In this issue of Blood, Nakajima-Takagi et al use human embryonic stem (ES) and induced pluripotent stem (iPS) cells to identify Sox17 as a regulator of hemogenic endothelium, and use conditional expression of Sox17 to capture endothelial cells at the critical moment of hemogeneic transition.1
That hematopoietic stem cells derive from specialized endothelial cells during embryogenesis is now well accepted, thanks to elegant early studies in avian systems,2,3 studies that have recently been backed up with direct observation in mouse and fish embryos.4,5 However, much remains mysterious about the endothelial origins of hematopoietic stem cells, not least the question of why the process needs to happen in this way at all. Only 2 components of the molecular mechanism have been investigated deeply to date, both in the mouse system: Runx1, a positive regulator, necessary for the hemogenic transition,6 and HoxA3, a negative regulator that forces hemogenic endothelial cells to stay endothelial.7 Understanding the mechanism by which embryonic endothelial cells undergo this hemogenic transition will provide important insight into the making of hematopoietic stem cells, and may even lead to that Holy Grail, the generation of repopulating hematopoietic stem cells in vitro from iPS cells.
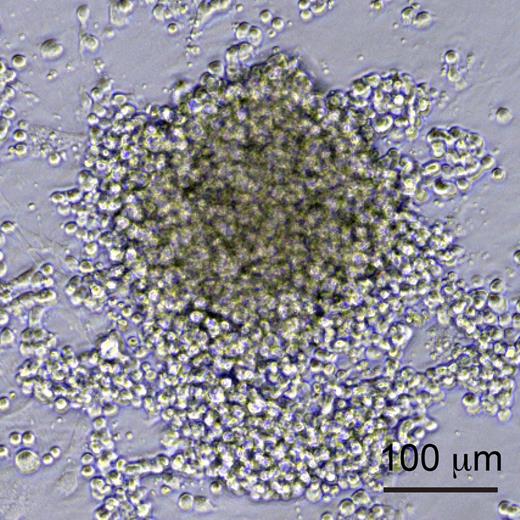
To this end, Nakajima-Takagi et al developed an improved culture system for the differentiation of human iPS cells allowing them to efficiently generate cells at different stages of hemogenesis: endothelial cells (defined as CD34+ CD43− CD45−), prehematopoietic progenitor cells (pre-HPCs), which have acquired CD43 expression,8 and HPCs, that have further acquired CD45. They then performed a functional screen of a panel of important regulators of early hematopoiesis by transducing sorted iPS-derived endothelial cells or pre-HPCs with different retroviruses, each encoding a different regulator, and following the growth and differentiation of these cells under the influence of the regulator in question. Remarkably, whereas genes like SCL, GATA2, and RUNX1 had no detectable effect of the further differentiation of these cells, they discovered that expression of 1 gene, Sox17, drove the expansion of an adherent cell type that expressed both endothelial and hematopoietic markers (see figure). To better characterize the activity of Sox17, Nakajima-Takagi et al then modified it by fusion to the estrogen receptor, to regulate its nuclear localization, and thus activity, by application of 4-OH-tamoxifen to the culture medium. This allowed Sox17 to be turned on, and then turned off at a later time point. When Sox17 was removed, these cells differentiated into hematopoietic progeny and down-regulated endothelial markers, demonstrating that Sox17 had locked them into a state intermediate between endothelial and hematopoietic.
Sox17-overexpressing hemogenic endothelial cells. iPS-derived endothelial cells or pre-HPCs transduced with Sox17 were locked into an adherent, proliferative state, and expressed both endothelial and hematopoietic markers. See Figure 2B in the article by Nakajima-Takagi et al that begins on page 447.
Sox17-overexpressing hemogenic endothelial cells. iPS-derived endothelial cells or pre-HPCs transduced with Sox17 were locked into an adherent, proliferative state, and expressed both endothelial and hematopoietic markers. See Figure 2B in the article by Nakajima-Takagi et al that begins on page 447.
The investigators then used these captured hemogenic endothelial cells to investigate the molecular mechanism underlying the activity of Sox17. Transcriptional profiling of the Sox17-induced population revealed that with regard to transcription factor expression, or general hematopoietic gene expression, the Sox17-induced population was more similar to pre-HPCs and HPCs than to endothelial cells. ChIP-chip analysis showed that overexpressed Sox17 bound to about 100 promoters and regulatory regions (about 1% of those surveyed), including a large number of hematopoietic transcription factor genes, but also several endothelial-specific genes, such as VE-cadherin. Thirty-six genes were in both datasets, that is, both direct targets of Sox17 and transcriptionally up-regulated.
It seems likely from the transcriptional profile and ChIP data that Sox17 is initiating a hematopoietic program while also maintaining the endothelial program, thus allowing expansion of a cell type stuck between 2 fates. This is distinct from the 2 previously studied regulators of endothelial hemogenesis: HoxA3, the negative regulator, promotes the endothelial program while preventing acquisition of the hematopoietic program; Runx1 on the other hand appears to erase the endothelial program while inducing the hematopoietic program.
It is interesting that several of the genes targeted and induced by Sox17 expression were actually screened in the initial experiment, and found to have no effect on proliferation of endothelial or pre-HPC cells. It may be that combinatorial effects explain the activity of Sox17. However, the genes in this initial panel were all selected for playing important or essential roles in early HPCs. Because hematopoietic differentiation occurs efficiently without intervention in this iPS culture system, pro-hematopoietic factors might not present a strong phenotype in this assay. On the other hand, anti-hematopoietic or pro-endothelial factors would be predicted to have clear phenotypes, the latter possibly explaining why Sox17 was found.
It is notable that in addition to a dual hematopoietic-endothelial phenotype, high levels of Sox17 expression drive the proliferation of this intermediate cell type. In theory at least, proliferation is not necessary for hemogenesis. Nakajima-Takagi et al show that under normal circumstances, high levels of Sox17 persist for only a short time, and in the earliest (endothelial) fraction, presumably at the moment of their hemogenic commitment. High levels of Sox17 thus appear to “kickstart” hemogenesis. Whether the concomitant proliferation might serve some biophysical purpose, facilitating bulging of cells out of the epithelial sheet for example, remains to be determined. In any event, the sustained proliferation of this transitional population by Sox17 is serendipitous in that it allows the generation of large numbers of these cells for study, which should facilitate a number of important experiments. For example, as the mechanism of hemogenesis is still very much a black box, it will be valuable to determine which of the 36 directly up-regulated targets are actually necessary for effects of Sox17 on differentiation and proliferation. It would also be interesting to study the cell biologic changes that occur as hemogenic endothelial cells convert into hematopoietic cells in a large scale culture synchronized en masse by Sox17 release. Finally, and most importantly, has Sox17 brought us any closer to the important goal of developing transplantable hematopoietic stem cells from human iPS cells in vitro? Having embryonic origins in a sustained hemogenic endothelium is a feature that distinguishes intra-embryonic definitive repopulating hematopoietic stem cells from their earlier yolk sac nonrepopulating cousins. It will therefore be very interesting to learn whether these Sox17-induced human iPS-derived hematopoietic progenitors have in vivo repopulating potential.
Conflict of interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal