Key Points
Role for LIMK1 in GPIb-IX–dependent cPLA2 activation, TXA2 synthesis, and platelet activation independent of its role in actin polymerization.
LIMK1 is important in arterial thrombosis in vivo but appears to be dispensable for hemostasis, suggesting a new antithrombotic target.
Abstract
Current antithrombotic drugs have an adverse effect on bleeding, highlighting the need for new molecular targets for developing antithrombotic drugs that minimally affect hemostasis. Here we show that LIMK1−/− mice have defective arterial thrombosis in vivo but do not differ from wild-type mice with respect to bleeding time. LIMK1−/− mice show a selective defect in platelet activation induced through the von Willebrand Factor (VWF) receptor, the glycoprotein Ib-IX-V complex (GPIb-IX), but not by GPIb-IX–independent platelet agonists. In fact, LIMK1−/− platelets show an enhanced reaction to certain GPIb-IX–independent agonists. The defect of LIMK1−/− platelets in GPIb-IX–mediated platelet activation is attributed to a selective inhibition in VWF/GPIb-IX–induced phosphorylation of cytosolic phospholipase A2 (cPLA2) and consequent thromboxane A2 (TXA2) production. Supplementing a TXA2 analog, U46619, corrected the defect of LIMK1−/− platelets in VWF-induced stable platelet adhesion. Although LIMK1−/− platelets also showed reduced actin polymerization after GPIb-IX–mediated platelet aggregation, actin polymerization inhibitors did not reduce TXA2 generation, but rather accelerated platelet aggregation, suggesting that the role of LIMK1 in GPIb-mediated platelet activation is independent of actin polymerization. Thus, LIMK1 plays a novel role in selectively mediating GPIb-IX–dependent TXA2 synthesis and thrombosis and represents a potential target for developing antithrombotic drugs with minimal bleeding side effect.
Introduction
LIM kinases (LIMKs) are a family of serine-threonine protein kinases that contain the LIM domain, a zinc-finger motif initially discovered in Lin-11, Isl-1, and Mec-3.1,2 The LIMK family has 2 members, LIMK1 and LIMK2.2 LIMKs are activated by phosphorylation at specific threonine residues (Thr508 for LIMK1 and Thr505 for LIMK2), which can be catalyzed by several protein kinases, including p21-activated kinase3 and Rho kinase.4 Once activated, LIMK is known to phosphorylate the actin-depolymerizing protein, cofilin,3 which allows the binding of 14-3-3 protein to cofilin, resulting in dissociation of cofilin from actin, thereby facilitating actin polymerization.5 LIMK1, but not LIMK2, is expressed in platelets and is activated during platelet activation.6 However, the function of LIMK1 in platelet activation and thrombosis remains unclear.
The platelet receptor for von Willebrand Factor (VWF), the glycoprotein Ib-IX-V complex (GPIb-IX), mediates the initial platelet adhesion to subendothelial-bound VWF at sites of vascular injury and transduces signals, leading to platelet activation, stable platelet adhesion, and thrombus formation.7,8 GPIb-IX is important under high shear rate flow conditions seen in arteries and arterioles. GPIb-IX has also been shown to be important under low shear rate conditions seen in veins.9,10 Previous studies indicate that GPIb-IX–induced platelet activation requires the sequential activation of the Src family kinase, Lyn,11,12 Rac1,13 PI3K/Akt,14,15 cGMP-dependent protein kinase,16 and mitogen-activated protein kinases (MAPKs).17 However, full platelet responses to VWF require amplification signaling mediated through the immunoreceptor tyrosine-based activation motif signaling pathway, involving either the Fc receptor γ-chain and/or Fcγ receptor IIA, Syk, SLP76, Bruton tyrosine kinase (Btk), and PLCγ2,11,18-20 followed by consequent thromboxane A2 (TXA2) and TXA2-dependent granule secretion of adenosine diphosphate (ADP).15,21,22 It remains unclear, though, how GPIb-IX signaling leads to TXA2 generation.
In this study, we demonstrate that LIMK1 is important for arterial thrombosis in vivo, but appears to be dispensable for hemostasis. Furthermore, we show that LIMK1 selectively promotes GPIb-IX–mediated platelet activation and stable platelet adhesion by mediating GPIb-IX–dependent cPLA2 activation and TXA2 synthesis. The role of LIMK1 in GPIb-IX–mediated TXA2 synthesis appears to be independent of its role in promoting actin polymerization. These results not only demonstrate a novel function of LIMK1 in GPIb-IX–mediated TXA2 synthesis, platelet activation, and thrombosis, but they also identify a new molecular target for developing an antithrombotic with minimal bleeding side effect.
Materials and methods
Preparation of platelets
Human blood was drawn by venipuncture from healthy volunteers. Institutional review board approval was obtained from the University of Illinois at Chicago, and informed consent from volunteers was obtained in accordance with the Declaration of Helsinki. Acid-citrate-dextrose was used as an anticoagulant and the platelets were prepared as previously described.15,23 The platelets were then allowed to rest in modified Tyrode’s buffer for at least 1 hour at 22°C before use.
The generation of LIMK1-knockout mice has been previously described.24 The mice were kept on a mixed 129R1(50%)/C57BL (50%) background. Wild-type control mice and LIMK1-knockout mice used in this study were 15- to 20-week-old littermates generated from heterozygous breeding. Washed platelets were prepared from blood drawn from the mouse inferior vena cava as previously described.23 Final concentrations of 1 U/mL apyrase and 0.1 µg/mL PGE1 were added to the freshly drawn whole blood. After washing, the platelets were resuspended in Tyrode’s buffer. In some experiments, the platelets were washed in the presence of apyrase and 5 mM ethylenediaminetetraacetic acid (EDTA) in modified Tyrode’s buffer.11 Hematologic parameters were routinely measured using a Hemavet 950FS (Drew Scientific, Dallas, TX).
Platelet aggregation and secretion
Washed platelets (3 × 108/mL) were allowed to rest for at least 1 hour at 22°C before use. Platelet aggregation and secretion of granule adenosine triphosphate were determined simultaneously in a Chronolog lumiaggregometer at 37°C with stirring (1000 rpm). For some experiments, washed human platelets were preincubated with either SB203580 (20 μM), U0126 (3 μM), Cytochalasin D (10 μM), Latrunculin A (5 μM) (all from Calbiochem), or vehicle control (0.1% dimethyl sulfoxide [DMSO]) for 5 minutes at 37°C.
Phosphorylation of LIMK, cofilin, cPLA2, and P38 MAPK
Washed platelets (3 × 108/mL) were stirred (1000 rpm) in a platelet aggregometer for various lengths of time after adding ristocetin (0.25 mg/mL) alone or ristocetin and VWF (10 µg/mL) (human), or after adding botrocetin (2 µg/mL) with or without VWF (10 µg/mL) (mouse). The reactions were stopped by the addition of an equal volume of 2× sodium dodecyl sulfate sample buffer, containing 0.2 mM E64, 2 mM phenylmethanesulfonyl fluoride and 34 µg/mL aprotinin. Samples were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes and immunoblotted with antibodies recognizing LIMK1/2 phosphorylated at threonine 508/505, phospho-cofilin at Ser,3 total cofilin, total LIMK1, p38 MAPK phosphorylated at threonine 180/tyrosine 182 (Cell Signaling, Danvers, MA), cPLA2, or cPLA2 phosphorylated at serine 505 (Abcam, Santa Cruz, CA).
Platelet adhesion under shear stress
The analysis of mepacrine (10 µM)-labeled platelet adhesion to VWF-coated surfaces under shear stress was performed essentially as previously described.15 A cone-plate rheometer (Rheostress 1; Thermo-HAAKE, Paramus, NJ) was used to introduce shear stress (800 s−1) to the platelets for 5 minutes. After washing, the slides were viewed with a Leica DMI RB fluorescence microscope (Leica) using an N PLAN L lens at 40/0.55 NA objective with 1.5× magnification. Stably-adherent platelets were counted in ≥20 randomly selected fields (mean ± standard error). A one-way analysis of variance was used for statistical analysis, and the significance between individual treatments was determined by using Bonferroni’s multiple comparison test.
VWF binding to platelets
Washed mouse platelets (1 × 108/mL) in a modified Tyrode’s buffer containing 1% bovine serum antigen and 5 mM EDTA were incubated with VWF (10 µg/mL) alone as control or VWF plus botrocetin (2 µg/mL) at 22°C for 30 minutes. After washing once in phosphate-buffered saline, VWF binding to platelets was detected using fluorescein isothiocyanate–labeled anti-VWF antibody, SZ-29, and flow cytometry.25
Platelet adhesion and spreading on immobilized VWF and fibrinogen under static conditions
Washed platelets (2 × 107/mL) were allowed to adhere and spread at 37°C for 1.5 hours on cover slides coated with fibrinogen (100 μg/mL) or VWF (30 μg/mL).26 For adhesion on VWF, 2.0 μg/mL botrocetin was added to the wells. The slides were rinsed, fixed with 4% paraformaldehyde, and then permeabilized. Adherent platelets were stained with Alexa Fluor 546-conjugated phalloidin (Invitrogen) and observed with a Leica DM IRB fluorescence microscope using a 100×/1.30 NA oil objective as previously described.15 Images were acquired using a Cool SNAP HQ CCD camera and processed with µManager software. To quantitate platelet adhesion, platelets were incubated in VWF-coated microtiter wells at 37°C for 1 hour. Adherent cells were quantified using a colorimetric acid phosphatase assay as previously described.27
TXB2 generation assay
Washed platelets (3 × 108/mL) were stimulated with agonists in an aggregometer at 37°C with stirring (1000 rpm). The reaction was stopped by adding 3 mM aspirin and 10 mM EDTA at 8 minutes on ice, centrifuged at 6000 g for 1 minute in a microfuge, and the supernatant stored at −70°C until analysis. A TXB2 EIA Kit (Assay Designs, Inc., Ann Arbor, MI) was used to determine the level of TXB2 in each sample. Experiments were repeated at least 3 times. All data are expressed as mean ± standard error. Statistical significance was determined using the Student t test.
F-actin measurement in platelets
Washed platelets (3 × 108/mL), either resting or stimulated with VWF/botrocetin, were fixed in 4% paraformaldehyde solution for 30 minutes at 37°C, permeabilized with a 0.2% Triton X-100 solution, and incubated with 10 U Alexa Fluor 488 phalloidin (Molecular Probes) for 1 hour at 37°C as previously described.28 The samples were analyzed by flow cytometry (FACSCalibur, BD). The data shown are fluorescence intensity of single platelets as gated by forward and side scatter.
In vivo thrombosis
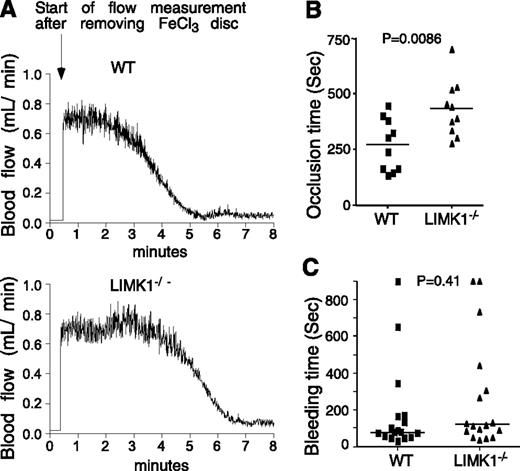
Mice were anesthetized with isoflurane. The left carotid artery was isolated, and a MA-0.5PSB nanoprobe (Transonic Systems, Ithaca, NY) was hooked to the artery to monitor blood flow using a TS420 flowmeter (Transonic Systems) as previously described.29 A filter paper disc (2-mm diameter) soaked with 1.2 μL of 10% (0.460 M) ferric chloride (FeCl3) (Sigma Aldrich, St. Louis, MO) was placed on top of the artery for 3 minutes. Blood flow was then monitored until 5 minutes after occlusion. Statistical analysis was performed using a parametric, unpaired Student t test assuming equal variances between treatments (F-test, P = .81; 2-tailed t test, P = .0086, n = 10 per group).
Bleeding time
Mice were anesthetized with isoflurane and their tails were then immersed in 0.15 M NaCl immediately after cutting 0.5-cm-long tail tips. Bleeding was followed visually, and time to stable cessation of bleeding (no rebleeding within 1 minute) was recorded. A nonparametric, unpaired t test was used to compare medians between treatments (Mann-Whitney U test: P = .41, n = 17 per group).
Results
LIMK1 promotes in vivo thrombosis but appears to be dispensable for hemostasis
We evaluated the effect of LIMK1 deficiency on tail bleeding time and FeCl3-induced carotid artery thrombosis, respectively. The time from FeCl3-induced arterial injury to occlusive thrombosis was significantly prolonged in LIMK1−/− mice compared with wild-type mice (Figure 1A-B), indicating that LIMK1 plays an important role in arterial thrombosis in vivo. The inhibitory effect shown in LIMK1−/− mice is in the period between FeCl3-induced injury and the onset of a rapid occlusive thrombosis phase (Figure 1A). Once entering the rapid occlusive thrombosis phase, there was no longer a difference between wild-type and LIMK1−/− mice in the rate of occlusive thrombus formation (WT: –ΔV/Δt = 0.295 ± 0.025; LIMK1−/−: –ΔV/Δt = 0.281 ± 0.038 (mL/min2), P = .763). Interestingly, we observed no statistically significant differences in tail bleeding time between wild-type and LIMK1−/− mice (Figure 1C). These data suggest that LIMK1 is important in promoting arterial thrombosis but appears to be dispensable in hemostasis.
The effects of LIMK1 knockout on in vivo thrombosis and hemostasis. (A-B) FeCl3-induced occlusive thrombosis in carotid arteries of LIMK1−/− and wild-type (WT) mice. (A) Typical charts of FeCl3-induced occlusive thrombosis in WT and LIMK1−/− mice as indicated by carotid artery blood flow. (B) The occlusion times for each mouse are shown as squares (WT, n = 10) or triangles (LIMK1−/−, n = 10). The horizontal bars represent the mean occlusion time (P = .0086, Student t test). (C) Tail bleeding times of each mouse are shown as squares (WT, n = 17) or triangles (LIMK1−/−, n = 17). The horizontal bars represent the median bleeding time (P = .41, Mann-Whitney U test).
The effects of LIMK1 knockout on in vivo thrombosis and hemostasis. (A-B) FeCl3-induced occlusive thrombosis in carotid arteries of LIMK1−/− and wild-type (WT) mice. (A) Typical charts of FeCl3-induced occlusive thrombosis in WT and LIMK1−/− mice as indicated by carotid artery blood flow. (B) The occlusion times for each mouse are shown as squares (WT, n = 10) or triangles (LIMK1−/−, n = 10). The horizontal bars represent the mean occlusion time (P = .0086, Student t test). (C) Tail bleeding times of each mouse are shown as squares (WT, n = 17) or triangles (LIMK1−/−, n = 17). The horizontal bars represent the median bleeding time (P = .41, Mann-Whitney U test).
LIMK1 is important in platelet secretion and the second wave of platelet aggregation induced by VWF
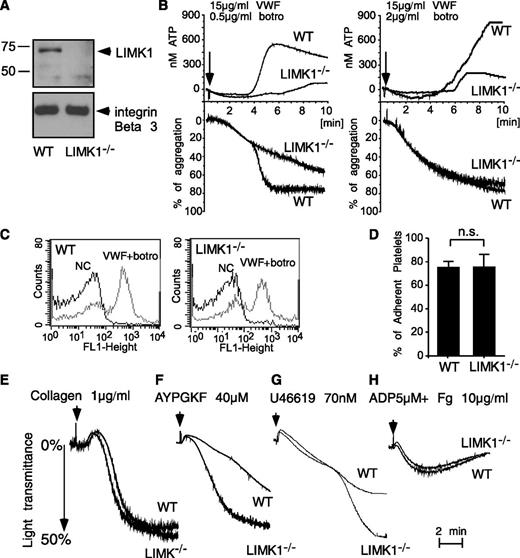
Consistent with a previous report,6 LIMK1 was detected by Western blot analysis in wild-type mouse platelets but was undetectable in platelets from LIMK1−/− mice (Figure 2A). There were no obvious differences between wild-type and LIMK1−/− mice in major hematologic parameters including platelet count and platelet volume. To study the role of LIMK1 in the GPIb-IX–dependent platelet response, wild-type and LIMK1−/− platelets were stimulated with VWF in the presence of botrocetin, a VWF-binding snake venom protein used in vitro to mimic the effect of the VWF-collagen interaction in increasing the affinity of VWF for GPIb-IX.30 As expected, at a low concentration (0.5 µg/mL), botrocetin induced 2 waves of platelet agglutination/aggregation in wild-type platelets in the presence of VWF (Figure 2B). The first wave is comprised mainly of GPIb-IX–mediated platelet agglutination, with GPIb-IX–induced, integrin-dependent platelet aggregation also playing a role.31 LIMK1 knockout did not affect the first wave of platelet agglutination/aggregation (Figure 2B). The second wave of platelet aggregation requires TXA2-dependent platelet granule secretion, secreted ADP, and the consequent amplification of integrin activation.21,31 The second wave of platelet aggregation and the secretion of dense granules were inhibited in LIMK1−/− platelets (Figure 2B), indicating that LIMK1 is important for GPIb-IX–mediated signaling, leading to granule secretion and the second wave of platelet aggregation. At a higher concentration of botrocetin, the initial agglutination/aggregation was very strong and the 2 phases of platelet agglutination/aggregation were no longer distinguishable (Figure 2B). However, LIMK1−/− platelets still showed decreased granule secretion, further indicating the importance of LIMK1 in VWF/botrocetin-induced granule secretion. The inhibitory effects observed in LIMK1−/− platelets were not caused by a defective ligand-binding function of GPIb-IX because botrocetin-induced VWF binding to platelets was not affected in LIMK1−/− platelets relative to wild-type (Figure 2C). Similarly, botrocetin-induced platelet adhesion to VWF under static conditions was not affected by knockout of LIMK1 (Figure 2D). These data suggest that LIMK1 plays an important stimulatory role in VWF/GPIb-IX–induced intracellular signaling, leading to granule secretion and integrin-dependent second-wave platelet aggregation.
LIMK1 stimulates platelet aggregation and secretion induced by VWF. (A) Immunoblots of platelet lysates from WT and LIMK1-knockout mice probed with an anti-LIMK1 antibody and an anti-β3 integrin antibody (loading control). (B) Aggregation and adenosine triphosphate (ATP) secretion traces of WT and LIMK1−/− platelets stimulated with VWF (15 μg/mL) and the indicated doses of botrocetin. (C) Flow cytometric analysis of the binding of VWF to WT and LIMK1−/− platelets. (D) Quantitation of WT or LIMK1−/− platelet adhesion to VWF-coated microtiter wells in the presence of botrocetin using an acid phosphatase assay. (E-H), Aggregation of WT and LIMK1−/− platelets in response to: collagen (E), PAR4AP (F), U46619 (G), and ADP and fibrinogen (H).
LIMK1 stimulates platelet aggregation and secretion induced by VWF. (A) Immunoblots of platelet lysates from WT and LIMK1-knockout mice probed with an anti-LIMK1 antibody and an anti-β3 integrin antibody (loading control). (B) Aggregation and adenosine triphosphate (ATP) secretion traces of WT and LIMK1−/− platelets stimulated with VWF (15 μg/mL) and the indicated doses of botrocetin. (C) Flow cytometric analysis of the binding of VWF to WT and LIMK1−/− platelets. (D) Quantitation of WT or LIMK1−/− platelet adhesion to VWF-coated microtiter wells in the presence of botrocetin using an acid phosphatase assay. (E-H), Aggregation of WT and LIMK1−/− platelets in response to: collagen (E), PAR4AP (F), U46619 (G), and ADP and fibrinogen (H).
LIMK1 plays differential roles in distinct platelet activation pathways
We also examined whether LIMK1 plays a role in stimulating platelet activation induced by collagen (Figure 2E), PAR4 (thrombin receptor) agonist peptide (AYPGKF) (Figure 2F), TXA2 analog U46619 (Figure 2G), and ADP (Figure 2H). Platelet aggregation responses induced by collagen or ADP were not affected by the loss of LIMK1 (Figure 2E,H). In contrast, to the defects observed in VWF-stimulated LIMK1−/− platelets (Figure 2B), LIMK1−/− platelets stimulated by low concentrations of PAR4 agonist peptide or U46619 showed an enhanced platelet aggregation response (Figure 2F-G). However, at higher concentrations of PAR4AP or U46619, no difference in aggregation was observed between wild-type and LIMK1−/− platelets. Altogether, these results suggest that LIMK1 selectively plays a stimulatory role in VWF/GPIb-IX–dependent platelet activation but negatively regulates some other platelet activation pathways.
The selective role of LIMK1 in VWF-induced TXA2 production
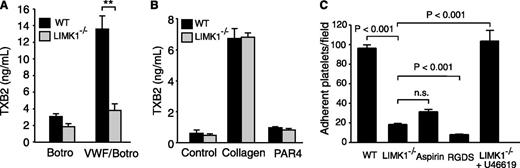
The secretion- and integrin-dependent second wave of platelet aggregation induced by botrocetin/VWF requires the cyclooxygenase/TXA2 signaling pathway and TXA2-dependent granule secretion.21,31 Thus, we further investigated whether LIMK1 is important in mediating VWF-induced stimulation of TXA2 synthesis by measuring the stable TXA2 metabolite, TXB2. As expected, VWF/botrocetin induced an increase in TXB2 in wild-type platelets. In contrast, VWF/botrocetin-induced TXB2 production was significantly reduced in LIMK1−/− platelets (Figure 3A), indicating that LIMK1 is important in VWF-induced TXA2 synthesis. We also assessed TXB2 synthesis after stimulation with the GPIb-IX-independent platelet agonists, collagen and PAR4AP. TXB2 was dramatically elevated by collagen in both wild-type and LIMK1−/− platelets, with no statistically significant difference noted (Figure 3B). Low-dose PAR4AP induced low, but significant (P < .05), amounts of TXB2 both in wild-type and LIMK1−/− platelets (Figure 3B), between which there was no significant difference. Thus, the stimulatory role of LIMK1 in TXA2 generation is selective for the GPIb-IX–dependent platelet activation pathway.
LIMK1 selectively promotes VWF-stimulated platelet TXB2 production and platelet adhesion under shear stress. (A-B) TXB2 production in WT and LIMK1−/− platelets stimulated with: (A) VWF and botrocetin or with botrocetin alone as a control, or (B) collagen or PAR4AP. All data are expressed as mean ± standard error. Statistical significance was determined using the Student t test, **P < .01. (C) Stable adhesion of mepacrine-stained WT and LIMK1−/− platelets to VWF-coated slides under a constant shear rate (800 s−1). The adherent platelets were photographed under a Leica fluorescence microscope. The number of adherent platelets per field in ≥20 randomly selected fields (4 experiments) is shown (mean ± SE). The first, third, and fourth columns are adhesion of WT platelets (with or without pretreatment with the integrin inhibitor RGDS or the cyclooxygenase inhibitor aspirin), and the second and fifth columns are LIMK1−/− platelets (with or without 40 nM U46619).
LIMK1 selectively promotes VWF-stimulated platelet TXB2 production and platelet adhesion under shear stress. (A-B) TXB2 production in WT and LIMK1−/− platelets stimulated with: (A) VWF and botrocetin or with botrocetin alone as a control, or (B) collagen or PAR4AP. All data are expressed as mean ± standard error. Statistical significance was determined using the Student t test, **P < .01. (C) Stable adhesion of mepacrine-stained WT and LIMK1−/− platelets to VWF-coated slides under a constant shear rate (800 s−1). The adherent platelets were photographed under a Leica fluorescence microscope. The number of adherent platelets per field in ≥20 randomly selected fields (4 experiments) is shown (mean ± SE). The first, third, and fourth columns are adhesion of WT platelets (with or without pretreatment with the integrin inhibitor RGDS or the cyclooxygenase inhibitor aspirin), and the second and fifth columns are LIMK1−/− platelets (with or without 40 nM U46619).
LIMK1−/− platelets are defective in stable platelet adhesion to VWF under shear stress, which is corrected by supplementing a TXA2 analog
In platelets, stable adhesion to VWF under shear stress involves (1) early GPIb-IX signaling leading to integrin activation and (2) its subsequent amplification by the second messenger TXA2.15,32 To differentiate in which of these 2 pathways LIMK1 participates, wild-type and LIMK1−/− platelets were stained with mepacrine and allowed to adhere to VWF-coated surfaces under flow conditions (800 s−1 shear rate) for 5 minutes. As expected, stable platelet adhesion to VWF under shear stress was almost completely inhibited by the integrin antagonist, RGDS peptide, and was significantly but partially inhibited by a saturating concentration of the cyclooxygenase inhibitor, aspirin (1 mM) (Figure 3C). Similarly, stable platelet adhesion to VWF was significantly reduced, but not totally abolished, in LIMK1−/− platelets (Figure 3C), indicating that LIMK1 is important in the secondary amplification of stable platelet adhesion to VWF under flow conditions. Furthermore, supplementing a TXA2 analog, U46619, at a concentration similar to that of TXA2 generated in platelets after VWF stimulation, corrected the adhesion defect in LIMK1−/− platelets, suggesting that LIMK1 promotes GPIb-IX–dependent stable platelet adhesion mainly by stimulating TXA2 generation (Figure 3C).
VWF-induced phosphorylation of LIMK1 and cofilin
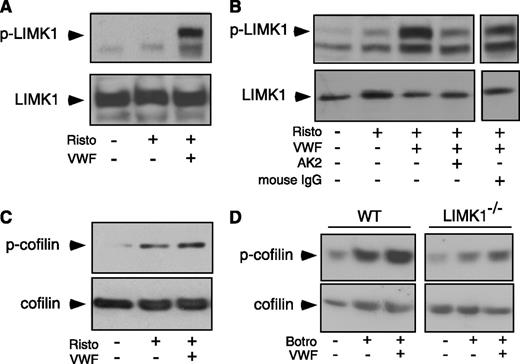
To determine whether LIMK1 is activated by GPIb-IX ligation, we immunoblotted VWF/ristocetin-stimulated human platelet lysates with an antibody recognizing LIMK1 phosphorylated at Thr508 (which indicates LIMK1 activation).4 LIMK1 became phosphorylated upon VWF stimulation (Figure 4A). The VWF/ristocetin-induced phosphorylation of LIMK1 was inhibited by a monoclonal anti-GPIbα antibody, AK2,33 which blocks VWF binding (Figure 4B), indicating that VWF binding to GPIb-IX induces the activation of LIMK1.
VWF-induced LIMK1 phosphorylation and LIMK1-dependent cofilin phosphorylation. (A-C) Immunoblots of washed human platelets, stimulated with VWF (10 μg/mL) in the absence or presence of ristocetin (0.25 mg/mL) in a platelet aggregometer at 37°C for 6 minutes with: (A and B) an antibody recognizing phosphorylated Thr508/505(T508/505) in LIMK and an anti-LIMK1 antibody (loading control); and (C) an antibody specific for Ser3 (S3 )-phosphorylated cofilin and an anticofilin antibody (loading control). In (B), platelets were preincubated with a function-blocking anti-GPIbα antibody, AK2 (10 μg/mL), or 10 µg/mL of control IgG for 6 minutes and then stimulated with VWF and ristocetin. (D) Immunoblots of WT and LIMK1−/− mouse platelets stimulated with VWF (10 µg/mL) in the presence of botrocetin (2 µg/mL) with an antibody against Ser3 -phosphorylated cofilin or an anticofilin antibody.
VWF-induced LIMK1 phosphorylation and LIMK1-dependent cofilin phosphorylation. (A-C) Immunoblots of washed human platelets, stimulated with VWF (10 μg/mL) in the absence or presence of ristocetin (0.25 mg/mL) in a platelet aggregometer at 37°C for 6 minutes with: (A and B) an antibody recognizing phosphorylated Thr508/505(T508/505) in LIMK and an anti-LIMK1 antibody (loading control); and (C) an antibody specific for Ser3 (S3 )-phosphorylated cofilin and an anticofilin antibody (loading control). In (B), platelets were preincubated with a function-blocking anti-GPIbα antibody, AK2 (10 μg/mL), or 10 µg/mL of control IgG for 6 minutes and then stimulated with VWF and ristocetin. (D) Immunoblots of WT and LIMK1−/− mouse platelets stimulated with VWF (10 µg/mL) in the presence of botrocetin (2 µg/mL) with an antibody against Ser3 -phosphorylated cofilin or an anticofilin antibody.
The enzymatic activity of LIMK1 may be assessed by evaluating the phosphorylation of cofilin at Ser,3 a known LIMK1 substrate.3 Phosphorylation of cofilin at Ser3 was induced by VWF/ristocetin in human platelets (Figure 4C), and by VWF/botrocetin in wild-type mouse platelets, which was attenuated in LIMK1−/− platelets (Figure 4D). Altogether, these data indicate that VWF binding to GPIb-IX induces activation of LIMK1 and phosphorylation of cofilin.
LIMK1 promotes VWF/GPIb-IX–mediated actin polymerization after platelet aggregation
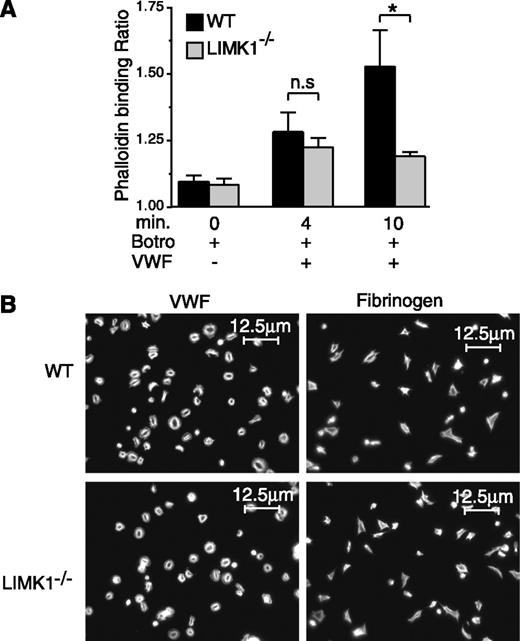
A well-known function of LIMK1 is to promote actin polymerization. Thus, we investigated whether knockout of LIMK1 affects VWF/GPIb-IX–induced actin polymerization in platelets. Stimulation of wild-type platelets with VWF/botrocetin, as expected,34 resulted in a significant increase in the amount of polymerized actin as determined by flow cytometric analysis (Figure 5A). By contrast, LIMK1−/− platelets were defective in GPIb-IX–induced actin polymerization after full platelet aggregation only at the 10-minute time point, but they responded normally at the early time point (4 minutes) during the first wave of agglutination/aggregation (Figure 5A). These data suggest that LIMK1 promotes GPIb-IX–mediated actin polymerization after full platelet aggregation, but not during early VWF/GPIb-IX–dependent agglutination/aggregation. However, LIMK1−/− platelets were neither different from wild-type control platelets in adhesion and spreading on VWF or fibrinogen nor in phalloidin staining of spread platelets (Figure 5B), suggesting that integrin outside-in signaling35 and associated actin polymerization do not require LIMK1 during platelet spreading.
The role of LIMK1 in VWF/GPIb-IX–mediated actin polymerization and in platelet spreading. (A) Flow cytometric analysis of the relative amounts of polymerized actin in WT and LIMK1−/− platelets stimulated with 1 µg/mL botrocetin in the presence or absence of 6.4 µg/mL VWF in an aggregometer at 37°C for 4 and 10 minutes. The ratio between the mean fluorescence intensity values of stimulated platelets versus the baseline phalloidin binding in control platelets is shown. Statistical significance was determined by one-way analysis of variance (*P < .05). (B) Alexafluor-546–labeled phalloidin staining of polymerized actin in WT and LIMK1−/− platelets spread on VWF (in the presence of 2 µg/mL botrocetin) or fibrinogen-coated coverslides (90 min at 37°C). The scale bars represent 12.5 µm.
The role of LIMK1 in VWF/GPIb-IX–mediated actin polymerization and in platelet spreading. (A) Flow cytometric analysis of the relative amounts of polymerized actin in WT and LIMK1−/− platelets stimulated with 1 µg/mL botrocetin in the presence or absence of 6.4 µg/mL VWF in an aggregometer at 37°C for 4 and 10 minutes. The ratio between the mean fluorescence intensity values of stimulated platelets versus the baseline phalloidin binding in control platelets is shown. Statistical significance was determined by one-way analysis of variance (*P < .05). (B) Alexafluor-546–labeled phalloidin staining of polymerized actin in WT and LIMK1−/− platelets spread on VWF (in the presence of 2 µg/mL botrocetin) or fibrinogen-coated coverslides (90 min at 37°C). The scale bars represent 12.5 µm.
Actin polymerization is not required for GPIb-IX–dependent regulation of TXA2 production
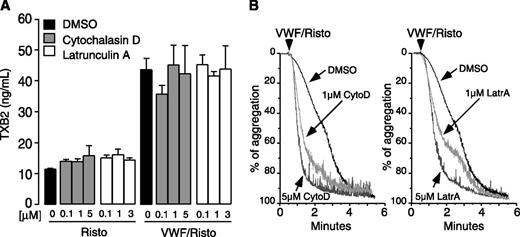
To determine whether the role of LIMK1 in stimulating GPIb-IX–induced TXA2 production and platelet activation is consequential to its function in promoting actin polymerization, we tested the effect of actin polymerization inhibitors on GPIb-IX–induced TXA2 production and platelet aggregation. Two well-known inhibitors of actin polymerization, cytochalasin D and latrunculin A, were used at concentrations known to inhibit actin polymerization. Neither had any effect on VWF/GPIb-IX–induced TXA2 production (Figure 6A), suggesting that GPIb-IX mediated TXA2 production does not require actin polymerization. Interestingly, cytochalasin D and latrunculin A did not inhibit, but rather accelerated, VWF-induced platelet aggregation (Figure 6B). Therefore, LIMK1 promotes GPIb-IX–mediated TXA2 synthesis and platelet activation via a mechanism that is independent of actin polymerization.
Actin polymerization inhibitors have no effect on VWF-induced TXB2 production but enhance platelet aggregation response to VWF. Human platelets, preincubated with the indicated doses of cytochalasin D or latrunculin A, were stimulated with 10 µg/mL VWF with or without 0.35 mg/mL ristocetin or unstimulated at 37°C for 6 minutes in the aggregometer. (A) Samples were analyzed for TXB2 and (B) aggregation traces were recorded.
Actin polymerization inhibitors have no effect on VWF-induced TXB2 production but enhance platelet aggregation response to VWF. Human platelets, preincubated with the indicated doses of cytochalasin D or latrunculin A, were stimulated with 10 µg/mL VWF with or without 0.35 mg/mL ristocetin or unstimulated at 37°C for 6 minutes in the aggregometer. (A) Samples were analyzed for TXB2 and (B) aggregation traces were recorded.
LIMK1 is important for phospholipase A2 activation during GPIb-IX signaling and is regulated by GPIb-IX–induced MAPK activity
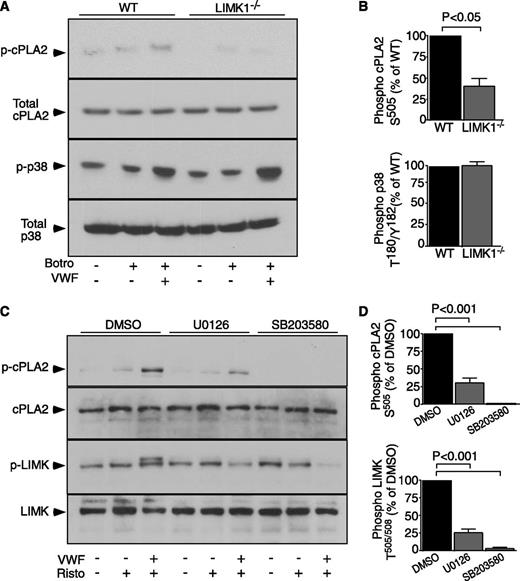
It is established that cytosolic phospholipase A2 (cPLA2) is a rate-limiting enzyme in TXA2 synthesis and that cPLA2 becomes activated upon phosphorylation at Ser505.36 Thus, we assessed whether LIMK1 regulates cPLA2 phosphorylation during botrocetin/VWF-induced platelet activation. cPLA2 phosphorylation was markedly reduced in LIMK1−/− platelets compared with wild-type (Figure 7A-B), indicating that LIMK1 plays an important role in promoting the activation of cPLA2 during GPIb-dependent platelet activation.
GPIb-IX–mediated cPLA2 activation in WT and LIMK1−/− platelets, and the effect of MAPK inhibitors on LIMK activation. (A) Immunoblot analysis of WT and LIMK1−/− platelets, stimulated in the presence or absence of 5 µg/mL VWF, 2 µg/mL botrocetin, or both, with antibodies recognizing phosphorylated cPLA2 Ser505(S505), total cPLA2, phosphorylated p38 Thr180/Tyr182(T180/Y182), or total p38. (B) Quantitative data from 4 experiments depicted in (A) using ImageJ (uncalibrated OD, mean ± SE). (C) Human platelets, preincubated with 25 μM SB203580, 3.5 μM U0126, or DMSO for 2 minutes, were stimulated with VWF (10 µg/mL) in the absence or presence of ristocetin (0.25 mg/mL) or stirred without stimulation in a platelet aggregometer at 37°C for 6 minutes. Lysates from these platelets were immunoblotted with antibodies against phosphorylated LIMK Thr505/508 or against phosphorylated cPLA2 Ser505, and also with antibodies against LIMK1 and cPLA2 as loading controls. (D) Quantitative data for (C) expressed as a percentage of DMSO control.
GPIb-IX–mediated cPLA2 activation in WT and LIMK1−/− platelets, and the effect of MAPK inhibitors on LIMK activation. (A) Immunoblot analysis of WT and LIMK1−/− platelets, stimulated in the presence or absence of 5 µg/mL VWF, 2 µg/mL botrocetin, or both, with antibodies recognizing phosphorylated cPLA2 Ser505(S505), total cPLA2, phosphorylated p38 Thr180/Tyr182(T180/Y182), or total p38. (B) Quantitative data from 4 experiments depicted in (A) using ImageJ (uncalibrated OD, mean ± SE). (C) Human platelets, preincubated with 25 μM SB203580, 3.5 μM U0126, or DMSO for 2 minutes, were stimulated with VWF (10 µg/mL) in the absence or presence of ristocetin (0.25 mg/mL) or stirred without stimulation in a platelet aggregometer at 37°C for 6 minutes. Lysates from these platelets were immunoblotted with antibodies against phosphorylated LIMK Thr505/508 or against phosphorylated cPLA2 Ser505, and also with antibodies against LIMK1 and cPLA2 as loading controls. (D) Quantitative data for (C) expressed as a percentage of DMSO control.
We have previously reported the importance of the MAPKs, p38 and ERK, in GPIb-IX–induced platelet activation.17,31 In addition, p38 and ERK are also known to stimulate cPLA2 activation by phosphorylating Ser505.36 To determine the relationship between MAPKs and LIMK1, we tested the effect of a p38 inhibitor, SB203580, and a MEK inhibitor, U0126, on LIMK activation. Both inhibitors abolished VWF/ristocetin-induced LIMK phoshorylation (Figure 7C-D). Conversely, GPIb-IX–induced p38 phosphorylation was not affected in LIMK1−/− platelets (Figure 7A-B). These data indicate that LIMK1 is activated downstream from p38 and ERK MAPKs during GPIb-IX–dependent platelet activation, and that LIMK1 likely promotes GPIb-IX–induced TXA2 synthesis by stimulating the activation of cPLA2.
Discussion
Antiplatelet drugs have been extensively used clinically to treat and prevent thrombosis, particularly arterial thrombosis. A major adverse effect of currently used antiplatelet drugs is bleeding. This is because the targets of current antiplatelet drugs, such as cyclooxygenase, ADP receptors, and integrins, are important for both hemostasis and thrombosis. It would be ideal if we could identify novel molecular targets for developing antithrombotics without significant bleeding side effects. In this study, we demonstrate that LIMK1 plays an important role in promoting arterial thrombosis but appears to be dispensable for hemostasis, as suggested by mouse tail bleeding time. This selective role in arterial thrombosis is associated with the novel function of LIMK1 to selectively promote GPIb-IX–dependent platelet activation and stable platelet adhesion under high shear flow conditions, while having no effect on or negatively regulating GPIb-IX–independent platelet activation pathways. Furthermore, LIMK1 mediates GPIb-IX–dependent cPLA2 activation and TXA2 synthesis, which serve as a secondary amplification signaling pathway important in occlusive arterial thrombus formation, but not in the initial GPIb-IX–mediated integrin activation and platelet adhesion. Thus, although GPIb-IX–mediated initial platelet adhesion and activation is important in both thrombosis and hemostasis, LIMK1 plays a selective role in occlusive arterial thrombosis without significantly affecting bleeding. It is important to note that LIMK1 knockout selectively affected GPIb-IX–dependent TXA2 production and signal amplification but had no negative effect on TXA2 production and platelet responses induced by GPIb-IX–independent platelet agonists. This feature is distinct from the effects of cyclooxygenase inhibitors (such as aspirin37-39 ) and TXA2 receptor knockout,40 which affect the TXA2 pathway induced by all platelet agonists and thus are important in both thrombosis and hemostasis. Our study therefore not only reveals a new mechanism for GPIb-IX signaling and a novel function of LIMK1 but also a potential molecular target for the development of an antithrombotic with minimal bleeding side effect, although it is important to further investigate the role of LIMK1 under various hemostatic and thrombotic conditions.
Our data provide the first direct evidence of an important role for LIMK1 in platelet activation. GPIb-IX–mediated platelet activation consists of 2 components: (1) the GPIb-IX–mediated early signaling leading to integrin activation and primary platelet response and (2) the secondary amplification pathways, mediated mainly by TXA2 and TXA2-dependent ADP secretion. Data from our laboratory and others indicate that GPIb-IX–mediated early integrin activation involves the sequential activation of the Src family kinase, Lyn,11,12 Vav/Rac1,13 PI3K,14 Akt,15 cGMP-dependent protein kinase,16 and MAPK pathways.17,31 However, LIMK1 does not appear to have a role in the initial integrin activation pathway because LIMK1−/− platelets show no defect in platelet spreading on VWF under static conditions, a process that requires GPIb-IX–dependent integrin activation, and because LIMK1−/− platelets show only a partial defect in platelet adhesion under flow similar to aspirin-treated platelets. We conclude that LIMK1 mediates GPIb-IX signaling mainly by stimulating GPIb-IX–dependent TXA2 synthesis and consequent TXA2-dependent platelet granule secretion. This conclusion is supported by data showing that GPIb-IX–dependent TXA2 synthesis was abolished in LIMK1−/− platelets, and that supplementation with the TXA2 analog, U46619, rescued the adhesion defect of LIMK1−/− platelets. Furthermore, LIMK1−/− platelets showed reduced VWF/GPIb-IX-mediated cPLA2 activation, suggesting that LIMK1 stimulates VWF-induced TXA2 synthesis by activating cPLA2. It is still unclear how LIMK1 stimulates cPLA2 activation. Previous studies suggest that ERK and p38 MAPKs can mediate cPLA2 activation.36,41 Our studies showed that both p38 and ERK are activated during GPIb-IX signaling and are important in GPIb-IX–mediated platelet activation.17,31 Interestingly, we show that LIMK is activated downstream from the MAPK signaling pathways. Thus, it will be interesting to further investigate how MAPK-dependent activation of cPLA2 is regulated by LIMK1 during GPIb-IX signaling.
To date, the major recognized function of LIMK1 is to phosphorylate and deactivate the cofilin family of actin depolymerizing factors, and thus promote actin polymerization.3,5 In agreement with this function, we observed a significant reduction in GPIb-IX–mediated actin polymerization in LIMK1−/− platelets after VWF-induced platelet aggregation. However, the role of LIMK1 in promoting actin polymerization is unlikely to be responsible for its role in selectively stimulating GPIb-IX–mediated TXA2 synthesis and consequent amplification of platelet activation, because both actin depolymerizing agents and LIMK1−/− platelets attenuate actin polymerization, but only knockout of LIMK1 affects GPIb-induced TXA2 synthesis. Moreover, actin polymerization inhibitors enhance VWF-induced platelet aggregation, as previously reported,42 which is opposite to the inhibitory effect of LIMK1 knockout. Finally, cofilin phosphorylation is induced by GPIb-IX–independent and –dependent agonists, yet deficiency in LIMK1 only inhibited VWF-induced platelet activation. Therefore, our results reveal a novel function of LIMK1 in stimulating GPIb-IX–mediated cPLA2 activation and TXA2 synthesis independent of its role in stimulating actin polymerization. Nevertheless, we do not exclude the possibility that LIMK1-mediated phosphorylation of cofilin and regulation of actin polymerization may also play a role in GPIb-IX–mediated platelet adhesion or in the regulation of platelet function independent of TXA2 synthesis, because LIMK1−/− platelets showed a further moderate decrease in platelet adhesion relative to aspirin-treated platelets. In addition, cofilin is phosphorylated during GPIb-IX–dependent activation and it was reported previously that actin depolymerization reduced the stability of platelet adhesion under shear stress.43
Overall, our data demonstrate a novel role for LIMK1 in selectively amplifying GPIb-IX–dependent platelet adhesion and activation by mediating GPIb-IX–induced cPLA2 activation and TXA2 synthesis, but not in other agonist receptor signaling pathways. Importantly, the selective role of LIMK1 in thrombosis, but not in hemostasis, suggests a potential new strategy for antithrombotic development.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Dr Tatyana A. Voyno-Yasenetskaya for collaboration on this study.
This work was supported by grants from the National Institutes of Health/National Heart, Lung and Blood Institute (HL062350, HL068819, HL080264) (X.D.).
Authorship
Contribution: B.E., A.S.-T., and M.K.D. performed experiments, created figures, analyzed results, and helped write the paper; K.A.O. performed experiments and helped edit the paper; M.C.B. and C.R. provided valuable suggestions, reagents, and manuscript editing; and X.D. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiaoping Du, Department of Pharmacology, University of Illinois College of Medicine, 835 S. Wolcott Ave, Chicago IL 60612; e-mail: xdu@uic.edu.
References
Author notes
B.E. and A.S.-T. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal