor the true incidence has been precisely defined.27
Withdrawal of treatments that stimulate platelet production may theoretically result in reoccurrence of thrombocytopenia with platelets decreasing to below pretreatment levels. In a pooled analysis of 3 prior eltrombopag studies, reoccurrence of thrombocytopenia after discontinuation of study medication occurred in 8% of both eltrombopag- and placebo-treated patients and was generally not associated with bleeding events28 ; similar results were observed in EXTEND. Nonetheless, after interrupting any therapy for thrombocytopenia, it is expected that platelets will return to baseline levels if not occasionally lower. Accordingly, platelet counts should be monitored weekly for 4 weeks after eltrombopag is discontinued.
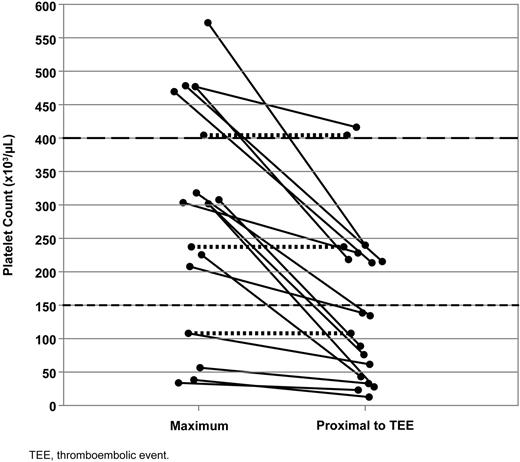
The concept that chronic ITP is a disease with not only hemorrhagic but also prothrombotic characteristics has gained gradual acceptance.29-33 Studies with different therapies that increase platelets in these patients through various mechanisms have reported TEEs, including IVIg,34 corticosteroids,35 anti-CD40 ligand,36 and other TPO-R agonists.37 Approximately 3% of patients had experienced TEEs before entering the eltrombopag ITP trials. In EXTEND, 5% of patients experienced TEEs. A single risk factor that would explain a majority of cases has not been identified. Both venous and arterial TEEs have been seen; the former is not typical of platelet effects. Even though in some cases it may be difficult to ascertain the accurate onset of a thrombotic event, and thus an accurate corresponding platelet count, 88% of the 16 patients who experienced TEEs did so with platelets lower than their maximum study platelet counts and half experienced TEEs at platelets below the normal range (Figure 4). This is consistent with published reports failing to document platelet activation with eltrombopag38 and do not demonstrate that high platelet counts per se are the main determinant of these events. Biomarkers of thrombophilia are being investigated to better understand the etiology of TEEs in eltrombopag-treated ITP patients.
Maximum and proximal platelet counts for patients experiencing TEEs on EXTEND. Lines connect platelet counts for individual patients with available platelet counts. Dotted lines indicate patients experiencing TEEs at their maximum platelet count.
Maximum and proximal platelet counts for patients experiencing TEEs on EXTEND. Lines connect platelet counts for individual patients with available platelet counts. Dotted lines indicate patients experiencing TEEs at their maximum platelet count.
An association between autoimmune diseases and hematologic malignancies has been recognized for decades.39-41 Thrombocytopenia can be the first indication of a hematologic malignancy and may precede its onset by years. Theoretically, stimulation of the TPO-R on hematopoietic cells may increase the risk of hematologic malignancies. However, preclinical studies in hematologic malignancies indicate that eltrombopag does not promote the proliferation of malignant cells.42,43 Data from EXTEND corroborate these laboratory findings by failing to suggest an increased risk of hematologic malignancy.
Cataracts were seen in preclinical studies of juvenile rodents but not in dogs. No significant increase in cataracts has been seen in any clinical trial with eltrombopag in chronic ITP, including EXTEND. The common use of steroids in patients with chronic ITP can confound these analyses, especially in single-arm studies.
Conclusions
In this interim analysis of 299 patients, eltrombopag was effective, safe, and well tolerated for up to 3 years in patients with previously treated chronic ITP. BM biopsy data on reticulin and collagen fibrosis, the potential risk for TEEs, and overall safety and efficacy are being assessed in this ongoing trial and in other studies as well. The data from this analysis and future data on long-term treatment with eltrombopag, including those derived from 2 ongoing trials in pediatric chronic ITP, will help physicians decide in which patients and when in the course of the disease to introduce TPO-R agonists in patient treatment paradigms. In addition, treatment algorithms need to be derived that include the TPO-R agonists that maximize clinical benefit while minimizing risks.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the following people for their contributions during the development of this manuscript on behalf of GlaxoSmithKline: Ted Everson, PhD, and Anne Lambert of AOI Communications for medical writing and editorial assistance; Bhabita Mayer of GlaxoSmithKline for statistical analysis; and Kimberly Marino, Rosanna Tedesco, and Manuel Aivado of GlaxoSmithKline for critical review.
Funding for this study (NCT00351468) was provided by GlaxoSmithKline. All authors listed meet the criteria for authorship set forth by the International Committee for Medical Journal Editors.
Authorship
Contribution: M.N.S., J.B.B., G.C., and O.M. (the principal investigators) and employees of the sponsor (GlaxoSmithKline) developed the protocol; the sponsor and the investigators collected and analyzed the data; and all authors participated in decisions related to the manuscript content and publication, had access to the primary data, and vouch for the completeness and accuracy of the data and analyses.
Conflict-of-interest disclosure: M.N.S. has speakers' bureau and consultancy relationships with and has received research funding from GlaxoSmithKline (GSK). J.B.B. has received research funding from Amgen, Cangene, GSK, Genzyme, InG of America, Immunomedics, Ligand, Eisai, Shionogi, and Sysmex; has participated in advisory boards for Amgen, GSK, Ligand, Shionogi, and Eisai; and had a 1-day consultation with Portola. G.C. is on the speakers' bureau and receives honoraria from GSK. O.M. has consultancy and honoraria relationships with GSK and Amgen. C.K.B., M.A., and A.B. are employees of and have equity ownership in GSK. M.A. also holds patents for certain uses of eltrombopag through GSK.
Correspondence: Mansoor N. Saleh, MD, Georgia Cancer Specialists, 5408 St Lyonn Pl, Marietta, GA 30068; e-mail: mansoor.saleh@ccc.uab.edu.

![Figure 1. Summary of patient disposition in EXTEND (intent-to-treat population). aPatients were considered to have completed this ongoing study if they were on study for at least 2 years and withdrew due to commercial availability of eltrombopag. bPatients may have had > 1 adverse event leading to withdrawal. cAccording to protocol specification. dOther adverse events leading to withdrawal were experienced by 1 patient each (Table 2). eOne of these patients, in the eltrombopag group and a responder in a prior study, withdrew due to other reason of “lack of response to drug.” Of these 30 patients, only 1 patient achieved platelets > 50 000/μL and > 2 × baseline without receiving rescue medication (1 platelet count of > 50 000/μL [54 000/μL], with baseline platelets of 15 000/μL). fOperation on cataract/lost to follow-up/protocol violation (n=1); lost effect of eltrombopag (n=1); stable platelets (n=1); Evan syndrome (n=1); pregnancy (n=1); and hemoglobin over the limit (n=1).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/3/10.1182_blood-2012-04-425512/4/m_zh89991201100001.jpeg?Expires=1769079902&Signature=C~E9GwIhrW3dVqPnvGd~aJ5Ufv~hct0cztrZwj6CurNV305Rg7y8cWFDgGuBm3seWQc7627kxog7i2KnD9q~7y9HwDteWdFYVh6BErqfXBDI0Z2E0ugRctbV8qwWjB1gCS0mdgs3qH6wRao0hd8GURRu-u~gqaj3cYEgflRS9sagUOmsKyenxCVLv~hssyPd8Uu4KsdCsPOKZgilLq17yiP7o8p2DVYUcRC1WwldW4hQQQcrDJbj8GVNCFgSdajQUwAQ6B3IROpj-vtotJEREl5YQUstuLBHIKnLeKXzvQQOjRFqb7WkICnbb2t68RIS5CWGLVinbm-ikqF1cF5DpA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)