Key Points
Low-count and high-count monoclonal B-cell lymphocytosis (MBL) have distinct immunogenetic signatures, with only the latter resembling CLL.
Rather than a true premalignant condition, low-count MBL may merely reflect immune senescence or result from persistent antigen stimulation.
Abstract
Chronic lymphocytic leukemia (CLL) –like monoclonal B-cell lymphocytosis (MBL) shares common immunophenotype and cytogenetic abnormalities with CLL, from which it is discriminated by a cutoff value of 5 × 109/L circulating clonal B cells. However, the clonal size in MBL is extremely variable and allows discrimination of two distinct entities (high-count [HC] and low-count [LC]-MBL) based on a cutoff value of 0.5 × 109/L clonal B cells. HC-MBL is associated with lymphocytosis and progresses to CLL requiring treatment at a rate of 1.1% per year, whereas LC-MBL is found in the general population only through high-sensitivity techniques and carries limited, if any, risk of progression. We performed an immunogenetic profiling of 333 cases with CLL-like MBL supplemented by detailed comparisons with CLL, focusing especially on CLL Rai stage 0 (CLL-0). LC- and HC-MBL had similar somatic hypermutation status, yet different IGHV gene repertoires and frequencies of B-cell receptor (BcR) stereotypy. In particular, stereotyped BcRs were infrequent in LC-MBL and were often not CLL specific. In contrast, HC-MBL exhibited clear immunogenetic similarities to CLL-0. These findings indicate that LC-MBL may not represent a true preleukemic condition, thus differing from HC-MBL/CLL-0 in which the identification of factors endowing malignant potential is strongly warranted.
Introduction
The existence of monoclonal B-cell expansions in the peripheral blood (PB) of healthy individuals has long been known1-3 but has recently been more clearly appreciated and dissected, thanks to advanced flow cytometry techniques.4-7 This condition falls under the general term “monoclonal B-cell lymphocytosis” (MBL), even though it may not always correspond to an increase in the absolute lymphocyte count. MBL is classified according to the immunophenotype of the clonal population. Thus, the majority of MBL cases (75%) have the immunophenotype of chronic lymphocytic leukemia (CLL; CD5+, CD23+, CD20dim, sIgdim) and are defined as having CLL-like MBL, whereas the remaining MBL cases are classified as atypical CLL-like (CD5+, CD20bright) and CD5− MBL.8,9
CLL-like MBL is reported to be a premalignant state of CLL, analogous to the relationship between monoclonal gammopathy of undetermined significance and multiple myeloma.10 Its frequency ranges between 3% and 12%, depending on the sensitivity of the techniques used, and reaches 17% among CLL family members.5,6,11
The World Health Organization has set a consensus cutoff value of 5 × 109/L clonal B cells in the PB to discriminate what constitutes disease and what does not.12 That notwithstanding, below this limit, the size of the clone in absolute numbers as well as in percentage of circulating B cells is very heterogeneous, and MBL is now usually subdivided into two categories: high-count MBL (HC-MBL, also known as clinical MBL) and low-count MBL (LC-MBL, also called population-screening MBL).8,13 The former is usually associated with lymphocytosis and is recognized in a clinical setting, thus explaining its initial name.14 The HC-MBL cases may progress to CLL requiring treatment at a rate of 1% to 2% per year.14 Conversely, LC-MBL can be detected only by applying highly sensitive cytometric techniques in otherwise healthy individuals13 and has been described as remaining stable over time, with no cases progressing to HC-MBL or CLL within a 34-month follow-up time.15
The jury is still out on what, if any, is the best cutoff value for discriminating MBL into subentities with truly distinct biological and clinical significance. In clinical MBL, the clonal size in the PB has a median value of about 2.9 × 109 B cells/L, and 95% of cases have more than 0.45 × 109 clonal B cells/L,16 whereas in LC-MBL, the median number of clonal B cells is only 0.001 × 109/L.13 For all these reasons, a cutoff value of 0.5 × 109 clonal B cells/L has been proposed for discriminating LC-MBL vs HC-MBL, based on the actual risk of progression.8,13
Thus, overall, the term MBL comprises a mixture of entities with different likelihood of representing a true preleukemic condition, as is also indicated by the clinical data.4,15 This article attempts to clarify the relationship between the different MBL subentities and CLL by analyzing the immunoglobulin (IG) gene repertoire within a cohort of 333 individuals who have CLL-like MBL, both LC (n = 60) and HC (n = 273), from collaborating institutions in Europe and the United States. Comparisons of LC-MBL and HC-MBL to a large cohort of CLL patients and to a subgroup of Rai stage 0 CLL (CLL-0) patients revealed that LC- and HC-MBL have different immunogenetic profiles and, in particular, that HC-MBL resembles CLL-0, whereas LC-MBL is clearly distinct from CLL. On these grounds, we believe that a reappraisal of MBL and its relationship to CLL is strongly warranted and clinically relevant.
Patients and methods
Study group
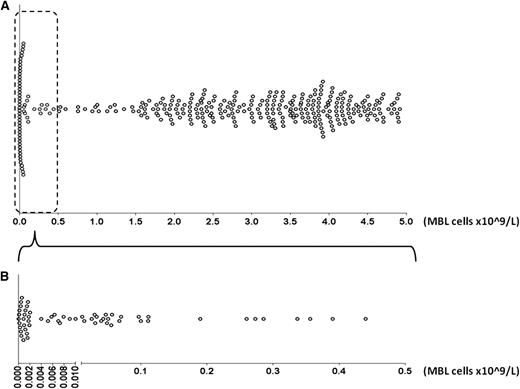
A total of 333 cases diagnosed with CLL-like MBL from collaborating institutions in Europe and the United States were included in this study. All cases met the formal criteria for the diagnosis of MBL established in 2005.9 In particular, CLL-like MBL was defined on the basis of an unbalanced κ/λ ratio (>3:1 or <1:3) within CD19+, CD5bright, CD23+, and CD20dim cells determined by flow cytometry. Within this cohort, 60 cases had a clonal B-cell count of <0.5 × 109/L (LC-MBL), whereas the remaining 273 cases had from 1.5 to <5.0 × 109/L clonal B cells with a CLL-like phenotype (HC-MBL) (Table 1). There were no cases with LC-MBL who had increased T cells or polyclonal B cells that would result in absolute lymphocytosis. The distribution of MBL cases according to clonal size is depicted in Figure 1. The study was approved by the local ethics review committee of each institution and was conducted in accordance with the Declaration of Helsinki.
Characteristics of the MBL cohort regarding absolute and relative clonal size
| MBL cohort charactertistics . | LC-MBL . | HC-MBL . |
|---|---|---|
| MBL cell range (×109/L) | 0-0.5 | 0.5-5.0 |
| Number of cases | 60 | 273 |
| Number of productive rearrangements | 72 | 283 |
| Median B-cell count (×109/L) | 0.14 | 3.52 |
| Median MBL cell count (×109/L) | 0.008 | 3.38 |
| MBL/B-cell percentage (average) | 21% | 93% |
| Median absolute lymphocyte count (×109/L) | 2.17 | 6.0 |
| MBL cohort charactertistics . | LC-MBL . | HC-MBL . |
|---|---|---|
| MBL cell range (×109/L) | 0-0.5 | 0.5-5.0 |
| Number of cases | 60 | 273 |
| Number of productive rearrangements | 72 | 283 |
| Median B-cell count (×109/L) | 0.14 | 3.52 |
| Median MBL cell count (×109/L) | 0.008 | 3.38 |
| MBL/B-cell percentage (average) | 21% | 93% |
| Median absolute lymphocyte count (×109/L) | 2.17 | 6.0 |
Distribution of MBL cases according to clonal size. Each case is represented by a dot. A) All MBL cases are depicted. The cohort is polarized toward carrying either very small clones or clones larger than 1.5 × 109 MBL cells/L. (B) Only LC-MBL cases are depicted. For better visualization, the x-axis is split in two differently scaled segments (<0.005 and ≥0.005 × 109 MBL cells/L). Most LC-MBL patients carry clones consisting of <0.1 × 109 MBL cells/L.
Distribution of MBL cases according to clonal size. Each case is represented by a dot. A) All MBL cases are depicted. The cohort is polarized toward carrying either very small clones or clones larger than 1.5 × 109 MBL cells/L. (B) Only LC-MBL cases are depicted. For better visualization, the x-axis is split in two differently scaled segments (<0.005 and ≥0.005 × 109 MBL cells/L). Most LC-MBL patients carry clones consisting of <0.1 × 109 MBL cells/L.
PCR amplification of IGHV-IGHD-IGHJ rearrangements
Sequence analysis and interpretation
PCR amplicons were subjected to either direct sequencing on both strands or, in 15 cases, after subcloning; in the latter case, at least 10 colonies were analyzed per amplicon.
Sequence data were analyzed by using the IMGT databases18 and the IMGT/V-QUEST tool (http://www.imgt.org).19 Codons and amino acid positions are designated according to the IMGT unique numbering for V domain.20 Only productive rearrangements were evaluated (n = 355).
Rearrangements using the IGHV4-59 and IGHV4-61 genes were considered as one group because the use of an FR2 primer for certain PCR amplification experiments precluded a clear assignment to either of the two IGHV genes, which differ mainly in the VH FR1-VH CDR1. To avoid misidentification of mutations when IGHV FR1 or FR2 consensus primers were used in the amplification reactions, nucleotide substitutions in the obtained sequences were evaluated from codon 27 in CDR1-IMGT or from codon 56 in the CDR1 region or from codon 56 in the CDR2 region in those sequences obtained with a VH FR2 primer.4
IG repertoire comparisons and VH CDR3 clustering
IG gene repertoire comparisons were performed for the IGHV-IGHD-IGHJ sequences from MBL cases included in this study and 7596 IGHV-IGHD-IGHJ sequences from a cohort of 7424 CLL patients from our recent publication.21 Comparisons were also performed between MBL cases and a subgroup of the CLL cohort including only CLL-0 patients from three collaborating institutions within our network (San Raffaele, Milan, Italy; G. Papanicolaou, Thessaloniki, Greece; Nikea General Hospital, Athens, Greece) that attend to patients with CLL found in daily clinical practice (ie, without any selection bias [“community CLL”]) (n = 544).
Furthermore, MBL sequences from our series were aligned and compared with a panel of 5494 nonredundant, well-annotated, and productive IGHV-IGHD-IGHJ sequences from various entities except CLL, which were retrieved from the IMGT/LIGM-DB sequence database (http://www.imgt.org/IMGTindex/LIGM.html); this panel has also been used for the same purpose in previous repertoire studies from our group.4,22,23
To assess B-cell receptor (BcR) IG stereotypy within the entire collection of IGHV-IGHD-IGHJ sequences (MBL, CLL, other entities), we followed a clustering process based on purpose-built bioinformatics as previously described.22
Statistical analysis
Descriptive statistics for discrete parameters included counts and frequency distributions. For quantitative variables, statistical measures included means, medians, and min–max values. Significance of bivariate and/or multivariate relationships between variables was assessed by using χ2. For all comparisons, a significance level of P = .05 was set, and all statistical analyses were performed with the statistical package GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA).
Results
LC- and HC-MBL have distinct IG gene repertoires
Overall, 355 productive IGHV-IGHD-IGHJ rearrangements were amplified from 333 cases with CLL-like MBL. When we differentiated MBL on the basis of the absolute clonal B-cell count—LC-MBL <0.5 × 109/L (median, 0.008 × 109/L); HC-MBL ≥0.5 × 109 to <5.0 × 109/L (median, 3.38 × 109/L) (Table 1)—we noted that a significantly higher proportion of LC-MBL vs HC-MBL cases carried multiple productive IGHV-IGHD-IGHJ rearrangements (7 [11.7%] of 60 vs 8 [2.9%] of 273 cases; P = .003). Among all studied rearrangements, IGHV3 was the predominant subgroup (175 [49.3%] of 355), followed by IGHV4 (87 [24.5%] of 355). Thirty-nine functional IGHV genes were identified. The most frequent gene was IGHV4-34 (42 rearrangements; 11.8%) followed by IGHV3-23 (8.7%), IGHV3-7 (8.5%), IGHV1-69 (6.8%), and IGHV4-59/61 (6.8%) (supplemental Table 1).
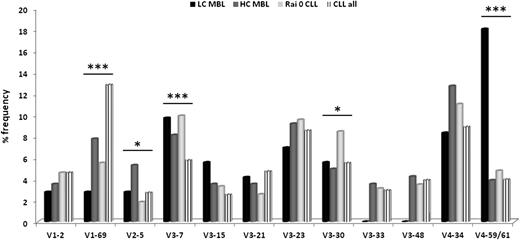
Significant differences were identified regarding the frequencies of IGHV genes between LC-MBL and HC-MBL. At the subgroup level, IGHV4 genes were significantly underrepresented (P = .02), and IGHV1 genes were significantly overrepresented (P = .04) in HC-MBL vs LC-MBL. At the individual gene level, IGHV1-69, IGHV2-5, IGHV3-23, IGHV3-33, IGHV3-48, and IGHV4-34 genes predominated in HC-MBL vs LC-MBL; however, the observed differences did not reach statistical significance, likely due to small sample size (at least for statistical purposes). Keeping the relatively low numbers in mind, it is indeed remarkable that the IGHV4-59/61 gene was highly significantly (P < .0001) more frequent in LC-MBL vs HC-MBL (Figure 2; supplemental Table 1).
IGHV gene repertoire comparison in LC-MBL vs HC-MBL vs CLL-0 vs CLL-all stages. *P < .05; ***P < .001.
IGHV gene repertoire comparison in LC-MBL vs HC-MBL vs CLL-0 vs CLL-all stages. *P < .05; ***P < .001.
Regarding VH CDR3 length and composition as well as IGHD and IGHJ gene usage, detailed results are provided in supplemental Tables 2 to 4.
Following the 98% germline identity cutoff value, 267 (75.2%) of 355 rearrangements were assigned to the mutated subgroup, whereas the remaining 88 (24.8%) of 355 were classified as unmutated, of which 52 (59.1%) of 88 had 100% sequence identity to the corresponding germline IGHV gene. No differences were identified in terms of somatic mutations in LC- vs HC-MBL, as evidenced by an overall similar distribution of cases to the mutated vs unmutated subgroups. Interestingly, in MBL cases with multiple productive IGHV-IGHD-IGHJ rearrangements, the mutational status was generally concordant in LC-MBL (5 [71.5%] of 7 cases with only mutated IGHV genes), whereas it was often discordant in HC-MBL (5 [62.5%] of 8 cases with coexisting mutated and unmutated IGHV genes).
LC-MBL have a distinct IG gene repertoire whereas HC-MBL resemble CLL-0
We next compared the IG gene repertoire between MBL and CLL. To this end, we used as reference two CLL datasets: one comprised 544 patients with CLL-0 only and the other comprised 7424 patients in all clinical stages (hereafter designated as CLL-all stages, as detailed in “Patients and methods”). In keeping with our previous report,4 the most pronounced difference concerned the IGHV4-59/61 gene, which was significantly overrepresented in LC-MBL (P < .0001) (Figure 2). A significantly different distribution was noted for the IGHV1-69 gene, which was rare in LC-MBL (2.8%); was used with similar, higher frequencies in HC-MBL (7.8%) and CLL-0 (5.5%); and peaked at 12.8% in CLL-all stages, being the most frequent gene overall (P < .0001). Other examples of IGHV genes with asymmetric usage in the compared groups are given in supplemental Table 5.
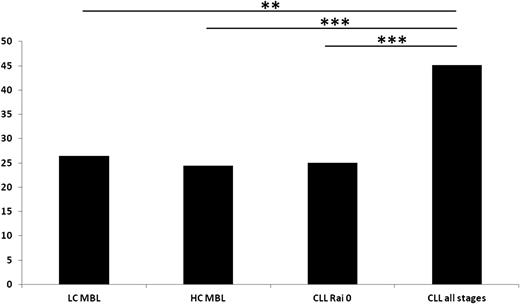
Regarding the mutational status of the IG rearrangements, no differences were identified among LC-MBL vs HC-MBL vs CLL-0 because the frequency of unmutated rearrangements (≥98% germline identity) was similar overall (LC-MBL, 26.4%; HC-MBL, 24.4%; CLL-0, 25%). Hence, all of the aforementioned subgroups differed significantly (P = .001, P < .0001, and P < .0001, respectively) from CLL-all stages in which almost 1 (45.1%) of 2 rearrangements carried unmutated IGHV genes (Figure 3). Interestingly, in both HC-MBL and CLL-0, a sizeable proportion (roughly one-third of rearrangements using the IGHV1-69 gene) were mutated (7 [31.8%] of 22 and 9 [30%] of 30, respectively), thus differing significantly (P < .001) from CLL-all stages, in which less than 10% of all such rearrangements were mutated. In the LC-MBL group, only 2 cases used the IGHV1-69 gene, and it was mutated in both.
The frequency (%) of unmutated IG rearrangements (≥98% germline identity) in MBL and CLL. **P < .01; ***P < .001.
The frequency (%) of unmutated IG rearrangements (≥98% germline identity) in MBL and CLL. **P < .01; ***P < .001.
BcR stereotypy is infrequent in LC-MBL, with the occasional stereotyped BcRs constituting “public” immunogenetic signatures
Analysis of the VH CDR3 region was possible in 349 of 355 rearrangements from the MBL cohort. To identify stereotyped VH CDR3s, we applied our purpose-built algorithm for the identification of shared VH CDR3 amino acid patterns and performed cluster analysis of the MBL sequences.22 Next, we compared the MBL VH CDR3 dataset against 7596 CLL sequences from our recent publication.21 Finally, we performed sequence comparisons with 5494 nonredundant, well-annotated, and complete VH CDR3 sequences from various entities except CLL, which were retrieved from the IMGT/LIGM-DB sequence database.
When we compared the frequency of CLL-specific BcR stereotypes between LC-MBL and HC-MBL, this was significantly higher in HC-MBL (P = .001), further underscoring a difference in the immunogenetic profile between these two entities. Specifically, only 4 (5.5%) of 72 LC-MBL IG sequences—none from cases with multiple productive IGHV-IGHD-IGHJ gene rearrangements—could be clustered with other sequences in subsets with stereotyped BcRs; they concerned cases with clonal B cells in the range of 0.004 to 0.055 × 109/L. In particular, (1) 1 LC-MBL case was clustered together with an HC-MBL case but with no CLL patients, thus forming a novel MBL-specific subset; (2) 1 LC-MBL case could be assigned to a CLL-specific subset (subset #239); and (3) 2 LC-MBL cases were clustered together with patients having sequences from various entities, including CLL; thus they were considered to carry public BcR stereotypes (see “Public BcR IG stereotypes in the MBL repertoire”).
In contrast, 62 (21.9%) of 283 HC-MBL rearrangements carried VH CDR3s similar to either other MBL or to CLL cases; 3 of 62 were detected in cases with multiple rearrangements. In particular, (1) 45 HC-MBL cases clearly belonged to well-established CLL-specific subsets (eg, #1, #2, #4, #16, #28); (2) 10 HC-MBL cases clustered together with CLL patients, forming novel MBL-CLL subsets; (3) 5 HC-MBL cases clustered together, forming MBL-specific subsets; and (4) 2 HC-MBL cases carried public IG BcR stereotypes (ie, they were assigned to clusters that included sequences from various entities (see “Public BcR IG stereotypes in the MBL repertoire”).
Furthermore, a progressive increase in the frequency of BcR IG stereotypy depending on the absolute count of CLL-like cells could be observed, starting with 5.5% in LC-MBL, rising to 21.9% in HC-MBL and 20.2% in CLL-0, and peaking at 30.4% in CLL-all stages (Figure 4).
The frequency (%) of BcR IG stereotypy in MBL and CLL. **P < .01; ***P < .001.
Public BcR IG stereotypes in the MBL repertoire
The first LC-MBL case with a public BcR IG stereotype (89VBB) was assigned to a subset of patients with sequences mainly from CLL (subset #13), but also including other sequences as well (a rheumatoid factor from a healthy individual, the clonotypic BcR of a patient with splenic marginal-zone lymphoma, two patients with Sjögren’s syndrome-associated myoepithelial sialadenitis, and a CLL patient with prior hepatitis C virus [HCV]–associated type II mixed cryoglobulinemia [HCV/MC-II]). As we recently reported,24 a number of patients assigned to this subset, regardless of the underlying diagnosis, had positive HCV serology; this was the case for 89VBB as well.
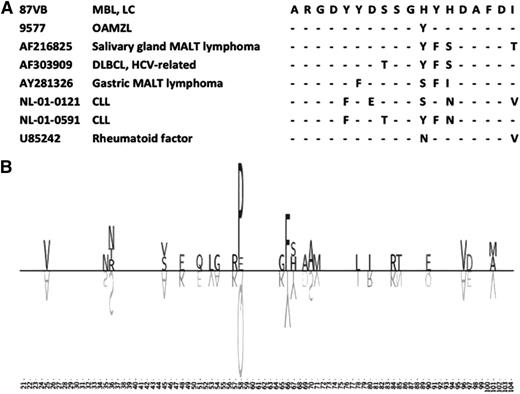
The second LC-MBL case with a public BcR IG stereotype (87VB) carried a VH CDR3 similar to the one recently reported in our study of ocular adnexa marginal-zone lymphoma (Figure 5A).25 This particular stereotype has so far been detected among various entities in addition to ocular adnexa marginal-zone lymphoma, including CLL (2 patients), salivary-gland mucosa-associated lymphoid tissue (MALT) lymphoma (1 patient), gastric MALT lymphoma (1 patient), diffuse large B-cell lymphoma (1 patient, positive for HCV), and a rheumatoid factor. All of these cases shared quasi-identical VH CDR3s and, furthermore, also exhibited a stereotyped somatic hypermutation in VH CDR2 codon 58, leading to substitution of proline for glutamine (Figure 5B).
A public BcR stereotype, as represented by the LC-MBL case 87VB. (A) VH CDR3 similarity between the LC-MBL cases 87VB and unrelated sequences. Dashes indicate identities. (B) Somatic hypermutation in the VH region of the LC-MBL case 87VB and co-clustered sequences, as visualized by using TeXshade peptide alignment tool (http://workbench.sdsc.edu/). The VH region is shown from IMGT positions 21 to 104. The letters above the line represent the amino acid changes, whereas the letters shown upside-down below the line represent the corresponding germ line amino acids of the IGHV gene. The size of the amino acid symbol represents the relative frequency of that amino acid at that position relative to all other mutations at that position in the certain IGHV group of sequences. Blank spaces represent amino acids that are unchanged in comparison with the germ line sequence.
A public BcR stereotype, as represented by the LC-MBL case 87VB. (A) VH CDR3 similarity between the LC-MBL cases 87VB and unrelated sequences. Dashes indicate identities. (B) Somatic hypermutation in the VH region of the LC-MBL case 87VB and co-clustered sequences, as visualized by using TeXshade peptide alignment tool (http://workbench.sdsc.edu/). The VH region is shown from IMGT positions 21 to 104. The letters above the line represent the amino acid changes, whereas the letters shown upside-down below the line represent the corresponding germ line amino acids of the IGHV gene. The size of the amino acid symbol represents the relative frequency of that amino acid at that position relative to all other mutations at that position in the certain IGHV group of sequences. Blank spaces represent amino acids that are unchanged in comparison with the germ line sequence.
Only 2 HC-MBL rearrangements were clustered together with sequences from different entities except CLL that were retrieved from the IMGT/LIGM-DB sequence database. The first carried a mutated BcR IG, which was quasi-identical to a rheumatoid factor. The second clustered with the BcR IG from a salivary MALT lymphoma and the autoreactive BcR IG from a patient with primary Sjögren’s syndrome.
Discussion
CLL-like MBL is generally considered to represent tout court a pre-CLL state, analogous to what monoclonal gammopathy of undetermined significance is for multiple myeloma.26 Along this line, a prospective cohort study demonstrated that CLL is always preceded by CLL-like MBL.10 However, the frequency of CLL-like MBL is particularly high among the general population, reaching 12% when advanced flow cytometric techniques are used,5 thereby being at least 100 times more frequent than CLL and highlighting the fact that not all MBL carry the same risk of clinical progression.15 It is therefore of crucial relevance to clearly distinguish those cases who are more likely to progress and require special clinical attention during follow-up. Studying the biological characteristics of MBL may help us identify prognostic markers that correlate with progression toward CLL, sparing the majority of cases from unnecessary psychological distress and continuous medical attention with its obvious social and economic implications.
So far, the absolute clonal B lymphocyte count is the only factor that was found to hold some prognostic value for the progression of MBL into CLL requiring treatment.13 On the basis of survival curves, it has been proposed that MBL may be subdivided into two groups—LC and HC—by being below or above a suggested cutoff of 0.5 × 109 clonal cells/L, respectively.14,16 Recently, our group demonstrated that LC-MBL carries distinct biological features, including the IG gene repertoire from CLL (both mutated and unmutated), and supported the idea that this entity may not always be equivalent to a premalignant state.4
Here, we analyzed a large, multinational cohort of CLL-like MBL (both LC and HC) cases, which allowed us to perform informative IG gene repertoire comparisons of LC-MBL, HC-MBL, CLL-0, and CLL-all stages. For all comparisons, we followed the cutoff of 0.5 × 109 clonal cells/L to discriminate LC- from HC-MBL. This cutoff was suggested by the literature and was also an educated choice; use of other cutoff values (namely 1.0 and 1.5 × 109/L) did not affect our conclusions since our MBL cohort was polarized toward carrying either very LC clones or >1.5 × 109 clonal B cells/L, and cases with a clonal B-cell count between 0.5 and 1.5 × 109/L were in essence very few (n = 17) (Figure 1).
Analysis of the IGHV genes expressed by LC-MBL revealed significant differences from HC-MBL as well as from CLL-all stages in terms of both gene usage and, perhaps more importantly, the frequency of BcR stereotypy. Interestingly, HC-MBL showed great similarity with CLL, especially CLL-0. In contrast, compared with both HC-MBL and CLL, LC-MBL exhibits pronounced differences such as suppressed frequency of the IGHV1-69 gene and overrepresentation of the IGHV4-59/61 genes, confirming our previous findings.4 The underrepresentation of the IGHV1-69 gene in LC-MBL may be associated with the overall paucity of stereotyped BcRs in this category, since this gene shows a remarkable propensity to be used in stereotyped rearrangements in CLL.22,23
The latter have been shown to be a distinctive feature of CLL, being present in almost one-third of patients.21,22 Hence, our finding that stereotyped receptors are indeed rare in LC-MBL is particularly noteworthy, again suggesting a phylogenetic distance from CLL, whereas HC-MBL is characterized by a much higher frequency of BcR stereotypes, surprisingly similar to that of CLL-0, to which again seem to be more closely related at the biological level.
One also has to consider that HC-MBL cases have been shown to carry a risk of evolution into CLL needing treatment at a sizeable frequency between 1% and 4% per year,14,16,27 whereas CLL-0 patients will require therapy at an approximate rate of 5% per year.28 This indicates that both entities comprise a mixture of cases with distinct overall propensity to progression and suggests the need to identify molecular or clinical signatures besides the official threshold of 5 × 109 clonal B cells/L in order to distinguish between progressive and nonprogressive cases, regardless of the actual MBL or CLL diagnosis.
From a clinical standpoint, our immunogenetic data further support the idea that HC-MBL should be managed as CLL not requiring treatment (ie, with regular follow-ups). It remains to be established in prospective studies whether it may be worth evaluating the IGHV gene usage, mutational status, and/or BcR stereotypy in an effort to obtain information that could be of use in identifying the small fraction of cases who will eventually progress. In this respect, it is worth mentioning that the most common subset recognized among this HC-MBL cohort was subset #2 (expressing IGHV3-21/IGHJ6 VH CDR3; 10%; 7 of 72 stereotyped HC-MBL clones). Subset #2 is conspicuous for its aggressive clinical behavior,29-31 so it would not be unreasonable to speculate that an HC-MBL case also expressing this stereotype should be followed more closely, though this remains to be proven.
Conversely, the overrepresentation of the IGHV4-59/61 genes in LC-MBL closely parallels the significant increase of this gene among elderly individuals,32 suggesting that it might simply mirror the age-related repertoire restriction occurring with age in healthy individuals.33-35 This further supports the possibility that LC-MBL may simply represent an aspect of the physiological process of immune senescence. Similar repertoire restrictions are well established for the T-cell peripheral pool, where the TCR diversity appears to collapse with age, especially in the CD8+ but also in the CD4highCD8low compartment.36-38 The frequent presence in the elderly of clonal expansions among the CD8+ T cells, but even more interestingly among normally negligible peripheral T-cell subsets, closely resembles CLL-like MBL in healthy individuals, the frequency of which is also age-related.5,39,40 The finding of public signatures among BcR stereotypes in LC-MBL further supports the notion that LC-MBL does not necessarily represent a preleukemic condition. Rather, it may reflect repertoire restriction and/or polarization due to immune senescence and/or chronic antigenic stimulation.41 Along the same line lies the conspicuously increased frequency of multiple productive rearrangements in LC-MBL. Expression of multiple antigen receptors has been reported for autoreactive B cells, in which constant exposure to self-antigens may ultimately lead to allelic inclusion as an editing mechanism (otherwise described as “receptor dilution”).42-44 In sharp contrast, the frequency of multiple productive rearrangements in HC-MBL was similar to the one reported for CLL.45 Altogether, these findings support the notion that LC-MBL might constitute another example in which clonality does not directly translate to malignancy and may cast serious doubts on its relation to CLL.
Against this contention—the seemingly indolent nature of LC-MBL—has to be reconciled with the finding of the same cytogenetic abnormalities that are frequently encountered in CLL (del13q, trisomy 12, del17p).15 However, as we recently reported, all LC-MBL cases, even those cases carrying cytogenetically abnormal clones, showed no evidence of progression from the LC state during a median follow-up time of 34 months.15 This may well stand in analogy to the detection of lymphoma-associated translocations in circulating B cells of healthy individuals, such as the follicular lymphoma-associated t(14;18) (with reported frequencies ranging from 30% to >70%)46-49 and the mantle cell lymphoma-associated t(11;14) (which is more rare, with reported frequencies ranging from 1% to 7%).50,51 Altogether, it is reasonable to argue that genetic aberrations may occur early in the timeline of MBL, perhaps as a result of persistent proliferative stimuli by antigen(s) and may relate more to the acquisition of the aberrant (or persistently activated?) phenotype rather than the malignant state itself.
One could argue that, since HC-MBL/CLL probably stems from a single cell that clonally expands as it gradually suffers malignant hits, it must transit through the LC-MBL range as well. We believe that in LC-MBL, the clonal population that results from persistent antigenic stimulation is contained despite the fact that the proliferative stimuli lead to the acquisition of genetic aberrations. Perhaps HC-MBL/CLL develops only when this microenvironmental drive occurs in a background of genetic predisposition, with the expanded clone having inherently different tendencies to quickly transit from the LC-MBL to the HC-MBL/CLL phase. This theory appears to be supported by the polarized distribution of the MBL cases in our cohort, in which few cases with MBL carry a clone size of 0.5 to 1.5 × 109 cells/L; instead, they carry either very LC clones (median clonal B-cell count of 0.008 × 109/L) or clones larger than 1.5 × 109 cells/L.
Because of such small clonal size, LC-MBL by itself causes very little perturbation in the blood count differential and requires application of high-sensitivity flow cytometry protocols in order to be identified. Therefore, a physician will not likely encounter this entity in daily practice. In any case, our immunogenetic data suggest that this condition is not clinically relevant and should not prompt either monitoring or analysis of biological prognostic markers except for research purposes.
In conclusion, although LC-MBL likely results from persistent antigenic stimulation, the relevant immune pathways can be distinct from those implicated in the ontogeny of CLL, especially the stereotyped category. On the contrary, HC-MBL has remarkable immunogenetic similarities to CLL-0, extending from similar IGHV gene usage to similar frequency of CLL-specific BcR stereotypes, which reinforce their immunobiological relatedness in addition to their clinical proximity. Therefore, thorough molecular analysis and characterization of BcR stereotypy combined with long-term clinical monitoring of HC-MBL may help clarify the degree to which CLL-biased stereotypy can serve as a predictor for evolution to CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the ENosAI project (code 09SYN-13-880) co-funded by the European Union and the Hellenic General Secretariat for Research and Technology (K.S.); Cariplo Foundation (Milan, Italy) (P.G. and K.S.); Program Molecular Clinical Oncology-5 (9965) and Investigator Grant, Associazione Italiana per la Ricerca sul Cancro (Milan, Italy) (P.G.); Progetti di Rilevanza Nazionale 2008, Ministero Istruzione Università e Ricerca (Rome, Italy) (P.G.); and Progetto Finalizzato 2010, Ministero della Salute (Rome, Italy) (P.G.).
Authorship
Contribution: A.V. performed research, analyzed data, and wrote the paper; A.D., L.S., D.J., D.N., F.B., J.A., A.R.-C., and S.A. performed research; M.L., A.C., E.O., S.V., M.M., A.R., T.S., and A.O. provided samples and associated clinicopathological data and supervised research; and K.S. and P.G. designed the study, supervised research, and wrote the paper.
Conflict-of-interest disclosure: Andy Rawstron is a consultant to Biogen Idec. The remaining authors declare no competing financial interests.
Correspondence: Kostas Stamatopoulos, Hematology Department and Hematopoietic Cell Transplantation Unit, G. Papanicolaou Hospital, 57010 Exokhi, Thessaloniki, Greece; e-mail: kostas.stamatopoulos@gmail.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal