One of the greatest challenges in all of medicine is to improve on the life of an asymptomatic patient; however, in this issue of Blood, Richter and colleagues share provocative new data taking steps toward accomplishing this goal.1
In this study, α-galactosylceramide (α-GalCer)–loaded dendritic cells (DC) were used in combination with lenalidomide to elicit multi-component, immune activation in patients with asymptomatic myeloma.1 Treatment appeared to be well tolerated and was associated with effects in several immune cell subsets. A reduction in M-protein was observed in 3 of 4 patients with measurable disease. This innovative approach builds nicely on prior studies and makes severalimportant, new contributions to ongoing efforts toward effective immunotherapy of myeloma.
Multiple myeloma proceeds from a “pre-malignant” phase termed “monoclonal gammopathy of uncertain significance” (MGUS) through an asymptomatic “smoldering” phase to active, clinically symptomatic disease.2 While the course of MGUS is variable, most patients with asymptomatic myeloma will inexorably progress to manifest signs and symptoms requiring therapy and ultimately die of their disease. Although a topic of ongoing investigation, the standard of care for patients with asymptomatic myeloma remains vigilant observation and withholding therapy until signs or symptoms of active disease appear.2 Development of clinical myeloma from asymptomatic precursor states is associated with worsening immune dysfunction, generating increasing interest in the opportunity for early intervention with immune-based therapies to prevent or delay this progression.2
When loaded into human DCs, α-GalCer, a potent ligand for Natural Killer T (NKT) cells, has been shown to activate and increase circulating invariant NKT cells in vivo.3,4 In a complementary manner, lenalidomide appears to induce favorable immunomodulation of T, natural killer (NK), and NKT cell subsets.5-7 Richter et al hypothesized that the combination of these therapies could lead to synergistic enhancement of immune activation against myeloma. After leukapheresis, monocytes isolated from patients with asymptomatic myeloma were cultured in granulocyte macrophage-colony stimulating factor and interleukin-4, and DCs were loaded with clinical grade α-GalCer (KRN7000; KHK). Patients received 3 cycles of low dose lenalidomide (10 mg by mouth days 1-21) and a fixed dose of 10 million DC (intravenously, day 8) on 28-day cycles. Extensive immune monitoring was performed, and clinical activity was determined 30 days after completion of all treatment.
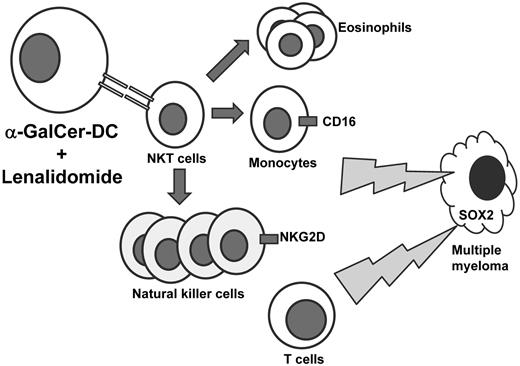
Therapy was generally well tolerated and associated with several immunomodulatory events (see figure). Interestingly, whereas α-GalCer alone was previously shown to expand circulating NKT cells, an early and persistent decrease in NKT cells was observed in the present trial in combination with lenalidomide. NK cell expansion occurred, which appeared to be related primarily to lenalidomide, but increased expression of the activating receptor NKG2D on NK cells was also seen, particularly after infusion of α-GalCer–loaded DCs. CD16 expression on CD14(+) monocytes increased, and eosinophilia was also documented. In addition, clinical activity may have also been associated established or induced T-cell mediated immunity against SOX2, an embryonal stem cell antigen previously identified as a possible immune target in plasma cell dyscrasias.8
Patients with asymptomatic myeloma received lenalidomide and autologous, α-GalCer–loaded DCs to stimulate NKT cells. While a reduction of NKT cells was observed in peripheral circulation, expansion of eosinophils and natural killer cells was observed. CD16 expression was increased on CD14(+) monocytes and natural killer cell expression of NKG2D was also increased. Evidence to suggest SOX2-directed T-cell immunity was also observed.
Patients with asymptomatic myeloma received lenalidomide and autologous, α-GalCer–loaded DCs to stimulate NKT cells. While a reduction of NKT cells was observed in peripheral circulation, expansion of eosinophils and natural killer cells was observed. CD16 expression was increased on CD14(+) monocytes and natural killer cell expression of NKG2D was also increased. Evidence to suggest SOX2-directed T-cell immunity was also observed.
The study by Richter and colleagues was small (n = 6, apparently because of lack of ongoing access to clinical grade α-GalCer) and raises several questions. First, it is unclear why α-GalCer alone led to NKT cell expansion in a prior study, but in combination with lenalidomide, NKT cells were decreased—perhaps this was related to trafficking of this subset out of peripheral circulation? Second, even though a low dose of lenalidomide (10 mg) was used, it is possible that this agent could have had a direct apoptotic effect on malignant plasma cells to account for the reduction of M-protein observed in some patients. Low dose lenalidomide may also modulate expression of NK cell ligands (eg, ULBP-1) to facilitate immune response against the disease.9 The study would have been strengthened with analyses of bone marrow plasma cells before and after therapy with α-GalCer and lenalidomide to detect any modulation of immune-cell ligand expression (such as CD1d and others) that may have contributed to immune-mediated rejection of myeloma tumor cells in a manner complementary to the immunomodulatory effects demonstrated. Third, the mechanism underlying the novel finding of eosinophil expansion in treated patients is also unclear. Finally, although requiring more extended follow up, it remains crucial to demonstrate that this approach would improve time-to-progression as a clinically relevant end point superior to M-protein reduction.
None-the-less, the study provides an important, first-in-human experience with α-GalCer and lenalidomide in myeloma. Lenalidomide has traditionally been paired with dexamethasone, a corticosteroid, which has formed the backbone of virtually every effective antimyeloma therapy to date. However, dexamethasone may attenuate lenalidomide's favorable immunologic properties,10 and corticosteroids are associated with substantial risk of side effects and toxicities (including hypertension, glucose intolerance, osteoporosis, and psychologic effects) in an already vulnerable patient population. Thus, the development of novel antimyeloma therapies, which are steroid-sparing and augment lenalidomide's immunomodulatory effects represents a compelling opportunity to advance the care of patients with myeloma particularly in the early intervention setting. Targeting NKT cells may also be particularly attractive in that this immune cell subset may serve to link innate and adaptive antitumor, immune resources.
Especially in attempts to improve the lives of asymptomatic patients, Hippocrates' advice to “do no harm” remains paramount. Nonetheless, achieving prevention of (or substantially delaying) the development of the associated morbidity and mortality of active myeloma in patients with asymptomatic, “smoldering” disease would represent significant progress. The findings of Richter et al are certainly encouraging and warrant further inquiry into this approach.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal