Key Points
Lenalidomide consolidation repairs T-cell immune synapses in CLL patients.
Lenalidomide consolidation improved the quality of response in CLL patients treated with chemoimmunotherapy.
Immunotherapy that facilitates endogenous T-cell activity has the potential to target therapy-resistant tumor clones. In vitro studies have demonstrated that lenalidomide repairs the T-cell immunologic synapse defect in chronic lymphocytic leukemia (CLL). Pentostatin, cyclophosphamide, and rituximab (PCR) in CLL is clinically active with modest toxicity, indicating suitability of this chemoimmunotherapy (CIT) platform for combination with immunotherapy. Here we report on a trial of PCR followed by lenalidomide consolidation. Of 34 patients who received lenalidomide, 24% improved their quality of response and 4 patients converted to minimal residual disease negative status. Retrospective comparison to a historical PCR trial indicated that lenalidomide consolidation extends time to progression requiring salvage therapy. Longitudinal analysis showed that antitumor T-cell immune synapse activity improved post-PCR and was further enhanced after lenalidomide consolidation. These novel data showing repair of T-cell defects provide proof-of-principle that lenalidomide-based consolidation after CIT could have a beneficial clinical and immunologic role in CLL.
Introduction
Immunotherapies designed to block cancer-mediated immunosuppressive mechanisms and repair antitumor T-cell function have the potential to target therapy-resistant tumor clones and reduce disease relapse.1 Recent studies have defined an immune evasion mechanism whereby tumor-exposed T cells from chronic lymphocytic leukemia (CLL) patients exhibit defective immunologic synapse formation.2 Immune synapses control assembly of signaling complexes and are master regulators of T-cell activation and effector function in response to antigen-presenting cells (APCs). The immunomodulatory drug lenalidomide has shown significant clinical activity as a single agent in CLL.3,-5 Although its mechanism of action is not fully defined, in vitro studies suggest lenalidomide modulates the tumor microenvironment including repair of T-cell synapse activity.2,6
Fludarabine, cyclophosphamide, and rituximab (FCR) or FCR-like chemoimmunotherapy (CIT) is regarded as the gold standard first-line platform for CLL patients.7 The fact that nearly all patients eventually relapse has generated interest in consolidation strategies, particularly using agents with a mechanism of action that is distinct from the agents used in CIT induction. The pentostatin, cyclophosphamide, and rituximab (PCR) regimen is an FCR-like CIT regimen that has clinical activity in CLL with acceptable toxicity,8 indicating suitability of this CIT platform to be combined with maintenance immunotherapy. Here we report the results of the first lenalidomide consolidation trial investigating both clinical activity and modulation of antitumor T-cell function.
Study design
Eligible patients were diagnosed with progressive CLL and in need of treatment.9 The protocol was approved by the Mayo Clinic Institutional Review Board. All patients provided written informed consent prior to study enrollment in accordance with the Declaration of Helsinki. Induction CIT (6 cycles) included pentostatin (2 mg/m2, day 1), cyclophosphamide (600 mg/m2, day 1), and rituximab (cycle 1: 100 mg, day 1; 375 mg/m2, day 2; cycles 2-6: 375 mg/m2, day 1) given intravenously every 21 days using the previously reported schedule.8 All patients completing PCR underwent complete restaging including minimal residual disease (MRD) evaluation.10 Following restaging and adequate recovery of blood counts, patients then received 6 months of lenalidomide consolidation by continuous daily administration. The starting lenalidomide dose was 5 mg/day, with escalation to 10 mg/day after the first cycle as tolerated. After 6 months of lenalidomide, patients again underwent complete restaging including MRD evaluation. MRD− patients entered observation. Those with residual disease continued on lenalidomide (“continuation”) and underwent repeat MRD assessment every 3 months until MRD−. Platelet and hemoglobin adverse events and criteria for response were graded according to the International Workshop on CLL (IWCLL) Working Group scales.9
The primary end point was the proportion of patients who achieved a complete response (CR). The study had 90% power, with a 9% type I error rate. Time to retreatment was defined as time from registration to initiation of subsequent treatment. The distribution of time to retreatment was assessed using the Kaplan-Meier method.
Quantitative T-cell synapse assays were performed and analyzed as previously described.6
Results and discussion
Thirty-eight of 44 (86%) eligible patients (supplemental Table 1 on the Blood website) completed 6 cycles of PCR induction. Adverse events included 18 (41%) patients with grade 3+ hematologic toxicity and 9 (20%) with grade 3+ nonhematologic toxicity. The overall response rate to induction was 93% (95% confidence interval: 81-99), with 14 achieving CR (8 MRD−), 3 complete clinical responses, and 24 partial responses (PRs; including 15 nodular [n]PRs).
Of the 38 eligible patients who completed induction, 34 (89%) initiated lenalidomide consolidation. Thus, by protocol intent-to-treat analysis, 34 of 44 (77%) patients starting CIT were able to receive consolidation therapy. The remaining patients were unable to initiate consolidation due to failure to complete induction (n = 6), disease progression (n = 1), and patient refusal (n = 3). Among patients initiating lenalidomide consolidation, the median number of cycles administered was 6 (range, 1-32). Only 9 of 34 (26%) patients had their lenalidomide dose increased to the 10-mg dose level, whereas 18 (53%) patients required a reduction to the 5 mg every other day dose level, of which 3 (9%) subsequently were reduced to 5 mg twice per week. Dose reductions were due to hematologic toxicity (n = 16), grade 3 myalgia (n = 1), and grade 2 dyspnea (n = 1). Only 1 patient went off lenalidomide because of persistent cytopenia. Adverse events included 22 (65%) patients with grade 3+ hematologic toxicity and 5 (15%) with grade 3+ nonhematologic toxicity (supplemental Table 2). No cases of tumor lysis syndrome or tumor flare reaction were observed. Among 34 patients who received ≥1 cycle of lenalidomide, 8 (24%) patients improved the quality of their response. This included 2 PR patients that improved to an nPR; 1 patient with a PR at completion of induction who became an nPR after 6 cycles of lenalidomide and improved to a MRD− CR after 12 cycles; 4 nPR patients who became CRs (2 MRD− and 2 MRD+); and 1 CR patient that improved from MRD+ to MRD−. Of note, 13 of 34 (38.2%) patients showed CRs (7 MRD− and 5 MRD+) prior to starting lenalidomide, whereas 18 of 34 (52.9%) achieved CR (11 MRD− and 7 MRD+) after lenalidomide (supplemental Table 3). These results show that lenalidomide consolidation is feasible, improved the depth of response, and converted some patients with residual disease after CIT induction to MRD− remissions.
Longitudinal changes in T-cell immune synapse activity were assessed by examining the cell conjugates formed between T cells from each treatment time point interacting with baseline untreated CLL cells pulsed with antigen, which acted as APCs. Results from a representative patient are shown in Figure 1A, C. Increased T-cell synapse activity was observed after PCR induction, with 23 of 28 (82%) patients showing significantly increased F-actin synapse polymerization (Figure 1B). Patients who achieved a better response to induction therapy (CR/complete cytogenetic response/nPR) had increased recovery of synapse activity compared with those who had partial remission or no response (stable disease/progressive disease), although this difference (median synapse area increase 0.88 vs 0.36; P = .075) did not reach the threshold of statistical significance (possibly because of the limited sample size). We postulate that clearance of highly immunosuppressive tumor cells may mediate repair of function, indicating suitability of the PCR CIT platform to be combined with immunotherapy. We believe our results highlight the potential utility of the T-cell immune synapse bioassay for the measurement and comparison of T-cell function following more intensive CIT regimens including FCR or emerging targeted therapies in CLL.11
Longitudinal analysis of T-cell immune synapse function during PCR induction-lenalidomide (Len.) consolidation therapy. (A) Confocal images of T-cell synapse formation for a representative patient: CD3+ T cells from each treatment time point indicated were conjugated with baseline untreated CLL B cells (pulsed with superantigen [sAg] and dyed blue with CellTracker). These identical APCs used for all time points allowed evaluation of changes in T-cell F-actin (dyed red with rhodamine phallodin) synapse activity (indicated with white arrows) during treatment. T-cell conjugates formed were analyzed by immunofluorescence and confocal microscopy. Blinded images (n = 10 per patient treatment sample) were analyzed using AxioVision (ASSAYbuilder) image analysis software (Zeiss). This measures the total area (μm2) of F-actin (red fluorescent channel) accumulation at all T-cell contact sites and synapses with CLL cells (minimum n = 50 synapses assessed per patient at each timepoint). (B) Mean change in T-cell synapse activity of 28 patients following PCR induction therapy compared with their respective (matched) baseline activity (C) Changes in T-cell synapse activity for a representative patient. Each data point represents the area (μm2) of F-actin polymerization at a T-cell:APC synapse contact site (n = 50 synapses per patient at each time point) measured using Axiovision software. The mean ± standard error of the mean is shown for each treatment time point in a representative patient (baseline, data points shown as black dots; post-PCR, blue; post-Len. 3 months, orange; post-Len. 6 months, red; matching colored squares denote the bar chart data shown in E and F), and (D) the mean change in T-cell synapse activity of 11 patients after 6 months (cycles) of lenalidomide consolidation compared with their respective (matched) post-PCR activity. Each data point represents the mean area (μm2) of T-cell F-actin synapse formation from ≥50 synapses for each patient. The mean for all patients is indicated by a bar. (E) Quantitative analysis of the percent of T-cell:APC conjugates (n = 50) showing phosphotyrosine synapse signaling by immunofluorescence (shown in yellow) comparing PCR induction therapy to the respective (matched) baseline activity (28 patients) and (F) cytolytic T lymphocyte granzyme B protein accumulation (shown in yellow) at the synapse site comparing 6 months (cycles) of lenalidomide consolidation to the respective (matched) post-PCR activity (11 patients). Data represent the combined mean ± standard deviation (baseline, black bar chart; post-PCR, blue; post-Len. 6 months, red). Original magnification, ×63. **P < .01 values are shown.
Longitudinal analysis of T-cell immune synapse function during PCR induction-lenalidomide (Len.) consolidation therapy. (A) Confocal images of T-cell synapse formation for a representative patient: CD3+ T cells from each treatment time point indicated were conjugated with baseline untreated CLL B cells (pulsed with superantigen [sAg] and dyed blue with CellTracker). These identical APCs used for all time points allowed evaluation of changes in T-cell F-actin (dyed red with rhodamine phallodin) synapse activity (indicated with white arrows) during treatment. T-cell conjugates formed were analyzed by immunofluorescence and confocal microscopy. Blinded images (n = 10 per patient treatment sample) were analyzed using AxioVision (ASSAYbuilder) image analysis software (Zeiss). This measures the total area (μm2) of F-actin (red fluorescent channel) accumulation at all T-cell contact sites and synapses with CLL cells (minimum n = 50 synapses assessed per patient at each timepoint). (B) Mean change in T-cell synapse activity of 28 patients following PCR induction therapy compared with their respective (matched) baseline activity (C) Changes in T-cell synapse activity for a representative patient. Each data point represents the area (μm2) of F-actin polymerization at a T-cell:APC synapse contact site (n = 50 synapses per patient at each time point) measured using Axiovision software. The mean ± standard error of the mean is shown for each treatment time point in a representative patient (baseline, data points shown as black dots; post-PCR, blue; post-Len. 3 months, orange; post-Len. 6 months, red; matching colored squares denote the bar chart data shown in E and F), and (D) the mean change in T-cell synapse activity of 11 patients after 6 months (cycles) of lenalidomide consolidation compared with their respective (matched) post-PCR activity. Each data point represents the mean area (μm2) of T-cell F-actin synapse formation from ≥50 synapses for each patient. The mean for all patients is indicated by a bar. (E) Quantitative analysis of the percent of T-cell:APC conjugates (n = 50) showing phosphotyrosine synapse signaling by immunofluorescence (shown in yellow) comparing PCR induction therapy to the respective (matched) baseline activity (28 patients) and (F) cytolytic T lymphocyte granzyme B protein accumulation (shown in yellow) at the synapse site comparing 6 months (cycles) of lenalidomide consolidation to the respective (matched) post-PCR activity (11 patients). Data represent the combined mean ± standard deviation (baseline, black bar chart; post-PCR, blue; post-Len. 6 months, red). Original magnification, ×63. **P < .01 values are shown.
T-cell function was further improved after 6 cycles (months) of lenalidomide consolidation (Figure 1D) in all patient samples examined. This immunostimulatory activity was maintained after 12 and 18 cycles (months) of lenalidomide. Four-color immune synapse bioassays showed that both CIT and lenalidomide increased the number of T-cell conjugates (data not shown), phosphotyrosine (early synapse signaling), and granzyme B (effector cytolytic activity) polarized expression (Figure 1E-F).12 Additional immunologic analysis showed that IgG levels and absolute numbers of T cells decreased after PCR induction, whereas there was a significant increase in these parameters following 6-month lenalidomide consolidation (P < .05; supplemental Figure 1).
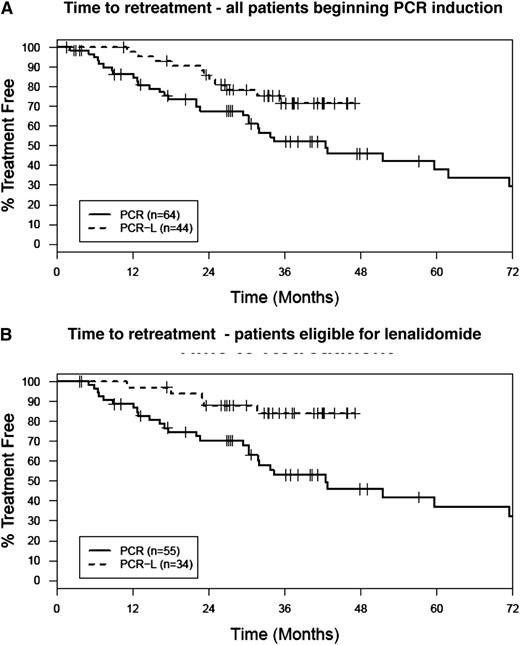
After a median follow-up of 37 months, 38 of 44 patients are alive, and the median duration of response has not been reached (11/44 [25%] of patients have required retreatment). Eligibility criteria, demographic, and prognostic characteristics of the patients were similar to our historical trial of patients treated with PCR alone (supplemental Table 4). Thus, we conducted an intent-to-treat retrospective analysis that compared all 44 patients in the present trial (regardless of whether they received lenalidomide) to our historical cohort of patients who received PCR without lenalidomide consolidation (n = 64).8 With the caveat that it represents a comparison across phase II trials, lenalidomide consolidation appeared to result in more durable remissions and prolonged time to salvage therapy in this analysis (Figure 2A-B).
Retrospective time to retreatment comparison of PCR-lenalidomide maintenance to a historical PCR induction alone study. (A) Intent to treat, all patients beginning PCR treatment. The proportion of patients free of retreatment at 36 months was 73% (95% CI: 60-88) for PCR followed by lenalidomide consolidation vs 52% (95% CI: 40-68) for PCR. (B) In a per protocol analysis of the patients in each trial that completed 6 cycles of PCR induction and therefore would have been eligible for lenalidomide consolidation, the proportion of patients free of retreatment at 36 months was 84% (95% CI: 72-98) for PCR-lenalidomide vs 53% (95% CI: 40-70) for PCR.
Retrospective time to retreatment comparison of PCR-lenalidomide maintenance to a historical PCR induction alone study. (A) Intent to treat, all patients beginning PCR treatment. The proportion of patients free of retreatment at 36 months was 73% (95% CI: 60-88) for PCR followed by lenalidomide consolidation vs 52% (95% CI: 40-68) for PCR. (B) In a per protocol analysis of the patients in each trial that completed 6 cycles of PCR induction and therefore would have been eligible for lenalidomide consolidation, the proportion of patients free of retreatment at 36 months was 84% (95% CI: 72-98) for PCR-lenalidomide vs 53% (95% CI: 40-70) for PCR.
The results of this phase II trial suggest that lenalidomide consolidation improves the depth of remission and supports future randomized testing. For the first time, our laboratory studies demonstrate enhancement of long-term T-cell function following CIT and lenalidomide consolidation in CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood commentary on this article in this issue.
Acknowledgments
T.D.S. is a Clinical Scholar of the Leukemia and Lymphoma Society.
This clinical trial was funded through a grant from Celgene. Associated correlative studies were supported by grant funding from Celgene and Hospira.
Authorship
Contribution: T.D.S., A.G.R., C.S.Z., J.F.L., H.W.T., T.G.C., B.L., D.F.J., C.A.H., and N.E.K. provided the concept and design; T.D.S., A.G.R., and N.E.K. provided financial support; T.D.S., A.G.R., C.S.Z., J.F.L., H.W.T., T.G.C., D.B., and N.E.K. provided the study materials and assisted with patient accrual; T.D.S., A.G.R., C.S.Z., J.F.L., H.W.T., T.G.C., B.L., D.B., A.P., D.F.J., C.A.H., and N.E.K. collected and assembled the data and provided data analysis and interpretation; T.D.S., A.G.R., and N.E.K. wrote the manuscript; and T.D.S., A.G.R., C.S.Z., J.F.L., H.W.T., T.G.C., B.L., D.B., A.P., D.F.J., C.A.H., and N.E.K. revised and approved the final manuscript.
Conflict-of-interest disclosure: T.D.S. receives funding for Mayo Clinic research from Celgene, Hospira, Genentech, Glaxo-Smith-Kline, Cephalon, and Polyphenon E International; A.G.R. receives research funding from Celgene; C.S.Z. receives funding for Mayo Clinic research from Genentech, Genzyme, Novartis, GlaxoSmithKline, and Biothera; H.W.T. receives funding for Mayo Clinic research from Celgene; and N.E.K. receives funding for Mayo Clinic research from Celgene, Genentech, Gilead, and Pharmacyclics and is a consultant for Janssen Research & Development and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Tait D. Shanafelt, Mayo Clinic, Division of Hematology, Department of Medicine, 200 First St, Rochester, MN 55902; e-mail: shanafelt.tait@mayo.edu.
References
Author notes
T.D.S. and A.G.R. contributed equally to this study.

![Figure 1. Longitudinal analysis of T-cell immune synapse function during PCR induction-lenalidomide (Len.) consolidation therapy. (A) Confocal images of T-cell synapse formation for a representative patient: CD3+ T cells from each treatment time point indicated were conjugated with baseline untreated CLL B cells (pulsed with superantigen [sAg] and dyed blue with CellTracker). These identical APCs used for all time points allowed evaluation of changes in T-cell F-actin (dyed red with rhodamine phallodin) synapse activity (indicated with white arrows) during treatment. T-cell conjugates formed were analyzed by immunofluorescence and confocal microscopy. Blinded images (n = 10 per patient treatment sample) were analyzed using AxioVision (ASSAYbuilder) image analysis software (Zeiss). This measures the total area (μm2) of F-actin (red fluorescent channel) accumulation at all T-cell contact sites and synapses with CLL cells (minimum n = 50 synapses assessed per patient at each timepoint). (B) Mean change in T-cell synapse activity of 28 patients following PCR induction therapy compared with their respective (matched) baseline activity (C) Changes in T-cell synapse activity for a representative patient. Each data point represents the area (μm2) of F-actin polymerization at a T-cell:APC synapse contact site (n = 50 synapses per patient at each time point) measured using Axiovision software. The mean ± standard error of the mean is shown for each treatment time point in a representative patient (baseline, data points shown as black dots; post-PCR, blue; post-Len. 3 months, orange; post-Len. 6 months, red; matching colored squares denote the bar chart data shown in E and F), and (D) the mean change in T-cell synapse activity of 11 patients after 6 months (cycles) of lenalidomide consolidation compared with their respective (matched) post-PCR activity. Each data point represents the mean area (μm2) of T-cell F-actin synapse formation from ≥50 synapses for each patient. The mean for all patients is indicated by a bar. (E) Quantitative analysis of the percent of T-cell:APC conjugates (n = 50) showing phosphotyrosine synapse signaling by immunofluorescence (shown in yellow) comparing PCR induction therapy to the respective (matched) baseline activity (28 patients) and (F) cytolytic T lymphocyte granzyme B protein accumulation (shown in yellow) at the synapse site comparing 6 months (cycles) of lenalidomide consolidation to the respective (matched) post-PCR activity (11 patients). Data represent the combined mean ± standard deviation (baseline, black bar chart; post-PCR, blue; post-Len. 6 months, red). Original magnification, ×63. **P < .01 values are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/20/10.1182_blood-2012-12-470005/2/m_4137f1.jpeg?Expires=1769182847&Signature=up5DwnHXiJRuriG-HUOqtUwpTk83Bj9SXsp4MgUdDKlCFmzeiBf7OpCMW9IiPSGqHEvR7-ZzOO1mIKpFjMvoXbvsRNaAYLnOeF2FnOpCbFW-wZn7IkMUQFSa1~20yzQbJATTfP-4LeqbAynG1lbSda4jl3TRNZH2rDDmHBIWBN7skS0yyHSlf8bSWeScMSWEd3Dt9qo8Ae9HH9iEoN7zpsgnp0XzxHCA3bgYEHV05d0YwKjBisI-P27Cfc-lQQ1J-Ad6n7EmZwsXz2JyN8zA6mVE~fQQNV4fIYx9QLFElzk-W4ProVrL33u8UddjQW0iZaCGkIWwKEu6zQlUToSU0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal