Abstract
In human peripheral blood, 4 populations of dendritic cells (DCs) can be distinguished, plasmacytoid dendritic cells (pDCs) and CD16+, CD1c+, and BDCA-3+ myeloid DCs (mDCs), each with distinct functional characteristics. DCs have the unique capacity to cross-present exogenously encountered antigens (Ags) to CD8+ T cells. Here we studied the ability of all 4 blood DC subsets to take up, process, and present tumor Ags to T cells. Although pDCs take up less Ags than CD1c+ and BDCA3+ mDCs, pDCs induce potent Ag-specific CD4+ and CD8+ T-cell responses. We show that pDCs can preserve Ags for prolonged periods of time and on stimulation show strong induction of both MHC class I and II, which explains their efficient activation of both CD4+ and CD8+ T cells. Furthermore, pDCs cross-present soluble and cell-associated tumor Ags to cytotoxic T lymphocytes equally well as BDCA3+ mDCs. These findings, and the fact that pDCs outnumber BDCA3+ mDCs, both in peripheral blood and lymph nodes, together with their potent IFN-I production, known to activate both components of the innate and adaptive immune system, put human pDCs forward as potent activators of CD8+ T cells in antitumor responses. Our findings may therefore have important consequences for the development of antitumor immunotherapy.
Key Points
Cross-presentation of both soluble and cell-associated tumor antigens by human DC subsets is enhanced by addition of adjuvant TLR agonists.
Ability to cross-present exogenous antigen with high IFNα secretion puts human pDCs as activators of CD8+ T cells in antitumor responses.
Introduction
Dendritic cells (DCs) are the professional antigen presenting cells (APCs) of the immune system with the unique capacity to attract and activate naive CD4+ and CD8+ T cells.1 After infection or inflammation, DCs undergo a complex maturation process and migrate into lymph nodes where they present antigens (Ags) to T cells. The DC family is very heterogeneous and consists of different DC subsets, each with distinct functional characteristics. In human peripheral blood, at least 2 main populations of DCs can be distinguished: CD11c positive myeloid DCs (mDCs) and CD11c negative plasmacytoid DCs (pDCs). Myeloid DCs can be further subdivided based on the expression of CD16, CD1c, and BDCA3.2 Transcriptional profiling revealed significant differences between the human blood DC subsets,3 probably reflecting differences in their Ag-presenting capacities. Furthermore, mDCs and pDCs show clearly different responses to products derived from pathogens, as a result of their distinct Toll-like receptor (TLR) expression profiles.4 Myeloid DCs have the capacity to produce IL-12 in response to microbial stimuli through TLRs, and thereby, induce Th1 responses.5,6 Plasmacytoid DCs (pDCs), in contrast, are the key effectors in innate immunity because of their capacity to produce large amounts of type I IFNs in response to bacterial or viral infections.7 Similar to mDC-derived IL-12, pDC-derived type I IFNs also participate in T-cell priming as Th1-inducing cytokines.8
In addition to directing CD4+ Th responses, DCs are also important for the generation of CD8+ cytotoxic T-cell responses against viruses and tumors. As professional APCs, DCs have the unique capacity to take up, process, and present exogenously encountered Ags for cross-presentation via MHC class I molecules to CD8+ T cells. Numerous studies have been performed to comprehend this cross-presentation process, and these have revealed 2 major pathways: (1) the “canonical” proteasome dependent cytosolic pathway, and (2) the TAP and proteasome independent pathway.9-12 Many studies however, made use of murine DCs to study cross-presentation capacities and mechanisms used by different DC subsets. There is ample evidence that identified the CD8α+ DC as the superior cross-presenting DC subset in mice.13,14 Recently, a lot of effort has been put toward finding the human counterpart of the murine cross-presenting CD8α+ DC subset. Despite basic similarities between human and mouse DCs, direct comparison is difficult because of large differences in cell-surface markers and TLR expression, in particular also for pDCs, which in contrast to mice are the sole TLR9-expressing subtype of DCs in man.15 Recent findings suggest, however, that the human BDCA3+ (XCR-1+, CLEC9A+) mDC subset represents the human homologue of the murine cross-presenting CD8α+ DC subset.16-19 In these studies comparative analysis of the different blood DC subsets suggested that the BDCA3+ mDC subset, similar to its murine counterpart, was the superior human cross-presenting DC subset, although also CD1c+ mDCs demonstrated to have cross-presenting abilities.20,21
In contrast to their myeloid counterparts, which efficiently endocytose extracellular particulates, pDCs are considered to primarily present endogenous Ags and are thought to minimally participate in the uptake and presentation of Ags from the extracellular environment.22 However, we and others recently showed that human pDCs can take up, process, and present particulate Ags.23 Furthermore, it was demonstrated that pDCs can cross-present exogenous Ags to CD8+ T cells in the context of viral infection.10,24,25 Cross-presentation of endocytosed tumor Ags to cytotoxic CD8+ T cells is also essential for the induction of antitumor immunity. In this study we assessed the capacity of all 4 described human blood DC subsets; CD1c+ mDCs, CD16+ mDCs, BDCA3+ mDCs, and pDCs, to present tumor Ags to T cells by studying (1) the uptake, (2) processing, and (3) cross-presentation of extracellular tumor Ags to cytotoxic CD8+ T cells. We show that, although they take up less Ags than myeloid DC subsets, human pDCs are also very well capable of cross-presenting soluble and cell-associated tumor Ags and should therefore be considered as potential inducers of antitumor immunity.
Methods
Cells
DCs were isolated from buffy coats obtained from healthy volunteers after written informed consent per the Declaration of Helsinki and according to institutional guidelines. Peripheral blood mononuclear cells (PBMCs) were purified from buffy coats via ficoll density gradient centrifugation (Lucron Bioproducts). To obtain peripheral blood leukocytes (PBLs), monocytes were depleted from PBMCs via adherence to plastic culture flasks. CD1c+ mDCs and CD16+ mDCs were isolated from PBMCs with a CD1c+ DC isolation kit and CD16+ monocyte isolation kit, respectively. BDCA3 myeloid DCs were isolated from PBLs by selection for BDCA3+ cells with a CD141 (BDCA3) isolation kit. pDCs were purified from PBLs by positive selection using anti–BDCA-4–conjugated magnetic microbeads (all Miltenyi Biotec). As previously described, DC purity was assessed by double staining CD11c+/CD1c+ for CD1c-mDCs (> 95%), CD11c+/CD16+ for CD16-mDCs (> 90%), CD11c+/BDCA3+ for BDCA3+ mDCs (> 95%), and BDCA2/CD123 for pDCs (> 95%; all Miltenyi Biotec).26,27 The yield from 1 buffycoat (∼500 × 106 PBMC) was approximately 0.15 × 106 for BDCA3+ mDCs, 5 × 106 for CD1c+ mDCs, 10 × 106 for CD16+ mDCs, and 0.7 × 106 for pDCs. DCs were cultured in X-VIVO-15 medium (Cambrex) supplemented with 2% human serum. DCs were stimulated with the following TLR ligands: 4 μg/mL R848 (Axxora) for pDCs and 4 μg/mL R848 and 2 μg/mL poly(I:C) (Sigma-Aldrich) for mDCs. If no activation stimuli were added, pDCs were cultured with 10 ng/mL IL-3 as a survival stimulus.
Microparticle preparation
PLGA [poly(lactic-co-glycolic acid)] microparticle formulations containing Atto647, DQ-BSA, or gp100272-300 peptide were prepared by double emulsion method as described.23 Particle size and polydispersity of the microparticles were measured by dynamic light scattering as described.23 Microparticles of 2 μm and with a relatively monodisperse diameter were produced.
Uptake of FITC-gp100272-300
Gp100272-300 long peptide was labeled with FITC through linking via a Lys-Lys cathepsin-like cleavage site as previously described.28 DCs were cultured for 0.5, 1, 4, and 18 hours in the presence of 5, 10, or 50μM FITC-gp100272-300. Uptake of gp100272-300 was determined by flow cytometry using FACS Calibur. Extracellularly bound, noninternalized Ag was quenched by the addition of trypan blue.
Uptake of PLGA particles
DC were incubated for 16 hours with 250 μg/mL PLGA particles encapsulating Atto647. Uptake of Atto647-containing PLGA particles was analyzed by flow cytometry and confirmed by confocal microscopy. For confocal microscopy, cells were fixed on poly-L-lysine coated glass slides and stained with anti-human MHC class II Ab (clone Q5/13), followed by a secondary goat anti–mouse IgG Alexa 488 mAb (Molecular Probes). Imaging was performed on a Olympus FV1000 confocal laser scanning microscope with a 60 × 1.35 NA oil immersion objective. Images were processed with National Institutes of Health ImageJ Version 1.46j software.
Ag processing
Ag processing was measured with DQ-BSA, a protein strongly labeled with a fluorescent BODIPY dye (Molecular Probes). DQ-BSA–containing microparticles23 (250 μg/mL) or soluble DQ-BSA (0.5 μg/mL) were added to pDCs, BDCA3+ mDCs, BDCA1+ mDCs or CD16+ mDCs (105) in the presence or absence of TLR ligands. Cells were cultured at 37°C and fluorescence was measured spectrophotometrically in a CytoFluor II.
Ag presentation to CD4+ T cells
PBLs from healthy donors were cultured for 8 to 10 days with TT830-844 peptide (3 μg/mL) and IL-2 (50 EU/mL) to increase the number of TT-responsive cells. Autologous pDCs, BDCA3+ mDCs, BDCA1+ mDCs, or CD16+ mDCs (104) were incubated overnight at 37°C with PLGA particles containing TT830-844 peptide (0.25 mg/mL) or equal amounts of soluble FITC-TT in the presence of absence of TLR ligands in 3-fold. After washing, prestimulated PBLs were added to the DCs at a ratio of 1:10. After 4 days, proliferative responses were determined by adding tritiated thymidine (1 μ Ci [0.037 MBq]/well; MP Biomedicals) to the cell cultures. Tritiated thymidine incorporation was measured after 16 hours in a scintillation counter.
Gp100-specific activation of CD8+ T cells
pDCs and mDCs from a HLA-A2.1+ donors were loaded with different concentrations of specific peptide (gp100280-288), irrelevant peptide (either gp100154-167 or tyrosinase369-376), gp100 long peptide (gp100272-300), or necrotic BLM cells transfected with full-length gp100 or tyrosinase in 96 well round bottom plates (7 × 103 per well). Necrotic BLM cells were generated by 3 subsequent snap freeze-thaw cycles in liquid nitrogen. After approximately 1 hour, CD8+ gp100280-288–specific T cells (5 × 104 per well)29,30 and TLR ligands were added. After overnight incubation, CD69 expression on the CD8+ gp100280-288–specific T cells was measured by flow cytometry using PeCy5-conjugated mouse anti–human CD69 (Pharmingen), and IFNγ production was measured using a standard sandwich ELISA (Pierce Endogen). Proliferation was measured after 4 days of culturing by adding (3H) thymidine and measuring incorporation with a scintillation counter.
Statistical analysis
Data were analyzed with either paired Student t test or with 1-way ANOVA followed by Newman-Keuls test using Prism Version 5.03 (GraphPad Software). Statistical significance was defined as < .05.
Results
Ag uptake by human circulating DC subsets
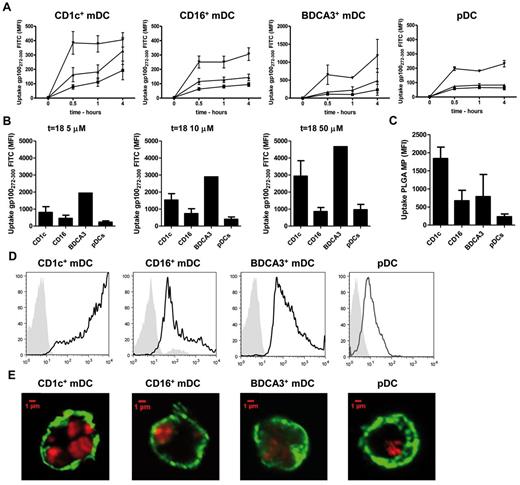
To present exogenous Ags to T cells, Ags need to be taken up from the extracellular environment, processed into peptides, and loaded onto MHC molecules. To study the ability of circulating DC subsets to take up soluble Ags, the uptake of fluorescently labeled Ag was analyzed by flow cytometry. As Ag we used short and long (which requires Ag processing) peptide fragments of gp100, a melanoma-associated tumor Ag that is commonly used as target Ag in immunotherapy against melanoma. The uptake of soluble gp100-long peptide by freshly isolated DC subsets was already detectable as early as 30 minutes after addition of the Ag (Figure 1A-B). Interestingly, CD16+ mDCs and pDCs showed only modest uptake at the highest Ag concentration, whereas CD1c+ mDCs and especially BDCA3+ mDCs in contrast displayed high uptake of soluble Ag after 4 hours (Figure 1A). This became even more evident after 18 hours of incubation, when the fluorescence of CD16+ mDCs and pDCs indicated a 3 to 5 times lower Ag uptake compared with CD1c+ mDCs and BDCA3+ mDCs (Figure 1B).
Ag uptake by human circulating DC subsets. (A-B) Uptake of soluble antigens (Ags). Human CD1c+ mDCs, CD16+ mDCs, BDCA3+ mDCs and pDCs were incubated with 5μM (■), 10μM (▴), or 50μM (▾) of FITC-labeled gp100272-300 long peptide. Ag uptake was measured by flow cytometry after 0.5, 1, and 4 hours (A) and 18 hours (B). Extracellularly bound, noninternalized Ag was quenched by the addition of trypan blue. The figures show mean MFI ± SEM of 3 independent experiments performed in duplicate. (C-D) Uptake of particulate Ags. Human CD1c+ mDCs, CD16+ mDCs, BDCA3+ mDCs and pDCs were incubated with Atto647-encapsulating PGLA microparticles for 16 hours. Uptake was analyzed by flow cytometry (C-D) and confocal microscopy (E). (C) The graph shows the mean MFI ± SEM of 3 independent experiments. Depicted in panel E are the merged pictures of the fluorescence of Atto647-containing PGLA particles (red) and surface MHC class II staining (green), magnification 60×. (D-E) The figures show the result from 1 representative experiment.
Ag uptake by human circulating DC subsets. (A-B) Uptake of soluble antigens (Ags). Human CD1c+ mDCs, CD16+ mDCs, BDCA3+ mDCs and pDCs were incubated with 5μM (■), 10μM (▴), or 50μM (▾) of FITC-labeled gp100272-300 long peptide. Ag uptake was measured by flow cytometry after 0.5, 1, and 4 hours (A) and 18 hours (B). Extracellularly bound, noninternalized Ag was quenched by the addition of trypan blue. The figures show mean MFI ± SEM of 3 independent experiments performed in duplicate. (C-D) Uptake of particulate Ags. Human CD1c+ mDCs, CD16+ mDCs, BDCA3+ mDCs and pDCs were incubated with Atto647-encapsulating PGLA microparticles for 16 hours. Uptake was analyzed by flow cytometry (C-D) and confocal microscopy (E). (C) The graph shows the mean MFI ± SEM of 3 independent experiments. Depicted in panel E are the merged pictures of the fluorescence of Atto647-containing PGLA particles (red) and surface MHC class II staining (green), magnification 60×. (D-E) The figures show the result from 1 representative experiment.
Receptor independent uptake of soluble antigens from the extracellular environment occurs via the nonspecific incorporation of extracellular fluid in a process called pinocytosis or “cell drinking.” However, to engulf larger particulates, such as synthetic microspheres, bacteria, or cellular debris, DCs exploit the mechanistically different process of phagocytosis. In addition, to study the capacity of freshly isolated DC subsets to take up particulate Ags, the DCs were incubated overnight with PLGA microparticles containing a fluorescent dye. Flow cytometric analysis indicated that all DC subsets engulfed PLGA microparticles after overnight incubation (Figure 1C), which was confirmed by confocal microscopy (Figure 1D). Z-stack analysis confirmed that the detected fluorescent signal was located intracellularly (data not shown). Interestingly, both flow cytometry and confocal analysis suggest that the CD1c+ mDCs are more efficient in engulfing exogenous particulate Ags through phagocytosis than the CD16+ mDCs and BDCA3+ mDCs, as in these cells we detected the strongest fluorescent signal. These findings support our current hypothesis that CD1c+ mDCs share key features with monocyte-derived DCs (moDCs), such as the capacity to engulf large amounts of particulate Ags. In accordance with our previous findings also human pDCs were able to phagocytose PLGA microparticles (Figure 1C),23 albeit to a lesser extent than the mDC subsets. Taken together, our data suggest that all DC subsets can clearly take up soluble as well as particulate Ags, but their uptake capacity differs greatly among the DC subsets.
Ag processing by human circulating DC subsets
To study the ability of human DC subsets to process soluble and particulate Ags, the model protein BSA, labeled with a fluorescent BODIPY dye (DQ-BSA), was used. DQ-BSA is labeled to such a high degree that the fluorescence is self-quenched. Quenching is relieved on processing of the Ag into fluorescent peptides by cellular proteases. Antigen degradation could only be monitored for maximally up to 4 days because of the limited lifespan of these rather fragile natural human DC subsets in vitro. As expected, almost no Ag degradation was detected when soluble or particulate DQ-BSA was incubated in culture medium in the absence of DCs. Moreover, DCs incubated in culture medium, without soluble or particulate DQ-BSA, displayed minimal auto-fluorescence (Figure 2). Although we observed that all DC subsets have the ability to take up soluble Ags (Figure 1A-B), in our hands only the CD1c+ mDCs and to some extend also the pDCs displayed the ability to process soluble DQ-BSA (Figure 2A). Particulate DQ-BSA in contrast was efficiently degraded by CD1c+ mDCs, BDCA3+ mDCs, and pDCs (Figure 2B). Interestingly, in spite of the significant uptake of particulates by the CD16+ mDCs, this DC subset showed only minimal antigen degradation of both soluble and particulate Ags (Figure 2B).
Ag processing by human circulating DC subsets. Human CD1c+ mDCs, CD16+ mDCs, BDCA3+ mDCs, and pDCs were incubated with the self-quenched model protein DQ-BSA and fluorescence, caused by uptake and subsequent degradation of DQ-BSA, was measured spectrophotometrically during 96 hours. (■) DCs were incubated with soluble DQ-BSA (A) or DQ-BSA encapsulated in PLGA particles (B). As a control, fluorescence of DCs only (·), or DCs activated with TLR ligands (▴; 4 μg/mL R848 for pDCs, 4 μg/mL R848 and 2 μg/mL poly(I:C) for mDC subsets) before adding DQ-BSA was measured. Data shown are mean ± SEM of 3 independent experiments performed in triplicate.
Ag processing by human circulating DC subsets. Human CD1c+ mDCs, CD16+ mDCs, BDCA3+ mDCs, and pDCs were incubated with the self-quenched model protein DQ-BSA and fluorescence, caused by uptake and subsequent degradation of DQ-BSA, was measured spectrophotometrically during 96 hours. (■) DCs were incubated with soluble DQ-BSA (A) or DQ-BSA encapsulated in PLGA particles (B). As a control, fluorescence of DCs only (·), or DCs activated with TLR ligands (▴; 4 μg/mL R848 for pDCs, 4 μg/mL R848 and 2 μg/mL poly(I:C) for mDC subsets) before adding DQ-BSA was measured. Data shown are mean ± SEM of 3 independent experiments performed in triplicate.
Pathogens or infected cells display TLR ligands that are known to affect DC antigen processing and presentation. Therefore we next analyzed the processing of exogenous Ags in the presence of TLR ligands known to activate the tested subsets: R848 alone (pDCs) or a combination of R848 and Poly I:C (mDCs). Previously we demonstrated that in pDCs TLR9-induced activation diminishes processing of exogenous Ags.23,31 Similarly, in CD1c+ and BDCA3+ mDCs R848 and Poly I:C–induced activation strongly diminished processing of soluble or particulate DQ-BSA by CD1c+ and BDCA3+ mDCs, although residual processing above background levels could still be observed (Figure 2A-B). In pDCs R848-induced activation, however, did not hamper the uptake and processing of particulate Ag (Figure 2B). Together, our findings unambiguously demonstrate that human CD1c+ mDCs, BDCA3+ mDCs, and also pDCs can process ingested particulate Ags.
MHC molecule surface expression on human blood DCs
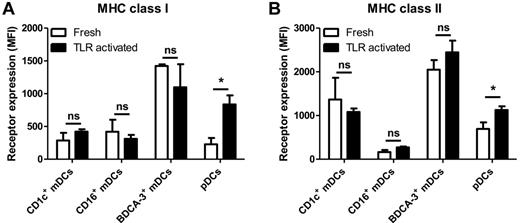
An effective antitumor immune response requires both MHC class I–restricted CD8+ and MHC class II–restricted CD4+ T cell responses. APCs present the Ags to T cells via MHC peptide complexes on their cell surface. Therefore, we compared the surface expression of MHC class I and MHC class II molecules. All blood circulating DC subsets express both MHC class I and MHC class II at their cell surface. However, Figure 3 illustrates that freshly isolated BDCA3+ mDCs express high levels of both MHC class I and MHC class II molecules compared with other subsets. Freshly isolated CD1c+ mDCs, CD16+ mDCs, and pDCs expressed lower but comparable levels of MHC class I (Figure 3A), whereas expression of MHC class II was exceptionally low on freshly isolated CD16+ mDCs (Figure 3B). Plasmacytoid DCs and CD1c+ mDCs expressed high levels of MHC class II. After overnight TLR-stimulation, pDCs exhibited a significant up-regulation of both MHC class I and MHC class II molecules (Figure 3B), which was modest for CD1c+ and BDCA3+ mDCs. On CD16+ mDCs in contrast, expression of MHC molecules was not altered. Thus, steady-state human blood DC subsets differentially express MHC molecules and also variably regulate their expression of MHC molecules in response to TLR activation. The up-regulation of MHC class I by pDCs may be linked to their role in viral defense, but may also indicate their importance for the cross-presentation of extracellular Ags.
Expression of MHC class I and MHC class II. Expression of (A) MHC class I (HLA-ABC) and (B) MHC class II (HLA-DR) was measured by flow cytometry on freshly isolated human CD1c+ mDCs, CD16+ mDCs, BDCA3+ mDCs, and pDCs and DC stimulated overnight with TLR ligands (4 μg/mL R848 for pDCs, 4 μg/mL R848 and 2 μg/mL poly(I:C) for mDC subsets). The graphs show the mean ± SEM of the MFI of at least 4 independent experiments. Significance was determined by unpaired Student t test (*P < .05).
Expression of MHC class I and MHC class II. Expression of (A) MHC class I (HLA-ABC) and (B) MHC class II (HLA-DR) was measured by flow cytometry on freshly isolated human CD1c+ mDCs, CD16+ mDCs, BDCA3+ mDCs, and pDCs and DC stimulated overnight with TLR ligands (4 μg/mL R848 for pDCs, 4 μg/mL R848 and 2 μg/mL poly(I:C) for mDC subsets). The graphs show the mean ± SEM of the MFI of at least 4 independent experiments. Significance was determined by unpaired Student t test (*P < .05).
Human CD1c+ and BDCA-3+ mDCs and pDCs present soluble and particulate Ag to CD4+ T cells
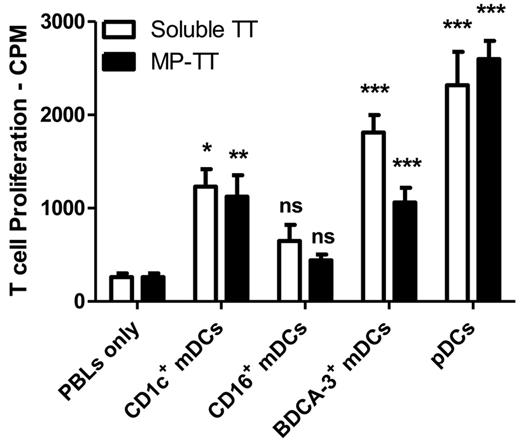
To determine whether pDCs could stimulate CD4+ T cells we next investigated the capacity of the blood DC subsets to induce tetanus toxoid (TT)–specific CD4+ T cell responses. Freshly isolated DCs, incubated with either soluble TT or with PLGA particles containing TT, induced higher proliferation of autologous TT-responsive T cells compared with unstimulated T cells (data not shown), indicating that the subsets processed and presented soluble and particulate Ags to CD4+ T cells, and were able to trigger an Ag-specific proliferative recall responses. The addition of TLR agonists strongly enhanced the ability of BDCA3+ mDCs but also pDCs to induce Ag specific proliferative recall responses (Figure 4), whereas only slightly and nonsignificantly increased T-cell proliferation induced by CD16+ mDCs was observed. These findings are in line with results shown in Figure 2A, where CD1c+ mDCs, BDCA3+ mDCs and pDCs, but not CD16+ mDCs, effectively processed particulate DQ-BSA. Taken together, our data suggest that the efficiency of Ag presentation by DC subsets is directly related to their ability to process incoming Ags and is for each subset differentially regulated by maturation.
Ag-specific CD4+ T cell activation. Human CD1c+ mDCs, CD16+ mDCs, BDCA3+ mDCs, and pDCs were incubated overnight with soluble TT peptide or with PLGA particles containing TT peptide in the presence of TLR ligands (4 μg/mL R848 for pDCs, 4 μg/mL R848, and 2 μg/mL poly(I:C) for mDC subsets). Subsequently, autologous TT-responsive PBLs were added. After 4 days, T-cell proliferation was measured by (3H)–thymidine incorporation. Data are mean values ± SEM of 1 representative experiment performed in triplicate. Significance was determined by ANOVA and Newman-Keuls testing (*P < .05; **P < .01; ***P < .001) compared with PBLs only.
Ag-specific CD4+ T cell activation. Human CD1c+ mDCs, CD16+ mDCs, BDCA3+ mDCs, and pDCs were incubated overnight with soluble TT peptide or with PLGA particles containing TT peptide in the presence of TLR ligands (4 μg/mL R848 for pDCs, 4 μg/mL R848, and 2 μg/mL poly(I:C) for mDC subsets). Subsequently, autologous TT-responsive PBLs were added. After 4 days, T-cell proliferation was measured by (3H)–thymidine incorporation. Data are mean values ± SEM of 1 representative experiment performed in triplicate. Significance was determined by ANOVA and Newman-Keuls testing (*P < .05; **P < .01; ***P < .001) compared with PBLs only.
Human circulating pDCs cross-present soluble and cell-associated Ags to CD8+ T cells
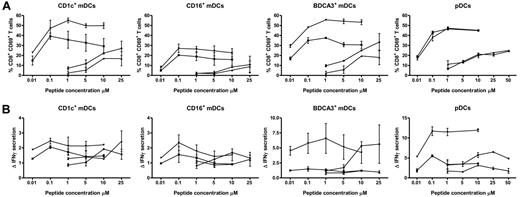
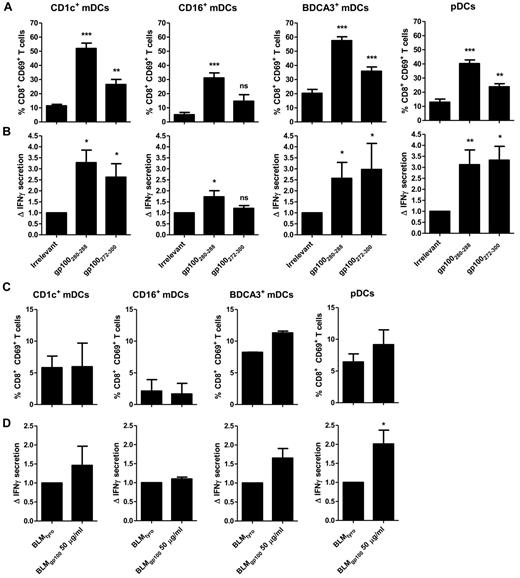
Cross-presentation of ingested Ags to cytotoxic CD8+ T cells is essential for the induction of antitumor immunity.32 In literature, there is a common census that BDCA3+ mDCs cross-present Ags derived from different sources. However, it remains controversial whether also the other DC subsets have the capacity to cross-present Ags to CD8+ T cells.10,16-21,24,25 Therefore, we next studied the capacity of all 4 blood DC subsets to cross-present soluble long gp100272-300 peptide that requires intracellular processing to release the T-cell cognate epitope gp100280-288. To control for MHC levels and DC activation we also assessed direct presentation of the short gp100280-288 peptide that does not require intracellular processing but directly binds extracellular HLA-A2 molecules. As expected, all blood DC subsets effectively presented the short gp100280-288 peptide to gp100280-288–specific CD8+ T cells in a dose-dependent manner, based on both the expression of the early activation marker CD69 (Figure 5A) and the secretion of IFNγ (Figure 5B). In accordance with previous studies, we observed that the use of TLR agonists strongly enhanced the ability of the blood DCs to specifically prime IFNγ secreting CD8+ T cells (Figure 5A-B).16,19,33 Intriguingly, CD1c+ mDCs, BDCA3+ mDCs as well as pDCs effectively cross-presented soluble long gp100272-300 peptide, in a dose-dependent manner in 2 different titration experiments (Figure 5A-B). In contrast, CD16+ mDCs displayed only minimal ability to activate gp100280-288–specific CD8+ T cells after incubation with the gp100272-300 long peptide, even in the presence of TLR ligands. The ability of CD1c+ mDCs, BDCA3+ mDCs and pDCs to cross-present was robust as it was found over a large number of different donors and T cell preparations (Figure 6 and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Thus, human pDCs can be regarded as efficient as CD1c+ mDCs and BDCA3+ mDCs in the cross-presentation of soluble long gp100 peptides and the subsequent cross-priming of CD8+ T cells.
TLR ligands enhance cross-presentation of extracellular Ags. Human CD1c+ mDCs, CD16+ mDCs, BDCA3+ mDCs, and pDCs were incubated with 0.01 to 10μM gp100 short peptide (gp100280-288; n and ▾), or 1 to 25μM gp100 long peptide (gp100272-300; ▴ and ♦). Next, DCs were cocultured overnight with allogeneic CD8+ T cells expressing gp100280-288–specific TCR in the absence (n and ▴) or presence (▾ and ♦) of 4 μg/mL R848 and 2 μg/mL poly I:C (mDCs) or 4 μg/mL R848 only (pDC). Ag-specific T-cell activation was assessed by analysis of CD69 expression (A) and IFN-γ production (B). IFNγ production is shown relative to irrelevant peptide. The graphs show mean ± SEM CD69 expression or relative IFNγ production of 2 independent experiments performed in duplicate with different donors.
TLR ligands enhance cross-presentation of extracellular Ags. Human CD1c+ mDCs, CD16+ mDCs, BDCA3+ mDCs, and pDCs were incubated with 0.01 to 10μM gp100 short peptide (gp100280-288; n and ▾), or 1 to 25μM gp100 long peptide (gp100272-300; ▴ and ♦). Next, DCs were cocultured overnight with allogeneic CD8+ T cells expressing gp100280-288–specific TCR in the absence (n and ▴) or presence (▾ and ♦) of 4 μg/mL R848 and 2 μg/mL poly I:C (mDCs) or 4 μg/mL R848 only (pDC). Ag-specific T-cell activation was assessed by analysis of CD69 expression (A) and IFN-γ production (B). IFNγ production is shown relative to irrelevant peptide. The graphs show mean ± SEM CD69 expression or relative IFNγ production of 2 independent experiments performed in duplicate with different donors.
Cross-presentation of extracellular Ags to CD8+ T cells. Human CD1c+ mDCs, CD16+ mDCs, BDCA3+ mDCs, and pDCs were incubated with 10μM irrelevant peptide (tyrosinase369-376), 10μM gp100 short peptide (gp100280-288), 25μM gp100 long peptide (gp100272-300) (A-B), or 50 μg/mL necrotic BLM cells expressing gp100 or tyrosinase (C-D). Next, DCs were cocultured overnight with allogeneic CD8+ T cells expressing gp100280-288–specific TCR in the presence of 4 μg/mL R848 and 2 μg/mL poly I:C (mDCs) or 4 μg/mL R848 only (pDC). Ag-specific T-cell activation was assessed by analysis of CD69 expression (A-C) and IFN-γ production (B-D). IFNγ production is shown relative to irrelevant peptide. For panels A and B the graphs show the mean ± SEM of CD1c+ mDCs (n = 5), CD16+ mDCs (n = 4), BDCA3+ mDCs (n = 6), pDCs (n = 12), and for panels C and D the graphs show CD1c+ mDCs (n = 2), CD16+ mDCs (n = 2), BDCA3+ mDCs (n = 2), and pDCs (n = 9) experiments with different donors. (A-B) Significance was determined by ANOVA and Newman-Keuls testing (*P < .05; **P < .01; ***P < .001) compared with irrelevant peptide. (D) Significance was determined by a paired Student t test.
Cross-presentation of extracellular Ags to CD8+ T cells. Human CD1c+ mDCs, CD16+ mDCs, BDCA3+ mDCs, and pDCs were incubated with 10μM irrelevant peptide (tyrosinase369-376), 10μM gp100 short peptide (gp100280-288), 25μM gp100 long peptide (gp100272-300) (A-B), or 50 μg/mL necrotic BLM cells expressing gp100 or tyrosinase (C-D). Next, DCs were cocultured overnight with allogeneic CD8+ T cells expressing gp100280-288–specific TCR in the presence of 4 μg/mL R848 and 2 μg/mL poly I:C (mDCs) or 4 μg/mL R848 only (pDC). Ag-specific T-cell activation was assessed by analysis of CD69 expression (A-C) and IFN-γ production (B-D). IFNγ production is shown relative to irrelevant peptide. For panels A and B the graphs show the mean ± SEM of CD1c+ mDCs (n = 5), CD16+ mDCs (n = 4), BDCA3+ mDCs (n = 6), pDCs (n = 12), and for panels C and D the graphs show CD1c+ mDCs (n = 2), CD16+ mDCs (n = 2), BDCA3+ mDCs (n = 2), and pDCs (n = 9) experiments with different donors. (A-B) Significance was determined by ANOVA and Newman-Keuls testing (*P < .05; **P < .01; ***P < .001) compared with irrelevant peptide. (D) Significance was determined by a paired Student t test.
So far literature suggests that the human BDCA3+ mDCs are superior in cross-presenting particulate Ags, such as cell-associated Ags.16,17,19 To investigate the ability of the blood DC subsets to cross-present cell associated Ags, we next compared the capacity of the DC subsets to cross-present the gp100280-288 epitope derived from whole gp100 protein-expressing BLM melanoma cell lysates, which were generated through 3 freeze-thaw cycles. Lysates of BLM melanoma cells expressing the irrelevant tyrosinase protein were used as control. Obviously, the release of the gp100280-288 epitope from whole gp100-expressing cell lysate is much more demanding on the cell's Ag uptake and processing machinery than for long peptides, also because the latter were offered in pure form and higher concentrations. Nonetheless, we detected small but reproducible increases in the expression of CD69 on gp100280-288–specific CD8+ T cells, incubated with BDCA3+ mDCs and pDCs that had ingested BLM cells expressing gp100 protein, but not in T cells incubated with CD1c+ mDCs and CD16+ mDCs (Figure 6C). Interestingly, only pDCs also effectively and significantly induced IFNγ secretion by gp100280-288–specific CD8+ T cells on exposure to tumor lysates (Figure 6D). Thus, our data suggest, pDCs at least share but may even outcompete the capacity of BDCA3+ mDCs to cross-present tumor cell-derived antigens to cytotoxic CD8+ T lymphocytes, also because their number circulating in the peripheral blood and lymph nodes is much higher.34
Discussion
The cross-presentation of exogenous antigens on MHC class I is essential for the initiation of an effective adaptive immune response against tumors. Therefore over the past decade cross-presentation has been extensively studied and the cell type and conditions best facilitating cross-presentation has become the holy grail of (tumor) immunology. Here we investigated not only the capacity of all 4 natural circulating DC subsets to cross-present exogenous Ags from various sources in combination with TLR agonists as adjuvants, but also their ability to take up and process different Ags both in soluble as particulate form. We show that, although human pDCs take up significantly less Ags than their myeloid counterparts, pDCs effectively cross-present and cross-prime soluble and cell-associated tumor Ags to CD8+ T cells.
The use of different Ags and Ag sources such as proteins, cell lysates, peptides, and virus infected apoptotic cells, in combination with small comparative studies has made it hard to compare the cross-presenting capacities of human blood DC subsets.10,16-21,24,25 The balance of different signaling pathways stimulated by the various pathogens may result in the initiation of the cross-presentation program, but currently, many aspects of this programming have not been elucidated. The identification of the dents on the keys that will kick-start each desired Ag-presentation pathway should lead to more efficient knowledge-based vaccine and adjuvant development. The stimulation of cross-presentation by specific adjuvants has been established using many different TLR-ligands and on many different DCs.16,19,33,35 The Ag-presenting capacities of each individual DC subset may vary with the stimulatory agents used in the different studies. Thus far, only Bachem et al directly compared the 4 known human DC subsets in their capacity to cross-present Ags.17 However, in that study the capacity to cross-present Ags was executed solemnly without the use of TLR agonists as adjuvants. Furthermore, DCs encounter Ags in many different shapes and sizes, derived from various sources, such as soluble peptides, particulates, immune complexes, and so on. The ability of DCs to handle all these different types of Ags is largely determined by the repertoire of Ag uptake receptors, and the ability to engulf Ags through phagocytosis or receptor independent processes, such as (micro) pinocytosis. The current paradigm of superior cross-presentation by human BDCA-3+ mDCs is mainly derived from the preferential use of specific types of Ags, such as cell-associated Ags, a restricted number of soluble model Ags or bead-bound Ags. DCs exploit endocytic receptors, such as DEC-205, to transport Ags into endosomes in which subsequently the cross-presentation machinery will be recruited. Previously, Bachem et al showed that human BDCA3+ mDCs induced strong T-cell activation on internalization of soluble protein, indicative for a potent cross-presenting machinery, whereas pDCs hardly induced T-cell activation.17 Nonetheless, the finding that pDCs did not cross-present whole protein is not surprising because previously we demonstrated that pDCs inefficiently take up soluble whole proteins in a receptor independent fashion. However, human pDCs very efficiently internalized and presented Ags from immune complexed proteins.36 Obviously, the capacity of blood DC subsets to cross-present antigens largely depends on their ability to exploit Ag uptake receptors to take up encountered Ags. This notion is supported by the study from Klechevsky et al where they demonstrated that all DC subsets tested cross-presented Ags when targeted trough the C-type lectin receptor DCIR.37 Hence, when analyzing the cross-presenting capacities of DCs, the capacity to handle different types of antigens should be carefully taken into account.
Here, we show that although human pDCs take up less Ags than their myeloid counterparts, pDCs effectively cross-present and cross-prime soluble and cell-associated tumor Ags to CD8+ T cells. This is in contrast to previous reports in mice where it was suggested that steady state spleen-derived pDCs do not cross-present cell-associated Ags,38 or soluble OVA and peptide-coated beads.35 In contrast, activation of murine pDCs by R848 and to a lesser extent CpG led to efficient cross-presentation of soluble and Ag-coated beads,35 emphasizing the significance and strength of specific stimulation of distinct DC subsets to induce the cross-presenting capacity. However, cross-presentation is not only dependent on Ag uptake and on adjuvants such as TLR agonists. Also Ag processing, intracellular routing of the Ag (containment in early endosomal compartments/entry into cross-presentation pathway), and loading onto MHC class I molecules are indispensable. Although pDCs take up less exogenous material compared with other DC subsets, they may (because of lower oxidative enzymes) preserve antigen for a prolonged period of time and efficiently transfer Ags into the cross-presentation pathway. We show that pDCs have developed a mechanism of efficient preserving and processing exogenous Ags. The Ags are present for prolonged periods of time within the cell and on stimulation strong induction of both MHC class I and II occurs, which explains their efficient activation of both CD4+ and CD8+ T cells. An in depth comparative proteomic analysis on the 4 subsets suggest that human pDCs express a specific set of Ag-derivative enzymes or transporters that could facilitate epitope generation and the transfer of Ags to the cytosol (unpublished results). Despite their lower Ag uptake potential, pDCs might specifically exploit these enzymes and transporters leading to more efficient Ag cross-presentation to equal levels induced by CD1c+ and BDCA-3+ mDCs despite lower antigen uptake.
Although human pDCs generally reside in the peripheral blood they also infiltrate solid tumors, such as breast cancer, head and neck cancer, and ovarian cancer.39 Soluble factors, secreted by the tumor and necrotic tumor material prevent differentiation and activation of tumor infiltrating pDCs (TIpDCs). In an inactivated tolerogenic state TIpDCs help to maintain the immunosupressive environment, which has been correlated with poor prognosis.39-41 In contrast to their suppressive counterparts, properly activated TIpDCs are able to directly lyse cancer cells or may take up the released tumor-associated Ags and present them to both CD4+ and CD8+ T cells.42,43 Furthermore, by their ability to secrete large amounts of type I IFN on activation, pDCs were already shown to mediate cross-talk with other immune cells, such as NK cells or T cells, leading to superior antitumor immunity.44,45 Therefore, TIpDCs are interesting targets when opting for the targeted delivery of Ags and TLR ligands to switch the balance from tolerance to immunity for the eradication of tumors. Our study thus underscores that pDCs can also generate CD8+ antitumor responses.
The important role for DCs in inducing immunity is the rationale for DC-based immunotherapy, in which DCs loaded with tumor antigens are injected into cancer patients to stimulate T cells to eradicate tumors.46,47 Because of the limited number of naturally circulating DCs, virtually all vaccination studies so far have used DCs differentiated ex vivo from monocytes or CD34+ progenitors.48-50 Although, clinical efficacy has been observed in a fraction of patients treated with these DCs,48,49 it has been postulated that moDCs might be less effective than naturally occurring DC subsets.30 The data presented here, in combination with other studies, urges for the re-evaluation of the role for human pDCs as professional APCs able to effectively induce both CD4+ and CD8+ T cells responses in addition to a high IFN-α production. Just recently, the worlds' first clinical study carried out in man (submitted) confirms the potency of vaccinating cancer patients with activated pDCs as it led to significant extension of patient survival.
Conclusion
The efficiency of Ag presentation is determined by several critical steps that are involved in this process: (1) presence of adjuvants, (2) Ag uptake, (3) processing, (4) loading onto MHC class I, and (5) presentation and cross-priming of CD8+ T cells. Here, we analyzed all these different steps involved and found that, although pDCs take up less tumor Ags than their myeloid counterparts, they can activate CD8+ T cells to a similar extend. Our findings thus have important consequences for the development of antitumor immunotherapy. All together, these findings underscore that human pDCs are indispensable and should be considered as potent activators of CD8+ T cells in antitumor responses, not only because of their capacity to efficiently present exogenous antigens, but also because of their potent IFN I production which potentiates both components of the innate and adaptive immune system.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Dutch Cancer Society (KUN2006-3699, KUN2009-4402), the European Union (The European Network for Cell Imaging and Tracking Expertise [ENCITE] Health-F5-2008-201842, Pharmachild FP7-HEALTH-2010-260353), The Netherlands Organization for Scientific Research (NWO-Vidi-917.76.363), and the Nijmeegs Offensief Tegen Kanker (NOTK) Foundation and a Radboud University Nijmegen Medical Centre PhD grant. C.F. received the NWO Spinoza award.
Authorship
Contribution: J.T. and G.S. designed and performed research, analyzed data, and wrote the paper; S.P.S., T.S.M.M., S.I.B., A.J.A.L., and L.J.C. contributed to experimental design and writing of the paper; and C.G.F., and I.J.M.dV. supervised the study and contributed to experimental design and writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: I. Jolanda M. de Vries, Deptartment of Tumor Immunology, Nijmegen Centre for Molecular Life Sciences, Radboud University Nijmegen Medical Centre, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: j.devries@ncmls.ru.nl.
References
Author notes
J.T., G.S., S.P.S., C.G.F., and I.J.M.d.V. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal