Key Points
Tolerance induction after in utero hematopoietic cell transplantation involves both direct and indirect antigen presentation.
Tolerance is achieved by deletion of effector T cells, which results in Treg enrichment without de novo Treg induction.
Abstract
In utero hematopoietic cell transplantation (IUHCTx) is a promising method to induce donor-specific tolerance but the mechanisms of antigen presentation that educate host T cells and the relative importance of deletion vs regulation in this setting are unknown. We studied the roles of direct and indirect antigen presentation (mediated by donor- and host-derived antigen-presenting cells [APCs], respectively) in a mouse model of IUHCTx. We found that IUHCTx leads to precocious maturation of neonatal host dendritic cells (DCs) and that there is early differentiation of donor-derived DCs, even after transplantation of a stem cell source without mature APCs. We next performed allogeneic IUHCTx into donor-specific T-cell receptor transgenic mice and confirmed that both direct and indirect antigen presentation lead to clonal deletion of effector T cells in chimeras. Deletion did not persist when chimerism was lost. Importantly, although the percentage of regulatory T cells (Tregs) after IUHCTx increased, there was no expansion in Treg numbers. In wild-type mice, there was a similar deletion of effector cells without expansion of donor-specific Tregs. Thus, tolerance induction after IUHCTx depends on both direct and indirect antigen presentation and is secondary to thymic deletion, without de novo Treg induction.
Introduction
Achieving donor-specific tolerance after hematopoietic stem cell (HSC) transplantation would minimize the need for chronic immunosuppression and expand the potential applications for HSC transplantation. In utero hematopoietic cell transplantation (IUHCTx) is a promising strategy to induce immune tolerance in the fetus without the inherent toxicities of preconditioning required for postnatal bone marrow transplantation (BMT). The therapeutic rationale for this approach is based on numerous advantages that the fetal environment may offer to support the proliferation and homing of allogeneic donor cells.1,2 In addition, the fetal environment may be inherently programmed to produce regulatory T cells (Tregs) instead of effector T cells (Teffs) upon exposure to alloantigens in utero.3 In mice, there is excellent engraftment of fully allogeneic HSCs in fetal recipients,4,5 with donor-specific tolerance in chimeras.6-8 In fact, it has been shown that the fetal immune response is not a barrier to engraftment as long as the maternal immune response is controlled.7,8
Ideally, the introduction of a new antigen in the fetal environment should recapitulate multiple mechanisms of self-tolerance and lead to durable engraftment. The mechanisms by which HSC transplantation leads to donor-specific tolerance have been studied extensively in the setting of postnatal BMT. Central thymic deletion and anergy of alloreactive T cells is the dominant mechanism for maintaining tolerance after BMT.9-12 Tregs, however, are likely not critical to engraftment after postnatal BMT, except when central deletion is incomplete.13 Unlike the setting of postnatal BMT, the mechanisms of tolerance after IUHCTx have not been fully elucidated. Successful engraftment after IUHCTx in mice leads to antigen-specific tolerance to the donor strain via deletion and anergy.6-8 Natural killer (NK) cell–mediated tolerance has also been described.14 However, although chimeric animals reportedly have donor-specific Tregs,7,15 whether these cells are critical for establishing or maintaining chimerism remains an open question.
Although neonatal antigen-presenting cells (APCs) are relatively immature,16 neonatal mice are capable of mounting effector T-cell responses to alloantigens in the right environment.17 Thus, defining host T-cell education and tolerance mechanisms after IUHCTx requires an understanding of the modes of antigen presentation in this setting. Host T-cell recognition of the allograft after transplantation can occur via direct antigen presentation, in which donor antigen is presented by donor APCs, or indirect antigen presentation, in which donor major histocompatibility complex (MHC) peptides are presented by recipient APCs. The respective roles and kinetics of direct and indirect antigen presentation have been well described in the setting of solid organ transplantation. Because donor-derived APCs are present in a solid organ graft, direct antigen presentation is the predominant pathway of allorecognition and early graft loss.18 The role of the direct pathway diminishes over time,19-22 and the indirect pathway has been associated with late allograft dysfunction,23-25 likely because recipient dendritic cells (DCs) constantly travel through the allograft and can present alloantigens throughout the life of the allograft.26 However, the kinetics of each of these pathways in the context of hematopoietic cell transplantation (which has the added complexity of APC differentiation from the graft itself) has not been studied. Because the frequency of directly reactive T cells is significantly higher than that of indirectly reactive T cells,18 whether transplanted hematopoietic cells can be recognized using the direct pathway early after transplantation is an important clinical question.
We investigated the fetal immune response to IUHCTx and the mechanisms that lead host T-cell education by using complementary approaches in wild-type and T-cell receptor transgenic (TCR-Tg) mice in which there is a high precursor frequency of donor-reactive T cells. We found that both direct and indirect antigen presentation are active after in utero transplantation and that thymic deletion, with resultant Treg enrichment, is the main mechanism of tolerance.
Materials and methods
Antibodies and flow cytometry
The following antibodies and reagents for flow cytometry were purchased from Becton Dickinson: CD3 (145-2C11), CD4 (RM4-5, GK1.5), CD8 (53-6.7), CD25 (PC61), CD40 (3/23), CD45R/B220 (RA3-6B2), CD80 (16-10A1), CD90.1 (HIS51), H-2Kb (AF6-88.5), H-2Kd (SF1-1.1), Vβ8 (F23.1), Vβ13 (MR12-3); eBioscience: CD4 (RM4-5), CD8 (53-6.7), CD11c (N418), CD86 (GL1), CD90.1 (HIS51), FoxP3 (FJK-16s), FoxP3 staining buffer set; Pharmingen: I-Ad (AMS-32.1), I-Ab (AF6-120.1); and Invitrogen: Qdot605 Streptavidin conjugate, 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI), LIVE/DEAD Cell Viability Dye (Invitrogen). Class II (N22) was a gift from Dr Max Krummel (University of California, San Francisco [UCSF]).
Mice
BALB/c and C57BL/6 (B6) mice were obtained from either the National Cancer Institute (NCI) or The Jackson Laboratory. TCR-Tg B6.Thy1.1.4C27 (B6 mice with CD4 T cells recognizing I-Ad presented on BALB/c MHC class II, direct pathway, “4C”) or B6.Thy1.1.TCR75 males28 (B6 mice with CD4 T cells recognizing H-2Kd presented on B6 MHC class II, indirect pathway, “TCR75”) were obtained from Dr Sang-Mo Kang (UCSF). All mouse experiments were performed according to the UCSF Institutional Animal Care and Use Committee–approved protocol.
In utero hematopoietic cell transplantation and determination of chimerism
Chimeras were generated by transplanting allogeneic fetal liver mononuclear cells (FLMCs) into recipient fetuses at embryonic days (E) 13.5 to 14.5 as previously described.8,29 Peripheral blood chimerism levels were determined at the time of harvest by flow cytometry after staining with antibodies against CD45, H-2Kb (B6), and H-2Kd (BALB/c). Animals with >1% circulating donor lymphocytes were considered chimeric.
Flow cytometric analysis to determine APC maturation
Lymphocytes were isolated from recipient mice 5 weeks after IUHCTx. Single-cell suspensions were prepared by mechanical dissociation of lymph nodes or collagenase and DNase digestion of spleen. The expression of maturation markers on APCs was analyzed using the following antibodies: B220, CD11c, class I (H-2Kb or H-2Kd), class II, B7-1, B7-2, and CD40. To account for experimental variation, the mean fluorescent intensity (MFI) of each costimulatory marker (except class I) on B cells (CD11clowB220Hi), plasmacytoid DCs (pDCs; B220HiCD11cdim), and conventional DCs (cDCs; CD11cHiB220low) was calculated relative to the MFI of CD11cnegB220neg lymphocytes. For experiments interrogating cross-presentation of donor antigen by host APCs, lymphocytes were stained with the following antibodies: CD45, B220, CD11c, Gr-1, class I (H-2Kb or H-2Kd), class II (I-Ab or I-Ad).
Thymic deletion and Treg induction in TCR-Tg mice
B6 mothers were bred to either 4C or TCR75 males and the resulting fetuses were transplanted with BALB/c FLMCs on E14.5. The genotype of the pups was determined by flow cytometric analysis of CD4, CD8, and Vβ13 (4C) and Vβ8 (TCR75). Deletion of alloreactive T cells was quantified by dividing the number of transgene Vβ8 or Vβ13 CD4 T cells by either CD4+CD8+ double-positive (DP) cells (thymus) or CD8+ T cells (spleen) after first gating on Thy 1.1.
In vivo mixed lymphocyte reaction
Donor-specific Teff and Tregs were detected by using a modified in vivo mixed lymphocyte reaction (MLR).8,30 Lymphocytes from spleens and peripheral lymph nodes from chimeric (>1%) and nonchimeric (<0.1%) mice were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE). Cells (25-50 × 106) were injected into secondary B6 × BALB/c F1 mice. For mice at the 2-week time point, 2 to 3 chimeras or nonchimeras were pooled to obtain enough cells. Recipient splenic lymphocytes were analyzed at 60 to 72 hours. The proliferation of CD4 cells (both Foxp3− and Foxp3+ fractions) was analyzed on an LSRII flow cytometer (BD).
Statistics
Comparisons between 2 groups were evaluated using either the χ2 test or the Student t test, and those involving multiple groups were evaluated using analysis of variance with the Tukey multiple comparison test. Correlations were performed using the Pearson correlation test. A P value < .05 was considered to be significant. Data represents mean ± SEM.
Results
Maturation of APCs in chimeras
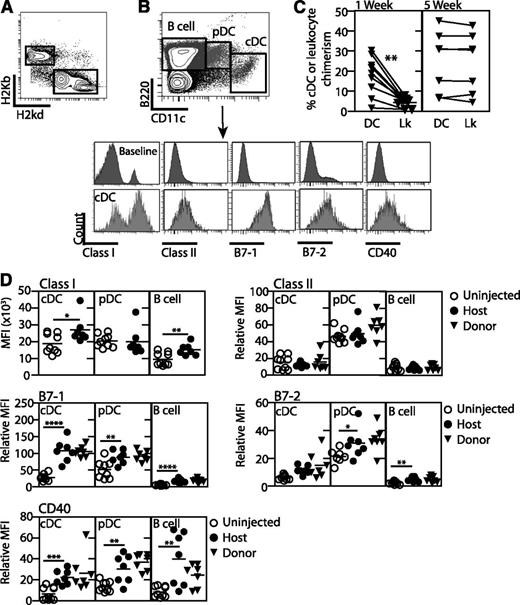
In our mouse model of IUHCTx, transplantation of allogeneic fetal liver cells leads to long-term, multilineage engraftment with donor-specific tolerance.8 We have phenotyped the donor FLMCs for the presence of APCs and determined that this stem cell source contains very few cDCs (supplemental Figure 1, available on the Blood website). To understand the relative timing of direct and indirect antigen presentation after IUHCTx, we next studied the differentiation and maturation of host and donor-derived APCs in animals that received transplants. We created chimeric mice by transplanting B6 fetal liver cells into E14.5 BALB/c fetuses, and, using flow cytometry, first determined whether donor-derived cDCs are present early after transplantation to participate in direct antigen presentation (Figure 1A-B). We detected donor-derived cDCs in spleens of these mice as early as 1 week after transplantation (Figure 1B-C). Strikingly, the cDC-specific chimerism (donor cDC/(donor + host cDC)) was higher than the total peripheral blood leukocyte chimerism (donor CD45+ cells/(donor + host CD45+ cells)) 1 week after IUHCTx (Figure 1C). Thus, as T cells are developing in neonatal chimeras, donor-derived APCs are already present to participate in direct antigen presentation. Five weeks after IUHCTx, however, the splenic cDC chimerism resembled the peripheral blood chimerism (Figure 1C). These findings indicate that donor-derived DCs differentiate rapidly, even when using a stem cell source with immature DCs, and may be available to participate in direct antigen presentation.
Allogeneic IUHCTx leads to maturation of host and donor-derived APCs. BALB/c fetuses were transplanted with B6 fetal liver hematopoietic cells and analyzed at 1 and 5 weeks after transplantation. (A) Detection of donor cell cells among host cells in peripheral blood after first gating on CD45+ leukocytes. Chimerism was calculated as donor-derived (H-2Kb) CD45+ cells/total CD45+ cells. (B) Gating strategy to detect host and donor-derived APCs. cDCs (CD11cHiB220Low), pDCs (CD11cdimB220Hi), and B cells (CD11c−B220Hi) were identified within the H-2Kd (host) or H-2Kb (donor) populations. (C) Comparison of splenic cDCs and circulating leukocyte chimerism (Lk) at 1 and 5 weeks. cDC chimerism was calculated by dividing the number of donor-derived cDCs by the total cDCs in the spleen of engrafted mice. (D) Relative MFI of class II, B7-1, B7-2, and CD40 on host- and donor-derived APCs in spleens of naive and chimeric animals was calculated relative to a baseline population (CD11c−B220−) to account for experimental variability. Uninjected, n = 9; chimera, n = 7. P values: *<.05, **<.01, ***<.001, ****<.0001 by Student t test.
Allogeneic IUHCTx leads to maturation of host and donor-derived APCs. BALB/c fetuses were transplanted with B6 fetal liver hematopoietic cells and analyzed at 1 and 5 weeks after transplantation. (A) Detection of donor cell cells among host cells in peripheral blood after first gating on CD45+ leukocytes. Chimerism was calculated as donor-derived (H-2Kb) CD45+ cells/total CD45+ cells. (B) Gating strategy to detect host and donor-derived APCs. cDCs (CD11cHiB220Low), pDCs (CD11cdimB220Hi), and B cells (CD11c−B220Hi) were identified within the H-2Kd (host) or H-2Kb (donor) populations. (C) Comparison of splenic cDCs and circulating leukocyte chimerism (Lk) at 1 and 5 weeks. cDC chimerism was calculated by dividing the number of donor-derived cDCs by the total cDCs in the spleen of engrafted mice. (D) Relative MFI of class II, B7-1, B7-2, and CD40 on host- and donor-derived APCs in spleens of naive and chimeric animals was calculated relative to a baseline population (CD11c−B220−) to account for experimental variability. Uninjected, n = 9; chimera, n = 7. P values: *<.05, **<.01, ***<.001, ****<.0001 by Student t test.
We next used flow cytometry to examine the maturation of host and donor-derived APCs at 5 weeks after transplantation, a time point when chimeric animals have become tolerant to donor antigen.7 We examined the expression of class I, class II, B7-1, B7-2, and CD40 on host and donor-derived cDCs (CD11chiB220low), pDCs (CD11cdimB220hi), and B cells (CD11clowB220hi) (Figure 1D). Analysis of host DC populations revealed significantly higher levels of class I, B7-1, and CD40 on cDCs, and significantly higher levels of B7-1, B7-2, and CD40 on pDCs and B cells in chimeras than in naive mice. These results demonstrate that IUHCTx leads to precocious maturation of host DCs (Figure 1D). Interestingly, the maturation of APCs from injected nonchimeras was indistinguishable from host APCs in chimeras, suggesting that antigen exposure without successful engraftment is sufficient to induce this maturation (supplemental Figure 2). The levels of all costimulatory molecules were equivalent on donor and host-derived APCs, indicating that both populations are mature and may be functional in antigen presentation 5 weeks after in utero transplantation (Figure 1D). Collectively, our results indicate that host T-cell recognition of the graft (with resultant tolerance induction) likely involves both direct and indirect modes of antigen presentation.
Host APCs may pick up intact donor MHC molecule-donor peptide complexes to stimulate directly reactive T cells, a pathway called “semidirect” presentation.31 To determine whether this mode of antigen presentation is active early after transplantation, we next analyzed the expression of donor-derived class II antigens on host APCs (supplemental Figure 3A). We did not observe expression of donor class II antigens on host B cells, cDCs, or pDCs in any lymphoid organ in chimeric animals. However, we cannot rule out the possibility that there is a small population of cells that coexpress both class I and class II markers. Importantly, we also observed some donor-derived cDCs in the thymus of transplanted animals at 2 weeks after transplantation (supplemental Figure 3B), indicating that donor-derived APCs could participate in direct antigen presentation with thymic deletion in this model.
Early induction of an immune response by indirect antigen presentation leads to in utero and neonatal demise after IUHCTx
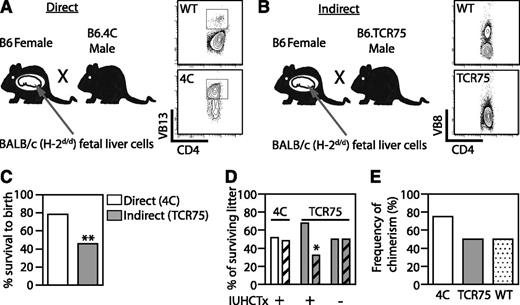
Using wild-type mice to understand host T-cell recognition of the graft entails several limitations. Allospecific T cells constitute a small percentage of the pool in a wild-type mouse and only an estimated 1% to 5% of alloreactive T cells respond to antigen via the indirect pathway.18 Although we previously showed, using an in vivo MLR, that allospecific T cells are decreased,8 this system does not allow us to explore whether direct or indirect antigen presentation is dominant at various times after transplantation, or to study whether antigen-specific Tregs are induced. We therefore used TCR-Tg systems in which host CD4 T cells respond to a BALB/c antigen via direct or indirect presentation and examined deletion and Treg induction in chimeric and nonchimeric animals after IUHCTx. We bred B6 mothers to either 4C males27 or TCR75 males28 and transplanted the resulting fetuses with BALB/c fetal liver cells on E14.5 (Figure 2A-B). 4C are B6 mice with CD4 T cells recognizing I-Ad presented on BALB/c MHC class II (direct pathway), whereas TCR75 are B6 mice with CD4 T cells recognizing H-2Kd presented on B6 MHC class II (indirect pathway). Because the males were heterozygous for the TCR transgene, half of the pups in each litter were expected to be TCR transgenic and half were expected to be wild type. This breeding scheme, in which the mother does not carry the transgene, was chosen to minimize the maternal response to transplanted cells.8 We predicted that because the transplanted fetal liver cells do not contain mature APCs, the indirect pathway would be functional immediately, but that direct presentation would be slightly delayed.
Indirect antigen presentation leads to in utero and neonatal demise after IUHCTx. (A) B6 mothers were bred to either 4C males (B6 mice with CD4 T cells recognizing I-Ad presented on BALB/c MHC class II, direct pathway) or (B) TCR75 males (B6 mice with CD4 T cells recognizing H-2Kd presented on B6 MHC class II, indirect pathway) and transplanted with BALB/c fetal liver hematopoietic cells. Flow cytometric analysis was used to genotype (A) 4C (CD4+Vβ13+) and (B) TCR75 (loss of bimodal distribution of Vβ8) transgenic animals. (C) The overall litter survival rate, including both wt and TCR-Tg pups. 4C, n = 37; TCR75, n = 83 injected. (D) Relative proportion of wt pups (plain bars) and TCR-Tg pups (diagonal bars) within each litter. 4C, n = 29; TCR75 with IUHCTx, n = 28; TCR75 without IUHCTx, n = 38. (E) The rate of chimerism of wt and TCR-Tg animals at 2 to 3 weeks after IUHCTx. TCR75, n = 8; 4C, n = 8; wt littermates, n = 26. P values: *<.05; **<.01 by χ2 test. wt, wild type.
Indirect antigen presentation leads to in utero and neonatal demise after IUHCTx. (A) B6 mothers were bred to either 4C males (B6 mice with CD4 T cells recognizing I-Ad presented on BALB/c MHC class II, direct pathway) or (B) TCR75 males (B6 mice with CD4 T cells recognizing H-2Kd presented on B6 MHC class II, indirect pathway) and transplanted with BALB/c fetal liver hematopoietic cells. Flow cytometric analysis was used to genotype (A) 4C (CD4+Vβ13+) and (B) TCR75 (loss of bimodal distribution of Vβ8) transgenic animals. (C) The overall litter survival rate, including both wt and TCR-Tg pups. 4C, n = 37; TCR75, n = 83 injected. (D) Relative proportion of wt pups (plain bars) and TCR-Tg pups (diagonal bars) within each litter. 4C, n = 29; TCR75 with IUHCTx, n = 28; TCR75 without IUHCTx, n = 38. (E) The rate of chimerism of wt and TCR-Tg animals at 2 to 3 weeks after IUHCTx. TCR75, n = 8; 4C, n = 8; wt littermates, n = 26. P values: *<.05; **<.01 by χ2 test. wt, wild type.
We first noted that survival to birth and survival of neonates after IUHCTx was strikingly lower in TCR75 than in 4C litters, suggesting that early induction of an immune response leads to in utero and neonatal demise in this model (Figure 2C: survival to birth: 4C [78.4%, n = 37] vs TCR75 [45.8%, n = 83], P = .001 by χ2 test). We observed further loss of pups as neonates from the TCR75 litter (10 of 38 live-born pups), but not from the 4C litter. Genotyping of these survivors indicated a preferential loss of TCR-Tg pups compared with their wild-type littermates (Figure 2D, TCR75 32% vs wild-type 68%, n = 28, P = .02 by χ2 test), while uninjected TCR75 litters had the expected 50% TCR75 pups (n = 38). In contrast, transplanted 4C litters demonstrated no neonatal demise and the expected even distribution of transgenic and wild-type pups in the litter (4C, 48.2%, n = 29, Figure 2D). The decreased survival of TCR-Tg pups in the indirect model suggests that the in utero and neonatal demise is secondary to loss of TCR-Tg pups, and that an early, indirect immune response to the transplant may influence survival of the fetus. The frequencies of chimerism in TCR75, 4C, and their wild-type littermates were equivalent (Figure 2E) and similar to what we previously reported for allogeneic transplantation.8
Both direct and indirect antigen presentation lead to thymic deletion of antigen-specific T cells in chimeric animals
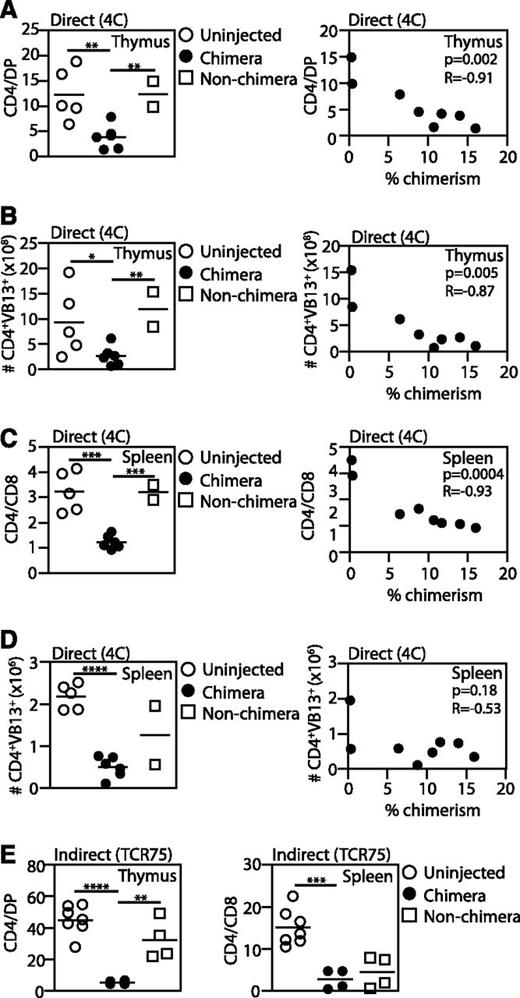
To determine whether successful chimerism leads to deletion of antigen-specific T cells, we harvested lymphoid organs 2 to 3 weeks after IUHCTx in both models and examined the percentage of TCR-Tg CD4 cells. We compared the percentage of Vβ13 (4C) and Vβ8 (TCR75) CD4 T cells (Teff) in chimeric mice to that in nonchimeras and age-matched uninjected controls. Deletion of Teff cells was detected as a decrease in the CD4/DP (thymus) or CD4/CD8 ratios (spleen). Chimerism was defined as >1% donor cells.
Analysis of 4C mice demonstrated a significant reduction in the CD4:DP ratio and the absolute number of Teff cells in chimeras compared with nonchimeras (Figure 3A-B). The observation of effective deletion in all chimeras indicates that 2 weeks is sufficient time for differentiation and thymic migration of fetal liver-derived APCs. There was a strong direct correlation between the level of chimerism and thymic deletion, as demonstrated both by a decrease in the CD4:DP ratio (Figure 3A) and decreased absolute number of Vβ13 Teff cells in the thymus (Figure 3B). In spleens, there was a marked decrease in the percentage and absolute number of Vβ13 CD4 cells in chimeric animals compared with uninjected animals (Figure 3C-D). As in the thymus, chimerism levels were inversely correlated with the CD4:CD8 ratio in the spleen (Figure 3C), whereas the absolute numbers of splenic Teff cells were low regardless of the level of chimerism (Figure 3D).
Both direct and indirect antigen presentation lead to deletion of antigen-specific T cells in engrafted mice. 4C (direct pathway, A-D) or TCR75 (indirect pathway, E) fetuses were transplanted with BALB/c fetal liver cells and the percentage of TCR-Tg CD4 cells was compared among chimeras, injected nonchimeras, and uninjected animals. TCR-Tg cells were identified by staining for CD4 and Vβ13 (4C hosts) or Vβ8 (TCR75 hosts). The proportion of TCR-Tg cells was quantified by either the CD4:DP ratio (thymus) or the CD4:CD8 ratio (spleen). Comparisons were made between (A,C,E) cell percentages or (B,D) absolute cell counts. Pearson correlations were used to compare the percentage of TCR-Tg cells and the percentage of chimerism. 4C: uninjected, n = 5; chimera, n = 6; nonchimera, n = 2. TCR 75: uninjected, n = 7; chimera, n = 4; nonchimera, n = 4. P values: *<.05; **<.01; ***<.001; ****<.0001.
Both direct and indirect antigen presentation lead to deletion of antigen-specific T cells in engrafted mice. 4C (direct pathway, A-D) or TCR75 (indirect pathway, E) fetuses were transplanted with BALB/c fetal liver cells and the percentage of TCR-Tg CD4 cells was compared among chimeras, injected nonchimeras, and uninjected animals. TCR-Tg cells were identified by staining for CD4 and Vβ13 (4C hosts) or Vβ8 (TCR75 hosts). The proportion of TCR-Tg cells was quantified by either the CD4:DP ratio (thymus) or the CD4:CD8 ratio (spleen). Comparisons were made between (A,C,E) cell percentages or (B,D) absolute cell counts. Pearson correlations were used to compare the percentage of TCR-Tg cells and the percentage of chimerism. 4C: uninjected, n = 5; chimera, n = 6; nonchimera, n = 2. TCR 75: uninjected, n = 7; chimera, n = 4; nonchimera, n = 4. P values: *<.05; **<.01; ***<.001; ****<.0001.
In TCR75 mice, CD4 cells were deleted in thymi and spleens of all chimeric animals, indicating the indirect pathway is also involved in central deletion (Figure 3E). Interestingly, deletion was detected in the spleens of nonchimeric animals. This observation suggests that even transient presence of donor antigen immediately after transplantation leads to some deletion, with delayed recovery of T cells in the periphery in all transplanted animals even if long-term chimerism is not established (Figure 3E).
Selective survival of Tregs after IUHCTx
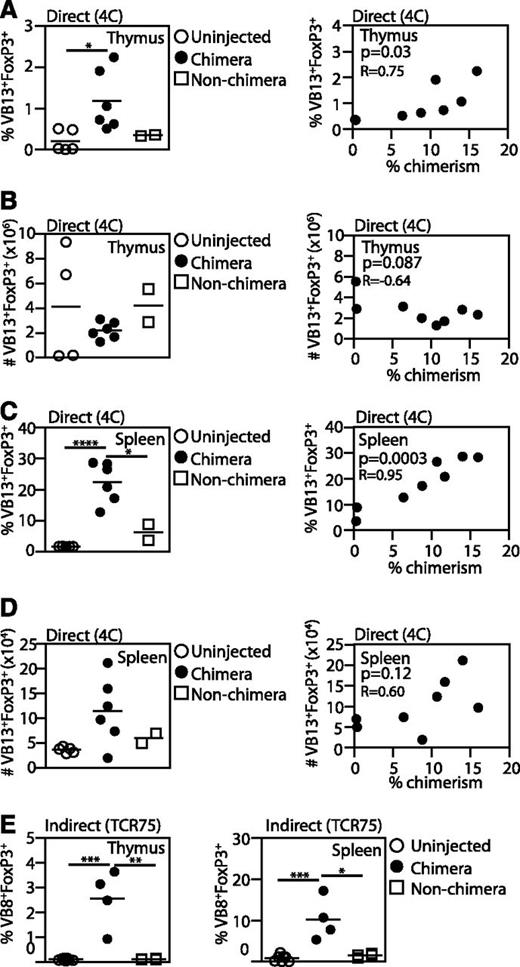
To determine whether antigen exposure in the fetus results in the generation of antigen-specific Tregs, we quantified the percentage of CD4+CD25+Foxp3+ Tregs in the thymus and spleens of injected mice. In 4C animals, successful chimerism led to a striking increase in the percentage of Tregs in both the thymus and spleen (Figure 4A,C). However, despite the increase in the proportion of Tregs, the absolute number of Tregs in the thymus was not increased compared with uninjected animals and injected nonchimeras (Figure 4B). We correlated the levels of chimerism with both the proportion of Tregs and the absolute number of Tregs and found a significant positive correlation with only the proportion of Tregs (Figure 4A-B), indicating that effective deletion of Teffs leads to an increase in percentage of Tregs without generation of new Tregs in the thymus. In the spleen, we found an increase in the proportion of Tregs in chimeras and a positive correlation between Treg percentages and levels of chimerism (Figure 4C). The absolute numbers of Tregs in the spleen was positively but not significantly correlated with chimerism levels (Figure 4D). Our results support the idea that Tregs are more resistant to thymic deletion than Teffs,32 and therefore antigen exposure in utero may result in the deletion of Teffs and an increase in the proportion of Tregs without the generation of new Tregs. However, this increase in the Treg:Teff ratio may still play a role in promoting engraftment.
Enrichment of antigen-specific Tregs in engrafted mice. 4C (direct pathway, A-D) or TCR75 (indirect pathway, E) fetuses were transplanted with BALB/c fetal liver cells and the percentage of TCR-Tg CD4+Foxp3+CD25+ Tregs among TCR-Tg CD4 cells was compared among chimeras, injected nonchimeras, and uninjected animals. TCR-Tg cells were identified by staining for CD4 and Vβ13 (4C hosts) or Vβ8 (TCR75 hosts). Comparisons were made between cell percentages (A,C,E) or absolute cell counts (B,D). Pearson correlations were used to compare the percentage of 4C Tregs and the percentage of chimerism. 4C: uninjected, n = 5; chimera, n = 6; nonchimera, n = 2. TCR 75: uninjected, n = 7; chimera, n = 4; nonchimera, n = 4. P values: *<.05; **<.01; ***<.001; ****<.0001.
Enrichment of antigen-specific Tregs in engrafted mice. 4C (direct pathway, A-D) or TCR75 (indirect pathway, E) fetuses were transplanted with BALB/c fetal liver cells and the percentage of TCR-Tg CD4+Foxp3+CD25+ Tregs among TCR-Tg CD4 cells was compared among chimeras, injected nonchimeras, and uninjected animals. TCR-Tg cells were identified by staining for CD4 and Vβ13 (4C hosts) or Vβ8 (TCR75 hosts). Comparisons were made between cell percentages (A,C,E) or absolute cell counts (B,D). Pearson correlations were used to compare the percentage of 4C Tregs and the percentage of chimerism. 4C: uninjected, n = 5; chimera, n = 6; nonchimera, n = 2. TCR 75: uninjected, n = 7; chimera, n = 4; nonchimera, n = 4. P values: *<.05; **<.01; ***<.001; ****<.0001.
In TCR75 mice, the percentage of Tregs increased in both spleen and thymi of surviving animals, indicating indirect antigen presentation also leads to an increase in the proportion of host Tregs (Figure 4E).
Deletion of Teff cells and selective Treg survival depend on continued chimerism
As expected in a TCR-Tg host with increased frequency of donor-reactive T cells, engraftment was not durable and analysis of 4C mice at 5 weeks demonstrated loss of chimerism in all pups, although donor cells were detected at low levels (0.4% ± 0.1%; n = 6). Accordingly, there was recovery of the CD4:DP ratio in the thymi and of the CD4:CD8 ratio in the spleens of these animals (supplemental Figure 4A). There were no differences in percentages or absolute numbers of Tregs in these animals compared with naive controls (supplemental Figure 4B).
Donor-specific Teffs and Tregs early after IUHCTx in wild-type mice
Given the nonphysiologic nature of T-cell avidity and antigen specificity in TCR-Tg mice, we questioned whether there would be enrichment of donor-specific Tregs in wild-type mice after IUHCTx. An increase in the percentage of peripheral Tregs has been reported in wild-type mice after IUHCTx.7 We also noted that the wild-type littermates of TCR-Tg mice had an increased percentage of Tregs in the spleens, but not thymi, at 2 weeks after transplantation, although absolute numbers were not increased (supplemental Figure 5). To directly measure the allofrequency of Teffs and Tregs in wild-type mice after IUHCTx, we modified the previously published in vivo MLR assay8,30 with a Foxp3 stain. We performed IUHCTx of B6 fetal liver cells into BALB/c fetuses and analyzed the transplanted pups (both chimeric and nonchimeric) at 2 and 3 weeks after IUHCTx using an in vivo MLR (Figure 5A). After determining peripheral blood chimerism (15.7% ± 11.8%; n = 9), we harvested lymphocytes, labeled them with CFSE, and injected them into BALB/c × B6 F1 secondary recipients to quantify donor-specific Teffs and Tregs (Figure 5B). Using this technique, we determined that donor-specific Teffs and Tregs were both present at 2 weeks after IUHCTx (Figure 5C). The percentage of proliferating CD4 cells was significantly lower in chimeric animals than in injected nonchimeras, although the difference was less pronounced than what we have reported for older animals.8 However, the proliferation of Tregs did not differ between chimeric and nonchimeric animals (Figure 5C) and proliferation did not differ with varying levels of peripheral blood chimerism (data not shown). Because proliferating Tregs in this assay should be specific for the donor antigen, these results indicate that donor-specific Tregs did not expand in chimeras, which is consistent with our findings in TCR-Tg mice. Collectively, these results suggest that Tregs have a minimal role, if any, in maintaining chimerism after IUHCTx. However, our results do not rule out the possibility that a shift in the Treg:Teff ratio is important early on in the establishment of engraftment.
Tregs in wild-type chimeras after in utero transplantation. (A) Experimental design and (B) gating strategy to enumerate donor-specific Tregs in chimeric mice. BALB/c fetuses underwent IUHCTx with B6 donor cells. Two to three weeks after IUHCTx, lymphocytes were harvested from chimeras and nonchimeras, labeled with CFSE, and transplanted into F1 (B6 × BALB/c) recipients. Primary host (B6) lymphocytes were identified as CD4+H-2Kb− and proliferating donor-specific CD4 T cells and Tregs (CD4+Foxp3+CFSElow) were (C) quantified in chimeras and nonchimeras. Chimera (n > 5), nonchimera (n > 2). *P < .05 by Student t test.
Tregs in wild-type chimeras after in utero transplantation. (A) Experimental design and (B) gating strategy to enumerate donor-specific Tregs in chimeric mice. BALB/c fetuses underwent IUHCTx with B6 donor cells. Two to three weeks after IUHCTx, lymphocytes were harvested from chimeras and nonchimeras, labeled with CFSE, and transplanted into F1 (B6 × BALB/c) recipients. Primary host (B6) lymphocytes were identified as CD4+H-2Kb− and proliferating donor-specific CD4 T cells and Tregs (CD4+Foxp3+CFSElow) were (C) quantified in chimeras and nonchimeras. Chimera (n > 5), nonchimera (n > 2). *P < .05 by Student t test.
Discussion
We have investigated the fetal immune response to IUHCTx and the mechanisms that lead to tolerance by using complementary approaches in wild-type and TCR-Tg mice. We found that IUHCTx leads to precocious maturation of neonatal host DCs and to early differentiation of donor-derived DCs, such that both host and donor APCs are available to participate in antigen presentation after IUHCTx. Our experiments in TCR-Tg mice confirm this finding and demonstrate that both mechanisms of antigen presentation lead to clonal deletion, with a resultant increase in the Treg:Teff ratio.
We have defined the relative timing of direct vs indirect antigen presentation in our model of fetal liver–derived hematopoietic cell transplantation. Because there are few DCs in the donor cell population, the indirect pathway is predominant immediately after transplantation. In TCR75 mice, this immediate T-cell response (which occurs when the animals are still in utero) likely resulted in the high rate of pregnancy loss we observed. However, donor-derived DCs differentiate and acquire maturation markers rapidly, and lead to deletion of directly reactive T cells in the thymus within 2 weeks after transplantation. For this pathway to be functional, the donor hematopoietic population must give rise to DCs and must be able to home to the thymus, which we have demonstrated. Although we did not observe appreciable evidence of semi-direct presentation31 of class II antigens early after in utero transplantation, it is possible that this pathway may become relevant in older mice or other animal models.
Our results indicate that the timing and relative importance of each pathway will depend on the stem cell source being used. For example, in adult BMT, there are mature DCs in the graft and DC chimerism is present early after transplantation,33 such that directly reactive T cells may recognize the graft soon after transplantation. However, if CD34+ HSCs are transplanted, there would be fewer DCs immediately after presentation, such that direct antigen presentation would be delayed until after DCs differentiate from the graft. Alternatively, if umbilical cord blood is transplanted, the DCs are immature and Th1 responses may be curtailed.34 Thus, the relative importance of each pathway must be determined empirically for newer donor cell populations such as embryonic stem cell–derived HSCs or stem cells which do not give rise to APCs.
We used both TCR-Tg and wild-type mice to probe the role of Tregs in engraftment and did not find a robust expansion of donor-specific Tregs in either model. These results support previously published findings that Tregs have a selective advantage in survival in the thymus in a TCR-Tg setting,32 and that the differentiation and generation of Tregs may not be triggered by the same cues that determine clonal deletion.32 It is possible that antigen-specific Tregs may undergo peripheral expansion in some settings, such as those with limited thymic deletion.35 It is also possible that Treg induction is important at very early time points, until thymic deletion is complete.
We have previously reported the use of a >1% threshold of chimerism in our analysis of chimerism of wild-type animals after IUHCTx.8 Our experiments confirm the validity of this threshold: although we could detect low levels of chimerism (<1%) in several 4C recipients, there was no deletion in these animals. We suggest that a possible biological explanation for this finding is that low-level chimeras do not have the required amount of thymic or peripheral antigen presentation to allow for adequate deletion of directly reactive T cells, which comprise the bulk of donor-specific T cells in wild-type mice.18 In addition to T-cell and DC interactions, other mechanisms such as NK-cell tolerance likely contribute to this threshold.14
The surprisingly high rate of fetal loss after transplantation of alloantigen into TCR75 fetuses suggests that there is a particularly robust early indirect response in these animals. Because T cells mature earlier in TCR-Tg animals, they may be present early after transplantation to induce an immune response, until thymic antigen presentation and tolerance may be established. Such an effect is likely not detected in 4C mice because of the slight lag in direct antigen presentation until donor-derived DCs differentiate and move into the thymus. In wild-type mice, an early response by indirectly reactive T cells should not hinder survival because the frequency of indirectly alloreactive T cells is much lower than that of directly reactive cells18 and their maturation is later than in TCR-Tg mice.36 We have also detected maternal CD11c+ cells in fetal blood8 and it is possible that trafficking maternal APCs could assist in antigen presentation, rendering the indirect pathway more effective. Induction of an innate immune response in the fetus has been associated with preterm labor37 but the finding that fetal T-cell activation leads to fetal demise is novel. Thus, our results may point to a unique mechanism for pregnancy complications after fetal intervention.
In conclusion, the remarkable ability to establish chimerism after IUHCTx in TCR-Tg mice supports the strategy of using the fetal environment to establish donor-specific tolerance, even in a host with restricted antigen specificity. Our results indicate that clonal deletion of alloreactive T cells (with resultant increase in Treg:Teff cell ratio) is the predominant mechanism leading to engraftment after IUHCTx. The respective roles and timing of direct vs indirect antigen presentation should be determined empirically for each donor hematopoietic cell type used in clinical applications.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Tang and Abbas laboratories for helpful discussions, and Dr Sang-Mo Kang for providing us with transgenic mice.
This work was supported by the California Institute for Regenerative Medicine (grants to A.N. and T.C.M.), National Institutes of Health/National Institute of Allergy and Infectious Diseases K08 (T.C.M.), the American College of Surgeons (T.C.M.), the UCSF Liver Center flow cytometry core (NIH P30 DK026743), and the Joslin Diabetes and Endocrinology Research Center flow cytometry core (NIH P30 DK063720).
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of California Institute for Regenerative Medicine or any other agency of the State of California.
Authorship
Contribution: A.N., C.D., T.L., E.J., L.N., and T.C.M. performed experiments; A.N., C.D., Q.T., and T.C.M. designed the research and analyzed results; and A.N., Q.T., and T.C.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tippi C. MacKenzie, Campus Box 0570, University of California, San Francisco, 513 Parnassus Ave, HSW 1601, San Francisco, CA 94143-0570; e-mail: Tippi.Mackenzie@ucsfmedctr.org.