Key Points
INTERIM treatment affects cytogenetic and molecular response, but not the outcome.
No patients treated with INTERIM progressed to accelerated or blast phase.
Abstract
We report a study of an alternative treatment schedule of imatinib (IM) in chronic myeloid leukemia (CML). Seventy-six Philadelphia-positive (Ph+), BCR-ABL–positive patients aged 65 years or older who had been treated with IM for more than 2 years and who were in stable complete cytogenetic response (CCgR) and major molecular response (MMR) were enrolled in a single-arm study to test the effects of a policy of intermittent IM (INTERIM) therapy for 1 month on and 1 month off. With a minimum follow-up of 4 years, 13 patients (17%) lost CCgR and MMR and 14 (18%) lost MMR only. All these patients resumed continuous IM and all but one (lost to follow-up) regained CCgR and MMR. No patients progressed to accelerated or blastic phase or developed clonal chromosomal abnormalities in Ph+ cells or BCR-ABL mutations. In elderly Ph+ CML patients carefully selected for a stable CCgR (lasting >2 years), the policy of INTERIM treatment affected the markers of residual disease, but not the clinical outcomes (overall and progression-free survival). This trial was registered at www.clinicaltrials.gov as NCT 00858806.
Introduction
Over the last decade, the goals of the treatment of Philadelphia chromosome-positive (Ph+), BCR-ABL–positive chronic myeloid leukemia (CML) with tyrosine kinase inhibitors (TKIs) have become more and more ambitious: delayed progression, prolonged survival, normalization of survival, and treatment discontinuation without molecular recurrence, or cure.1-6 With imatinib (IM) at the standard dose of 400 mg/d, 80% to 90% of patients are alive at 8 years, but only a small proportion of patients (∼5%) can discontinue the treatment without suffering a molecular recurrence.6 Thus, the great majority of responsive patients would be destined to continue the treatment indefinitely at the same standard dose. Although elderly patients have cytogenetic and molecular responses comparable with younger ones, they are less tolerant of IM, and this may reduce the benefit of therapy by inducing higher rates of therapy discontinuation and lower adherence to chronic treatment. In this setting, it may be important to improve tolerability without impairing the benefit. Because the median age of CML patients at diagnosis is ∼60 years with a life expectancy of ∼20 years, it is particularly important to devise and test age-related policies of treatment.3,4 Results have been improved by the use of second-generation TKIs, but their toxicity remains an issue and even if they prove to be very successful, the majority of patients are expected to undergo a lifelong treatment.7-12 Although the concept of cure remains more attractive, it is time to wonder whether the choice should still be restricted to either “white” (discontinuation) or “black” (treatment of ever). Therefore, the issue is not only which of these two policies is better, but also if, between these two extremes (lifelong treatment vs treatment discontinuation), there are other potential or possible policies that can be affordable in specific settings, particularly in elderly CML patients. One such strategy could be the identification of a schedule of IM that would be sufficient to maintain response and avoid progression but might improve compliance.
We report here on a study, the INTERIM study, that was designed 5 years ago to investigate if an alternative policy of treatment, namely intermittent administration of IM, would affect the surrogate markers of outcome, namely the cytogenetic and molecular response, as well as the outcome itself (progression-free survival [PFS] and overall survival [OS]).
Patients and methods
Patients
Patients with Ph+, BCR-ABL+ CML who were 65 years or older, were treated with IM frontline, had been on IM for 2 years or longer, and who were in stable complete cytogenetic response (CCgR) were eligible for the study. The stability of CCgR had to be documented by negativity of a chromosome banding analysis (CBA) of at least 20 marrow cell metaphases within 1 year before enrollment, and again at enrollment. Written informed consent was requested. The study was conducted in accordance with the Declaration of Helsinki. The EudraCT protocol number is 2007-005102-42 and it was approved by the Ethical Committee of the Spedali Civili Hospital in Brescia, Italy.
From April 2008 to November 2009, 114 Ph+, BCR-ABL+ CML patients were screened for the study. Nineteen patients (17%) denied consent and another 19 (17%) were not enrolled either because they had been pretreated with interferon-α (7 patients) or because the CCgR status was not confirmed in the baseline sample (12 patients). Seventy-six patients were enrolled. The main characteristics of these patients are reported in Table 1. At least 3 of these characteristics are worth noticing: the long duration of IM treatment, with a median of more than 60 months; the high proportion (81%) of the patients on a 400-mg dose in spite of the age and the long treatment duration; and the low proportion of high-risk patients in spite of the advanced age. The great majority of the patients reported one or more comorbidities, including cardiovascular in 72% of cases (Table 1). Also, 71% of patients were taking concomitant medications which in 28% of the cases were drugs known to be metabolized by the CYP450 isoenzymes, CYP2D6 and CYP3A4.
Main characteristics, comorbidities, and concomitant medications of the patients who have been enrolled in the INTERIM study
| No. of patients | 76 |
| Gender, M/F, n | 41/35 |
| Age at enrollment, y, median (range) | 72 (65-83) |
| Patients >80 y old, n (%) | 8 (11%) |
| Time from diagnosis, mo, median (range) | 64 (25-98) |
| Sokal risk (at diagnosis), n (%) | |
| Low (<0.80) | 25 (33%) |
| Intermediate (0.80–1.20) | 42 (55%) |
| High (>1.20) | 9 (12%) |
| IM therapy duration, mo | |
| Median (range) | 60 (24-94) |
| No. of patients with more than 48 mo (%) | 51 (67%) |
| IM dose, mg, median | 400 |
| No. of patients at 200 mg (%) | 2 (3%) |
| No. of patients at 300 mg (%) | 11 (14%) |
| No. of patients at 400 mg (%) | 62 (81%) |
| No. of patients at 600 mg (%) | 1 (1%) |
| Patients with comorbidities, n (%) | 68 (89%) |
| Cardiovascular | 55 (72%) |
| Prostatic | 14 (18%) |
| Gastrointestinal | 14 (18%) |
| Kidney | 12 (16%) |
| Arthritis | 7 (9%) |
| Respiratory | 6 (8%) |
| Neurological | 6 (8%) |
| Diabetes mellitus | 5 (7%) |
| Thyroid | 5 (7%) |
| Other | 20 (26%) |
| Patients with concomitant medications, n (%) | 54 (71%) |
| 1-2 drugs | 20 (26%) |
| 3-4 drugs | 24 (31%) |
| >4 drugs | 10 (13%) |
| Cy3A4 drugs metabolized | 21 (28%) |
| No. of patients | 76 |
| Gender, M/F, n | 41/35 |
| Age at enrollment, y, median (range) | 72 (65-83) |
| Patients >80 y old, n (%) | 8 (11%) |
| Time from diagnosis, mo, median (range) | 64 (25-98) |
| Sokal risk (at diagnosis), n (%) | |
| Low (<0.80) | 25 (33%) |
| Intermediate (0.80–1.20) | 42 (55%) |
| High (>1.20) | 9 (12%) |
| IM therapy duration, mo | |
| Median (range) | 60 (24-94) |
| No. of patients with more than 48 mo (%) | 51 (67%) |
| IM dose, mg, median | 400 |
| No. of patients at 200 mg (%) | 2 (3%) |
| No. of patients at 300 mg (%) | 11 (14%) |
| No. of patients at 400 mg (%) | 62 (81%) |
| No. of patients at 600 mg (%) | 1 (1%) |
| Patients with comorbidities, n (%) | 68 (89%) |
| Cardiovascular | 55 (72%) |
| Prostatic | 14 (18%) |
| Gastrointestinal | 14 (18%) |
| Kidney | 12 (16%) |
| Arthritis | 7 (9%) |
| Respiratory | 6 (8%) |
| Neurological | 6 (8%) |
| Diabetes mellitus | 5 (7%) |
| Thyroid | 5 (7%) |
| Other | 20 (26%) |
| Patients with concomitant medications, n (%) | 54 (71%) |
| 1-2 drugs | 20 (26%) |
| 3-4 drugs | 24 (31%) |
| >4 drugs | 10 (13%) |
| Cy3A4 drugs metabolized | 21 (28%) |
All patients were aged ≥65 years and were in CCgR for at least 2 years.
INTERIM treatment plan
This was a multicenter, phase 2 study (EudraCT no. 2007-005102-42) aimed to investigate how an intermittent treatment with standard-dose IM would modify the surrogate markers of outcome, namely the cytogenetic and molecular response, and how it would affect the treatment outcome, namely the PFS and OS.
The term “standard imatinib therapy” is used for daily administration of IM at any dose, whereas “intermittent imatinib treatment” (INTERIM) is used to define a treatment where the same dose of IM was given according to the following intermittent schedule: 1 week on/1 week off for the 1st month (weeks 1-4); 2 weeks on/2 weeks off for the 2nd and 3rd months (weeks 5-12); 1 month on/1 month off from the 4th month (week 13) to the 12th month and thereafter. The patients who lost the CCgR resumed the prestudy, daily IM treatment. The patients who lost the major molecular response (MMR) alone within the first year had to continue the intermittent schedule. After the first year, the patients who lost MMR alone were allowed to go back to the prestudy continuous treatment.
Objectives of the study
The first objective of the study was to evaluate the proportion of patients who remained in CCgR after 1 year on INTERIM. The study was designed 5 years ago when CCgR was universally recognized as the most solid surrogate marker of survival.13 Because we anticipated that a proportion of patients would lose CCgR, all patients were carefully monitored to evaluate if and when the standard continuous treatment had to be resumed. Other objectives were to determine the proportion of patients who would lose MMR, who would lose complete hematologic response, who would develop BCR-ABL kinase domain (KD) point mutations, and who would progress to accelerated or blast phase (AP or BP). PFS and OS were calculated for all patients, with a minimum follow-up of 48 months.
Response monitoring
Clinical evaluation, physical examination, blood counts, and differential, together with relevant biochemical tests, were performed at the time of enrollment (baseline) and every 3 months during the study.
Cytogenetics
Karyotypes were performed at each participating center and were examined after G or Q banding techniques according to the International System for Human Cytogenetic Nomenclature.14 The evaluation of CCgR was based on CBA of marrow cell metaphases. CCgR was defined as the absence of the Ph chromosome in at least 20 marrow metaphases. CBA of marrow cell metaphases was performed at enrollment, then only in the patients who scored positive by fluorescence in situ hybridization (FISH) during the study.
Interphase FISH analysis of buffy-coat blood cells was performed at each center every 3 mo during the first year (study core) and every 6 months thereafter. The Locus Specific Identifier BCR/ABL Dual Color, Dual Fusion (DF) Translocation Probe (Abbott Molecular-Vysis) or the Double-Fusion Signal D-FISH BCR/ABL Probe (Oncor-QBiogene) was employed.15 FISH was defined as either negative or positive if the percent of BCR-ABL–positive nuclei was ≤1% or >1%, respectively, counting at least 200 nuclei.15 Therefore, in the patients who became FISH-positive during the study, a CBA of bone marrow metaphases was performed to confirm the loss of CCgR and to detect if there were clonal chromosomal abnormalities in Ph+ cells (CCA/Ph+).
BCR-ABL transcript level
Real-time quantitative reverse transcription-polymerase chain reaction (RT-Q-PCR) for BCR-ABL transcripts was performed at baseline and every 3 months during the study. Samples were collected at investigational sites and centralized in one of the reference laboratories (Bologna) of the Gruppo Italiano Malattie Ematologiche dell’Adulto network. Leukocytes were isolated from 20 mL of peripheral blood after red blood cell lysis, and RNA was extracted and converted to cDNA according to conventional methods. RT-Q-PCR was performed with the TaqMan technology (Applied Biosystems, Foster City, CA) as previously set up and standardized within the framework of the Europe against Cancer program.16 Results were expressed as the ratio of BCR-ABL/ABL% on the International Scale17-19 using a laboratory-specific conversion factor. The conversion factor was derived by the reference laboratory in Bologna in the framework of the European Treatment and Outcome Study standardization initiatives.16,19 MMR, corresponding to a 3-log reduction in BCR-ABL transcript level from the standardized baseline, was thus defined as BCR-ABL ≤0.1%IS and is also indicated as MR3.0.
Statistics
Based on previous studies showing that the CCgR rate during continuous treatment would range between 85% and 95%, and using the optimal Simon’s 2-stage procedure,22 we set p0 (as the proportion of responses below which the treatment would be considered ineffective) at 85% and p1 (as the proportion of responses above which the treatment would be considered effective) at 95%. With an α error of 0.05 and a power 1-β of 80%, the number of patients to be enrolled was 65. To account for dropouts and withdrawals, the number of patients was adjusted to 76. The study would have been stopped if >2 of the first 13 patients (15.4%) had lost CCgR within the first year (first stage) and >7 of 76 patients (9.2%) had lost CCgR in the second stage. Standard descriptive statistics, such as mean, median, range, and proportions, were used to summarize the patient sample. The χ2 test was used to compare differences in percentages and the Mann-Whitney U test was used to compare continuous variables. The Kaplan-Meier method23 was used to estimate PFS, OS, CCgR loss (CBA-positivity), and MMR (MR3.0) loss from the first day on INTERIM. Death by any cause and progression to AP or BP were the events of interest for PFS. CCgR loss (CBA-positivity), MMR (MR3.0) loss, and the probability of continuing INTERIM were calculated using the cumulative incidence procedure.24 Death was considered the competing risk for CCgR and MMR loss, whereas death and refusal were the competing risks for the probability of continuing INTERIM. The Cox proportional hazard regression model was used for univariate and multivariate analysis of factors associated with CCgR and MMR loss. The following variables were analyzed: age, sex, Sokal risk group, baseline BCR-ABL transcript level, and duration of IM therapy before INTERIM and BCR-ABL transcript level. Variables found to be significant at the P < .10 in univariate analysis were tested in multivariate analysis. All P values were 2-sided and P < .05 was considered statistically significant.
Results
At the time of the start of the INTERIM study, the 76 enrolled patients were in CCgR and all but one was in MMR (MR3.0). Table 2 shows the flow diagram of the INTERIM study in which all the events occurring during the 48 mo of intermittent treatment with IM are reported.
Thirteen patients (17%) lost CCgR and MMR and all but one (a patient who was lost to follow-up) regained CCgR and MMR after they had discontinued INTERIM and resumed continuous IM. The monitoring and outcome of these patients are detailed in supplemental Tables 3 and 4. The first loss of CCgR (CBA-positivity) was detected after 6 months. Six patients (8%) lost CCgR during the first 12 months and the remaining 7 patients lost CCgR between the 13th and 27th months. Thus, at 12 and 48 months, the probabilities of maintaining CCgR were 92% (95% CI: 86% to 98%) and 81% (95% CI: 71% to 90%), respectively (Figure 1). The loss of MMR occurred 3 to 9 mo before the loss of CCgR in 9 cases. All 13 patients discontinued INTERIM and resumed continuous IM treatment at the same dose and, with the exception of one patient lost to follow-up, all of them regained the CCgR and MMR after a median of 7 and 6 months from resuming IM daily, respectively; they are still in chronic phase, in CCgR and in MMR after a median follow-up of 48.5 months (range 39-66) (supplemental Table 3).
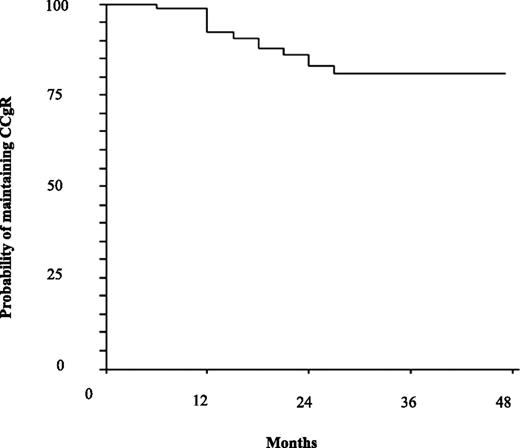
Probability of maintaining the CCgR on INTERIM. Estimated CCgR loss was 92% (95% CI: 86-98) at 12 months and 81% (95% CI: 71-90) at 48 months.
Fourteen patients (18%) lost MMR alone. Nine of these discontinued INTERIM and resumed daily IM; all regained MMR (Table 2). The first loss of MMR alone was detected after 3 months. Seven patients (9%) lost MMR during the first 12 months and the remaining 7 patients lost MMR between the 13th and 36th month (supplemental Table 4). Thus, at 12 and 48 months, the probabilities of maintaining MMR (MR3.0) were 80% (95% CI: 71% to 89%) and 63% (95% CI: 52% to 74%), respectively (Figure 2). The intermittent schedule of treatment was discontinued in 9 patients who had lost MMR alone; all of these regained a MMR after a median of 3 months from resuming daily IM. All 14 patients who lost MMR alone are still in the chronic phase and in MMR after a median follow-up of 48 months (range 33-60) (supplemental Table 4).
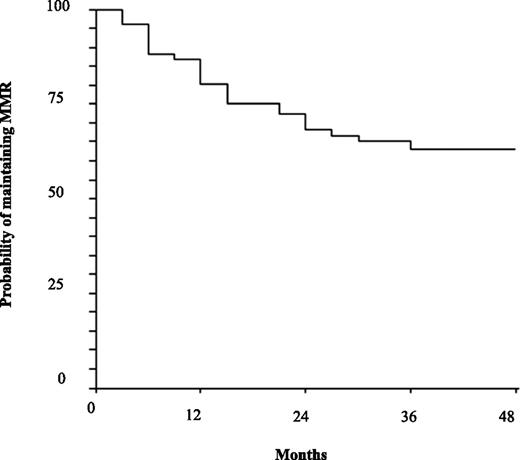
Probability of maintaining the MMR on INTERIM. Estimated MMR loss was 80% (95% CI: 71-89) at 12 mo and 63% (95% CI: 52-74) at 48 mo.
Age, sex, Sokal risk group, baseline BCR-ABL transcript level, and duration of IM therapy before INTERIM were not associated with loss of CCgR or MMR when they were examined by univariate and multivariate analyses.
One patient developed atrial fibrillation at 15 months and one refused to continue INTERIM at 24 months, but all the patients were in CCgR and MMR when the events occurred. No patients developed CCA in Ph+ cells or BCR-ABL point mutations; moreover, none of the patients progressed to AP or BP, but 2 died at 24 and 36 months because of acute myocardial infarction and intracranial hemorrhage, respectively (Table 2). Therefore, the probabilities of continuing INTERIM were 92% (95% CI: 86% to 98%) and 70% (95% CI: 60% to 80%) at 12 and 48 months, respectively (Figure 3), and the estimated PFS was 100% at 12 months and 96% (95% CI: 91-100) at 48 months (Figure 4).
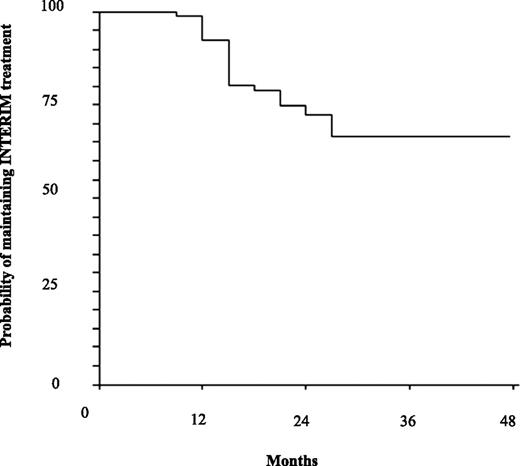
Probability of maintaining INTERIM treatment. The estimated probability of maintaining INTERIM treatment was 92% (95% CI: 86% to 98%) at 12 months and 70% (95% CI: 60% to 80%) at 48 months.

PFS. Estimated PFS was 100% at 12 months and 96% (95% CI: 91-100) at 48 months. The events were 2 deaths in remission (CCgR and MMR). No patients progressed to AP or BP.
No patient complained of new or more severe side effects during the “on” months. Three patients reported grade 2 side effects (0 adverse events) at enrolment into INTERIM. In one of these, fluid retention, muscle cramps, and skin erythema disappeared after 10 months. In the second patient, skin erythema disappeared after 8 months. The third patient continued to complain of fluid retention and skin erythema and this remained stable during INTERIM. Seventeen patients reported grade 1 side effects at enrolment in INTERIM (supplemental Table 5). Muscle pain or cramps and fluid retention disappeared in 4 of 5 patients. Fluid retention disappeared in 2 of 8 patients. Overall, the side effects disappeared in 11 of 20 patients (supplemental Table 5).
Discussion
This is a report of a phase 2, single-arm study testing prospectively, for the first time, the effects of a policy of TKI treatment reduction in patients who were optimal responders to IM but who did not fit the current requirements for a trial of treatment discontinuation. The first selection was based on age, and the study was limited to patients who were 65 years or older (the median age was 72 years). Elderly patients respond to IM as well as young patients,25 but they have more comorbidities, take more concomitant drugs, and are less tolerant of TKI. Furthermore, compared with younger patients, elderly patients may suffer less from living with leukemia, have fewer problems related to family planning and career, but may have more financial problems in those countries where the cost of TKI cannot yet be completely covered by a public health system or private insurance. These reasons can be more or less general, but in many diseases, treatment is modulated according to age and there are no good reasons why CML should be an exception.
The schedule of 1 month on and 1 month off was selected from several different possibilities. For the sake of simplicity, the cycle was set to 1 mo, but a posteriori, this choice looks fairly reasonable and appropriate, because the discontinuation studies have shown that most molecular relapses occur rapidly.6,26 Dose reduction instead of the intermittent administration was not taken into consideration, as prolonged exposure to a low IM concentration may favor the emergence and selection of resistant clones.27-29 When the study was designed, we anticipated that some patients could lose the molecular and CCgRs that were the most reliable surrogate markers of survival, at least at that time. Therefore, all patients were carefully monitored, and the protocol required that resuming the continuous schedule of treatment was always mandatory if CCgR was lost and was advisable if MMR was lost after the completion of the first year of INTERIM. With these recommendations and stringent cytogenetic and molecular monitoring, all the patients who had lost CCgR or MMR regained CCgR or MMR with daily IM treatment. No BCR-ABL KD point mutation emerged, and all patients remained free from progression to AP or BP, the ultimate goal of the study protocol.
This study cannot prove that a policy of INTERIM treatment was better or worse than the standard policy of continuing treatment. Furthermore, it cannot lead to the recommendation to extend this policy either to the patients who are believed to be ineligible for treatment discontinuation or to patients undergoing less stringent conventional monitoring of cytogenetic and/or molecular response. However, these results help to open a small door to alternative and yet unexplored policies of treatment. Currently, the internationally shared standard policy of treatment of CML with TKI is to continue the treatment, at the same dose, indefinitely, with the goal of ensuring survival close to that of the general population. More recently, a more ambitious policy is gaining support with the goal of achieving a condition of minimal residual disease such that remission can be maintained even after treatment discontinuation.6,26 This latter policy requires sensitive molecular assessment of minimal residual disease and foresees that the depth of the molecular response is the best surrogate marker of outcome, but the major obstacle to its success is the small proportion of patients who can achieve and maintain a deep molecular response and do not relapse after treatment discontinuation.6,26 In the patients who have been treated with IM for years, the probability of achieving a MMR or better is high, but the proportion of patients who maintain a MR4.0 or more for a sufficiently long period is lower. There are not many data, but a recent analysis of a multicentric series by the Gruppo Italiano Malattie Ematologiche dell’Adulto CML WP has shown that the proportion of patients who maintained a stable MR4.0 or better for ≥1 year was ∼22%.30 A similar analysis of a single center series reported that the proportion of patients who would have fit the requirements for entering the STIM study was 21%.31 Although it is believed, and expected, that the introduction of second-generation TKI as first-line treatment will mark a significant improvement, that proportion would hardly exceed 20% to 30%, leaving the majority of responders with a policy of chronic treatment at the same standard schedule and dose.
In conclusion, it has been shown that in the setting of optimal responders to IM, a 50% dose reduction of the drug results in loss of CCgR in 17% of patients and of MMR alone in 18% of patients, but that all patients can achieve the same level of response again by reassuming continuous IM. What is particularly striking is that, with a follow-up of 48 months, no patient has progressed or died of leukemia. In elderly CML Ph+ patients carefully selected for a stable CCgR (long lasting, ie, >2 years) and carefully monitored, the policy of INTERIM treatment affected the markers of residual disease, but not the clinical outcomes (OS and PFS). The results of this study do not allow us to definitively conclude that intermittent treatment can be offered to optimal and stable responders but opens a window to alternative treatment policies, even in the patients for whom a very deep molecular response cannot be achieved.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Special thanks to the following authors for their participation in the development of the manuscript: Francesco Albano (Bari), Miriam Fogli (Bologna), Chiara Colombi (Brescia), Giovanni Quarta and Mariella Girasoli (Brindisi), Emilio Usala and Emanuele Angelucci (Cagliari), Alberto Bosi and Valeria Santini (Firenze), Gianluca Gaidano and Monia Lunghi (Novara), Giuseppe Visani and Giuseppina Nicolini (Pesaro), Giuseppe Fioritoni and Roberto Di Lorenzo (Pescara), Monica Bocchia and Francesco Lauria (Siena), and Francesco Rodeghiero and Anna D’Emilio (Vicenza). Many thanks to Multilingue Srl Translations Services, Brescia, Italy for the English revision.
This work was supported in part by EuropeanLeukemiaNet (contract LSHC-CT-2004-503216) through the European Treatment and Outcome Study supported by Novartis Oncology Europe, and COFIN 2009.
Authorship
Contribution: D.R. and M. Baccarani designed the study; D.R., G.M., G.R., M.M., C.S., S.S., I.I., A.D.V., N.T., F.C., G.G., D.T., M. Bergamaschi, P.P., E.P., F.S., M. Breccia, B.M., T.I., C.F., E.A., M.T., C.B., B.M.C., and M. Baccarani collected the data; G.M., I.I., S.S., and N.T. performed the molecular and cytogenetic analysis; D.R., M. Baccarani, G.R., M.M., C.S., A.D.V., and B.M.C. analyzed the data; and D.R. and M. Baccarani wrote the manuscript.
Conflict-of-interest disclosure: D.R. received research funding from Celgene and Gilead, is a paid expert testimony for Novartis, and serves on the speakers’ bureaus of Novartis. G.M. serves on the speakers’ bureaus of Novartis, Bristol-Myers Squibb, and Pfizer. G.R. is a consultant for Novartis, Bristol-Myers Squibb, and ARIAD and serves on the speakers’ bureaus of Novartis, Bristol-Myers Squibb, and Roche. S.S. is a consultant for Novartis, Bristol-Myers Squibb, and Ariad and received honoraria from Novartis, Bristol-Myers Squibb, and Ariad. F.C. is a consultant for Novartis and Bristol-Myers Squibb and received honoraria from Novartis and Bristol-Myers Squibb. D.T. provides expert testimony for Novartis and Bristol-Myers Squibb and received other remuneration from Novartis and Bristol-Myers Squibb. M. Breccia is a consultant for Bristol-Myers Squibb. E.A. is a consultant for Novartis. M.T. is a consultant for Novartis and Bristol-Myers Squibb and received honoraria from Novartis and Bristol-Myers Squibb. M. Baccarani received honoraria from Novartis, Bristol-Myers Squibb, Pfizer, and Ariad and serves on the speakers’ bureaus of Novartis and Bristol Myers-Squibb. The remaining authors declare no competing financial interests.
Correspondence: Domenico Russo, Chair of Hematology, Unit of Blood Diseases and Stem Cell Transplantation, University of Brescia, AO Spedali Civili Brescia, P.le Spedali Civili 1, 25100 Brescia, Italy; e-mail: russo@med.unibs.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal