Key Points
IFNα targets Jak2V617F MPN stem cells.
Abstract
Interferon-α (IFNα) is an effective treatment of patients with myeloproliferative neoplasms (MPNs). In addition to inducing hematological responses in most MPN patients, IFNα reduces the JAK2V617F allelic burden and can render the JAK2V617F mutant clone undetectable in some patients. The precise mechanism underlying these responses is incompletely understood and whether the molecular responses that are seen occur due to the effects of IFNα on JAK2V617F mutant stem cells is debated. Using a murine model of Jak2V617F MPN, we investigated the effects of IFNα on Jak2V617F MPN-propagating stem cells in vivo. We report that IFNα treatment induces hematological responses in the model and causes depletion of Jak2V617F MPN-propagating cells over time, impairing disease transplantation. We demonstrate that IFNα treatment induces cell cycle activation of Jak2V617F mutant long-term hematopoietic stem cells and promotes a predetermined erythroid-lineage differentiation program. These findings provide insights into the differential effects of IFNα on Jak2V617F mutant and normal hematopoiesis and suggest that IFNα achieves molecular remissions in MPN patients through its effects on MPN stem cells. Furthermore, these results support combinatorial therapeutic approaches in MPN by concurrently depleting dormant JAK2V617F MPN-propagating stem cells with IFNα and targeting the proliferating downstream progeny with JAK2 inhibitors or cytotoxic chemotherapy.
Introduction
JAK2V617F is the most common molecular alteration in the BCR-ABL negative myeloproliferative neoplasms (MPNs).1-4 To definitively cure JAK2V617F-mediated MPN in humans, it will be necessary to eradicate all JAK2V617F mutant hematopoietic stem cells (HSCs) that solely possess the capacity to self-renew and therefore maintain the disease over time. Interferon-α (IFNα) is an effective therapy currently used in MPN patients and, importantly, it appears to be more effective than JAK2 kinase inhibitors, which inhibit the key molecular target in MPNs, at achieving molecular remissions in MPNs.5-8 Despite years of observational clinical data, the mechanism by which IFNα induces complete molecular remission (CMR) in MPN patients remains unknown. In this study, we use a conditional Jak2V617F/+E2ACre+ (hereafter Jak2VF) knockin murine model, in which we previously characterized the MPN-initiating stem cell population,9 to investigate the effects of IFNα on Jak2VF MPN stem cells in vivo.
In MPN patients, the JAK2V617F mutation is detectable in the most primitive HSCs in the bone marrow10 and in all mature cell lineages.11,12 JAK2V617F is also found in long-term culture initiating cells, and JAK2V617F mutant SCID repopulating cells are multi-potent and skewed toward myeloid differentiation,13 indicating that JAK2V617F is present in functionally competent long-term HSCs (LT-HSCs). Studies in retro-viral murine models demonstrate that JAK2V617F alone is sufficient to confer an MPN disease phenotype14-17 and using a conditional Jak2V617F knockin model, we previously demonstrated that MPN-propagating cells are contained exclusively in the LT-HSC compartment.18 All of these lines of evidence indicate that, analogous to chronic myelogenous leukemia (CML),19 JAK2V617F-mediated MPN is maintained by a reservoir of disease-propagating stem cells that represent the ultimate therapeutic target for a definitive cure of the disease.
IFNα has a long history of efficacy in the treatment of hematological malignancies. Early studies demonstrated robust improvement in blood counts in response to IFNα treatment in patients with polycythemia vera (PV) and essential thrombocythemia.20,21 More recent clinical trials have demonstrated that in addition to normalizing blood counts in the majority of PV and essential thrombocythemia patients treated, IFNα reduces JAK2V617F allelic burden and, in a significant proportion (15%), renders the JAK2V617F mutant clone undetectable by sensitive molecular assays.5,6,22,23 The development of long-acting pegylated IFNα combined with the modest results shown by JAK kinase inhibitors in reducing JAK2V617F mutant allele burden in patients with myelofibrosis8,24 has renewed interest in the use of IFNα for the treatment of MPN.25 Until recently, it was thought that IFNα therapy acted primarily through immunomodulatory or antiproliferative effects.26 However, 2 studies published in 2009 employed murine models to demonstrate that IFNα can directly activate the cell cycle in quiescent, LT-HSC populations.27,28 These data suggest a novel mechanism by which IFNα may target primitive JAK2V617F MPN stem cell populations, leading to long-term disease eradication. In support of this hypothesis, 2 recent randomized clinical trials examined the addition of pegylated IFNα to imatinib therapy in patients with chronic-phase CML and observed a significantly higher rate of molecular response when compared with patients receiving imatinib alone,29,30 suggesting that IFNα targets CML-maintaining stem cells and depletes them over time.
Using a chimeric bone marrow transplant (BMT) model generated with Jak2VF and wild-type (WT) HSCs, we assessed the effects of IFNα on Jak2VF disease-propagating stem cells in vivo.
Materials and methods
A mouse model of Jak2VF MPN
Jak2V617F/+E2Acre+ (hereafter Jak2VF) and PCR genotyping primers were previously described.9 Jak2VF mice were backcrossed and maintained on C57Bl/6 background (minimum 7-8 generations). B6.Ifnar1 knockout mice were generated by Paul Hertzog.31 CD45.1 Ptprca, CD45.2 C57Bl6/J, and C57Bl6/JxPtprca.F1 mice were obtained from Animal Resources Centre, Australia and Taconic, NY. All mice were maintained in pathogen-free facilities at the Queensland Institute of Medical Research and Children’s Hospital Boston, MA. All mouse experiments were approved by institutional ethics committees Queensland Institute of Medical Research protocol A11605M and Children’s Hospital Boston 10-04-1601R.
Generation of chimeric Jak2VF MPN
Purified HSC-enriched populations (lineagenegKithighSca1+, LKS+) were isolated from Jak2VF (CD45.2) or WT (CD45.1) mice aged 6-12 weeks. These LKS+ populations were mixed at an ∼1:2 ratio (Jak2VF:WT) and injected into lethally irradiated C57Bl6/JxPtprca.F1 (CD45.1/2 double positive) recipient mice. For secondary transplants, 0.5-2 × 106 bone marrow cells were mixed with 1 × 106 competitor bone marrow cells (CD45.1/2 double positive) and injected into lethally irradiated recipient mice (CD45.1/2 double positive). Hematocrit (HCT) and blood parameters were measured 4 weeks after transplantation. Chimerism was determined 4 and 16 weeks after injection. For Ifnar1−/−Jak2VF transplants, we used unfractionated Jak2VF or Ifnar1−/−Jak2VF bone marrow (post red cell lysis) that was normalized to an input of 2 × 104 LKS+ cell content per recipient. Each recipient received an equivalent amount of CD45.1 helper bone marrow in an approximate cell ratio of Jak2VF:WT or Ifnar1−/−Jak2VF:WT of 2:1. Recipient mice received 11 Gy total body irradiation in equal divided doses, at least 3 hours apart within 24 hours of transplantation. All transplanted cells were injected by lateral tail vein. Mice were maintained on antibiotic (Baytril) water for 2 weeks after transplantation.
Blood analysis
Blood was collected into EDTA-coated containers and was analyzed on a Hemavet 950 analyzer (Drew Scientific).
IFNα treatment
Recombinant murine IFNα-4 was purchased (catalog no. 12110; PBL Interferon Source, Piscataway, NJ). A total of 10 000 units (correlated with the published specific activity of each batch) IFNα was diluted in phosphate buffered saline (PBS) containing 0.1% bovine serum albumin and administered by the subcutaneous route daily for 4 weeks or alternate daily for 2 weeks.27 For the long-term (8-10 weeks) experiments, 20 000 units IFNα (Miltenyi Biotech, Teterow, Germany) was administered by subcutaneous route daily (Monday-Friday) for 8-10 weeks. Vehicle control mice received 0.1% bovine serum albumin in PBS injections.
Flow cytometry
Bone marrow, spleen, or peripheral blood were collected and prepared for staining by red blood cell lysis (BD Pharmlyse, BD Biosciences) and homogenization through a 70-μm filter. Erythroid precursor cell stainings were not pretreated with red cell lysis. All samples were analyzed by flow cytometry using FACS LSR Fortessa or LSR II (BD Biosciences). All staining steps were performed in ice-cold PBS containing 2% fetal bovine serum. Postacquisition analyses of data were performed with FlowJo software V9.2.3 (Treestar, CA). For peripheral blood chimerism studies, the following antibodies were used (clone in parentheses): CD45.1 (A20) and CD45.2 (104). For erythroid precursor cells, the following antibodies were used: CD71 (RI7217) and Ter119 (Ter-119). For stem cell and progenitor analysis, the following antibodies were used: lineage cocktail containing CD3ε (145-2C11), CD5 (53-7.3), Ter-119 (TER-119), Gr-1 (RB6-8C5), Mac-1 (M1/70), and B220 (30-F11); Kit (2B8), Sca-1 (D7), CD150 (TC15-12F12.2), CD48 (HM48-1), CD135 (A2F10), CD34 (Ram34), and CD16/32 (93) in addition to CD45.1 (A20) and CD45.2 (104). All antibodies were from Biolegend except CD45.1 Alexa 700 and CD45.2 Horizon V500 (BD Biosciences) and CD16/32 (eBioscience). For dead cell discrimination, Sytox blue (Invitrogen) was used. For cell cycle staining, cells were stained with the lineage cocktail. Samples were depleted of lineage-positive cells by biotin-binder Dynabeads (Invitrogen) and stained with secondary antibodies as above. Samples were then fixed and permeabilized (Fix & Perm, GAS-004; Invitrogen) as per the manufacturer’s instructions and stained with anti-Ki-67 (B56; BD Bioscience) during the permeabilization stage. Finally, cells were stained with Hoechst 33342 (Invitrogen) at a final concentration of 20 μg/mL in PBS containing 2% fetal bovine serum. For apoptosis analysis, the Vybrant FAM Poly Caspases Assay Kit (catalog no. V25117; Molecular Probes, Invitrogen) was used per the manufacturer’s instructions. Cells were first stained with lineage and stem cell antibodies and then with the cell permeant FAM-VAD-FMK poly caspases reagent, which detects active intra-cellular caspases, is fluorescent, and can be quantified using flow cytometry. The gating strategy used to determine LT-HSC frequency and chimerism is shown in supplemental Figure 1. In all cases, percentage chimerism was defined as the proportion of Jak2VF cells as a percentage of total donor cells, ie, (%CD45.2 Jak2VF)/(%CD45.2 Jak2VF + %CD45.1 WT) × 100%. Total engraftment (Figure 5) was measured as the total percentage of CD45.2 cells in the blood of secondary transplant recipients either 4 or 16 weeks after transplantation.
Gene expression analysis
Purified Jak2VF and WT HSC (CD150+LKS+) populations were isolated from IFNα-treated chimeric recipient mice or vehicle-treated controls (4 weeks of IFNα, n = 4 in each group). RNA was extracted using the Picopure RNA extraction kit according to the manufacturer’s instructions (Invitrogen). The samples were amplified with the Illumina Total Prepamp kit (Invitrogen) and hybridized on Illumina MouseRef-8 gene expression arrays (V2_0_R0_11278551_A). The data were analyzed with GenePattern online analysis software including quantile normalization.32 Gene set enrichment analysis (GSEA) v2.0 was used for analysis against datasets of molecular signature datasets (www.broadinstitute.org/msigdb).33 The microarray dataset reported in this article has been deposited in NCBI’s Gene Expression Omnibus and is accessible through GEO Series accession number GSE44961 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE44961).
Statistical analysis
Data were analyzed and figures were generated with Prism for Mac OsX, V5.0 (Graphpad Software Inc.). For comparison between 2 groups, a Student 2-tailed t test was performed. Results given are mean ± standard deviation unless otherwise specified.
Results
Generation of a chimeric BMT model using Jak2VF and WT HSCs
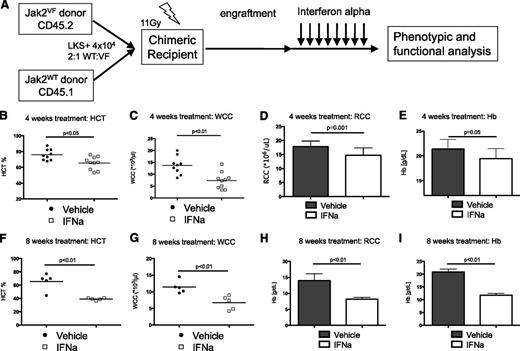
In this study, we used the Jak2VF knockin model to evaluate the effects of IFNα on Jak2VF mutant and WT hematopoietic stem and progenitor cells (HSPCs) in vivo. Because hematopoiesis in patients with MPN is comprised of a mix of both clonal MPN cells bearing somatic genetic mutations and residual polyclonal hematopoietic cells,34 we first undertook to accurately model this heterogeneity in vivo. To do this, we generated a chimeric model of Jak2VF-induced MPN by isolating HSC-enriched LKS+ populations from CD45.2 Jak2VF mice and CD45.1 Ptprca control mice. LKS+ were mixed at a 2:1 ratio (WT:Jak2VF) and injected into lethally irradiated B6xPtprca.F1 (expressing CD45.1 and CD45.2) (Figure 1A). After engraftment (at least 4 weeks), mice were treated with IFNα 10 000 U/d for 4 weeks or 10 000 U on alternate days for 2 weeks. To examine the effects of longer term IFNα, chimeric mice received 20 000 U/d on weekdays for 8 weeks.
Murine IFNα is effective treatment in Jak2VF-induced MPN. (A) Schematic representation of generation of chimeric recipient mice. Purified HSC-enriched populations (lineagenegKithighSca1+) were isolated from Jak2VF (CD45.2) or Jak2WT (CD45.1) and injected into lethally irradiated B6xPtprca.F1 (CD45.1/2 double positive) recipient mice. (B) Effects of 4 weeks of daily IFNα on Jak2VF chimeric mice. Reduced HCT after IFNα (vehicle 76.0 ± 6.9% vs IFNα 65.5 ± 8.1%; P < .05; n = 9-10). (C) Reduced peripheral blood WCC after IFNα (vehicle 13.9 ± 3.7 × 109/L vs IFNα 7.5 ± 3.5 × 109/L; P < .01; n = 9-10). (D) Reduced peripheral blood RCC after IFNα (vehicle 17.8 ± 0.5 × 1012/L vs IFNα 14.7 ± 0.7 × 109/L; P < .01; n = 9-10). (E) Reduced hemoglobin (Hb) after IFNα (vehicle 21.4 ± 0.6 g/L vs IFNα 19.4 ± 0.6 g/L; P < .05; n = 9-10). Each data point represents an individual mouse. Data shown are pooled results from 2 independent experiments. (F) Effects of 8 week daily IFNα on Jak2VF chimeric mice. Reduced HCT after IFNα (vehicle 65.6 ± 5.6% vs IFNα 39.1 ± 0.8%; P < .01; n = 5). (G) Reduced peripheral blood WCC after IFNα (V = vehicle 11.4 ± 0.8 × 109/L vs IFNα 6.7 ± 3.5 × 109/L; P < .01; n = 5). (H) Reduced peripheral blood RCC after IFNα (vehicle 14.0 ± 1.0 × 1012/L vs IFNα 8.2 ± 0.2 × 109/L; P < .01; n = 5). (I) Reduced Hb after IFNα (vehicle 20.9 ± 0.5 g/L vs IFNα 11.8 ± 0.3 g/L; P < .01; n = 5). Each data point represents an individual mouse. Results given are mean ± standard deviation. RCC, red cell count; WCC, white cell count.
Murine IFNα is effective treatment in Jak2VF-induced MPN. (A) Schematic representation of generation of chimeric recipient mice. Purified HSC-enriched populations (lineagenegKithighSca1+) were isolated from Jak2VF (CD45.2) or Jak2WT (CD45.1) and injected into lethally irradiated B6xPtprca.F1 (CD45.1/2 double positive) recipient mice. (B) Effects of 4 weeks of daily IFNα on Jak2VF chimeric mice. Reduced HCT after IFNα (vehicle 76.0 ± 6.9% vs IFNα 65.5 ± 8.1%; P < .05; n = 9-10). (C) Reduced peripheral blood WCC after IFNα (vehicle 13.9 ± 3.7 × 109/L vs IFNα 7.5 ± 3.5 × 109/L; P < .01; n = 9-10). (D) Reduced peripheral blood RCC after IFNα (vehicle 17.8 ± 0.5 × 1012/L vs IFNα 14.7 ± 0.7 × 109/L; P < .01; n = 9-10). (E) Reduced hemoglobin (Hb) after IFNα (vehicle 21.4 ± 0.6 g/L vs IFNα 19.4 ± 0.6 g/L; P < .05; n = 9-10). Each data point represents an individual mouse. Data shown are pooled results from 2 independent experiments. (F) Effects of 8 week daily IFNα on Jak2VF chimeric mice. Reduced HCT after IFNα (vehicle 65.6 ± 5.6% vs IFNα 39.1 ± 0.8%; P < .01; n = 5). (G) Reduced peripheral blood WCC after IFNα (V = vehicle 11.4 ± 0.8 × 109/L vs IFNα 6.7 ± 3.5 × 109/L; P < .01; n = 5). (H) Reduced peripheral blood RCC after IFNα (vehicle 14.0 ± 1.0 × 1012/L vs IFNα 8.2 ± 0.2 × 109/L; P < .01; n = 5). (I) Reduced Hb after IFNα (vehicle 20.9 ± 0.5 g/L vs IFNα 11.8 ± 0.3 g/L; P < .01; n = 5). Each data point represents an individual mouse. Results given are mean ± standard deviation. RCC, red cell count; WCC, white cell count.
Murine IFNα is effective treatment in Jak2VF-induced MPN
To determine the effects of murine IFNα on Jak2VF MPN in vivo, we treated chimeric recipient mice with IFNα 10 000 U/d for 28 days. IFNα treatment was associated with a modest, but significant reduction in HCT, white cell count (WCC), red cell count (RCC), and hemoglobin in Jak2VF chimeric recipients. (Figure 1B-E). There was no significant change in platelet count or total bone marrow cellularity (supplemental Figure 2A-B). IFNα treatment was well tolerated at this dose as evidenced by the absence of a significant reduction in mouse body weight (supplemental Figure 2C). Consistent with human clinical data indicating that molecular responses require prolonged IFNα treatment to be achieved, there was no significant reduction in the percentage of Jak2VF peripheral blood chimerism following this 28-day IFNα treatment schedule in chimeric recipient mice (supplemental Figure 2D). Prolonged treatment with IFNα for 8 weeks (5 d/weeks, 20 000 U/d) demonstrated sustained and progressive normalization of HCT, WCC, RCC, and hemoglobin in Jak2VF chimeric recipients (Figure 1F-I). Together, these data confirm that recombinant murine IFNα treatment induces hematological responses in Jak2VF-mediated MPN.
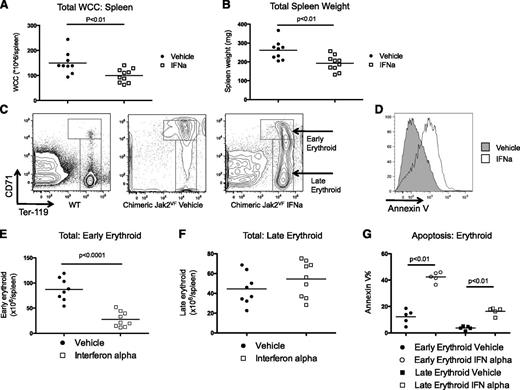
IFNα reverses Jak2VF-induced pathological extramedullary hematopoiesis
Splenomegaly and extramedullary hematopoiesis are cardinal features of JAK2V617F-induced MPN in patients and murine models of disease.9,35-38 We found that IFNα treatment decreased extramedullary hematopoiesis as evidenced by a significant reduction in the total spleen WCC and splenic weight (Figure 2A-B). There was also a significant reduction in the percentage of mobilized progenitor cells (lineagelowKithigh) in the spleens after IFNα treatment (supplemental Figure 3). To evaluate the effects of IFNα treatment on extramedullary erythropoiesis, we assessed erythrocyte differentiation using flow cytometry (Figure 2C for representative plots). Immature or early erythroid precursor cells (CD71highTer119mid-high) were expanded in Jak2VF transplant mice as previously described in Jak2VF primary mice.9 IFNα treatment resulted in a marked reduction in the total immature erythroid population of Jak2VF chimeric mice (Figure 2E), whereas late erythropoiesis (CD71low-midTer119high) was relatively preserved (Figure 2F). We found increased apoptosis in erythroid precursors after IFNα treatment, and again this effect was most pronounced in immature erythroid precursors (Figure 2D,G). To determine if these effects were specific for the extramedullary erythropoiesis seen in Jak2VF MPN, we treated nontransplanted primary Jak2VF or WT mice with IFNα. We found a relative reduction in immature erythropoiesis in IFNα-treated Jak2VF mice; however, no changes were observed in IFNα-treated WT mice (supplemental Figure 4). In aggregate, these findings demonstrate that IFNα reverses splenomegaly due to extramedullary hematopoiesis and that IFNα induces apoptosis in Jak2VF erythroid precursor cells, with immature erythroid precursors (CD71highTer119mid-high) demonstrating enhanced sensitivity compared with mature erythroid precursors (CD71low-midTer119high).
Effects on extramedullary hematopoiesis of 4 weeks of daily IFNα on Jak2VF chimeric mice. (A) Reduced spleen WCC after IFNα (vehicle 149.6 ± 44.1 × 106 vs IFNα 100 ± 27.1 × 106; P < .01; n = 9-10). (B) Reduced spleen weight after IFNα (vehicle 262 ± 51.2 mg vs IFNα 192 ± 40.6 mg; P ≤ .01; n = 9-10) (C) Representative flow cytometry plots showing maturation of erythropoiesis in WT and vehicle- and IFNα-treated mice. Early erythropoiesis is CD71high, late erythropoiesis is CD71mid-lowTer119+. (D) Representative histogram plots demonstrating annexin V staining on early erythroid cells. (E) Total early erythropoiesis reduced in IFNα-treated mice (vehicle 87.2 ± 8.0 × 106/spleen vs IFNα 27.6 ± 5.0 × 106/spleen; P < .01; n = 9-10) (F) No change in total late erythropoiesis (vehicle 44.3 ± 5.7 × 106/spleen vs IFNα 54.4 ± 6.0 × 106/spleen; P = .24; n = 9-10). Each data point represents an individual mouse. Data shown are pooled results from 2 independent experiments. (G) Apoptosis in early erythroid precursors increased after IFNα treatment (vehicle 12.3 ± 2.4% vs IFNα 42.4 ± 1.7; P < .01; n = 5). Apoptosis in late erythroid precursors increased after IFNα treatment (vehicle 3.7 ± 0.6% vs IFNα 16.4 ± 1.3; P < .01; n = 5). Results given are mean ± standard deviation.
Effects on extramedullary hematopoiesis of 4 weeks of daily IFNα on Jak2VF chimeric mice. (A) Reduced spleen WCC after IFNα (vehicle 149.6 ± 44.1 × 106 vs IFNα 100 ± 27.1 × 106; P < .01; n = 9-10). (B) Reduced spleen weight after IFNα (vehicle 262 ± 51.2 mg vs IFNα 192 ± 40.6 mg; P ≤ .01; n = 9-10) (C) Representative flow cytometry plots showing maturation of erythropoiesis in WT and vehicle- and IFNα-treated mice. Early erythropoiesis is CD71high, late erythropoiesis is CD71mid-lowTer119+. (D) Representative histogram plots demonstrating annexin V staining on early erythroid cells. (E) Total early erythropoiesis reduced in IFNα-treated mice (vehicle 87.2 ± 8.0 × 106/spleen vs IFNα 27.6 ± 5.0 × 106/spleen; P < .01; n = 9-10) (F) No change in total late erythropoiesis (vehicle 44.3 ± 5.7 × 106/spleen vs IFNα 54.4 ± 6.0 × 106/spleen; P = .24; n = 9-10). Each data point represents an individual mouse. Data shown are pooled results from 2 independent experiments. (G) Apoptosis in early erythroid precursors increased after IFNα treatment (vehicle 12.3 ± 2.4% vs IFNα 42.4 ± 1.7; P < .01; n = 5). Apoptosis in late erythroid precursors increased after IFNα treatment (vehicle 3.7 ± 0.6% vs IFNα 16.4 ± 1.3; P < .01; n = 5). Results given are mean ± standard deviation.
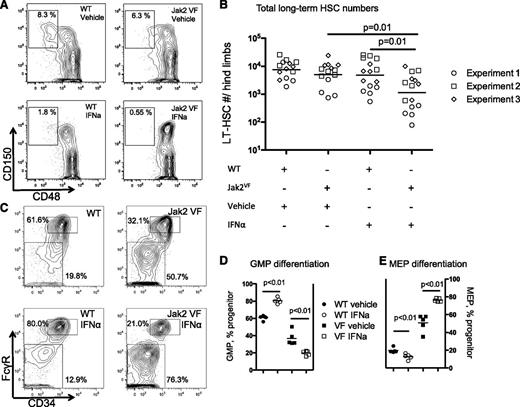
IFNα depletes Jak2VF disease-inducing long-term stem cells
Oncogenic Jak2VF is sufficient to engender MPN, and the expression of Jak2VF in LT-HSC is required for the full manifestation of disease in vivo.18 This identifies Jak2VF LT-HSC as the exclusive reservoir of disease-initiating stem cells in vivo. IFNα has been shown to exhaust long-term WT HSC function in murine models27,28 and the observation that molecular responses in MPN patients can be sustained even after discontinuation of IFNα therapy23,25,39 suggests that IFNα may act by depleting Jak2VF disease-propagating stem cells. To determine the effects of IFNα treatment on Jak2VF LT-HSC, we determined the relative frequency of LT-HSC in chimeric mice treated with IFNα or vehicle by using flow cytometry (CD45.1 WT or CD45.2 Jak2VF lineagelowKithighSca1+CD150+CD48− cells; Figure 3A). Jak2VF LT-HSCs were significantly depleted after 4 weeks of IFNα treatment (Figure 3B). Next, we determined the effects of IFNα treatment on myeloid-specified progenitor cell compartments using flow cytometry (Figure 3C). Surprisingly, IFNα treatment impaired megakaryocyte-erythroid progenitor (MEP) cell numbers in WT cells and increased granulocyte-macrophage progenitor (GMP) differentiation. In contrast, IFNα selectively increased MEP differentiation in Jak2VF mice at the expense of GMP numbers (Figure 3D-E). The overall levels of apoptosis in LT-HSC populations were low and there was no significant induction of apoptosis in Jak2VF compared with WT LT-HSC populations (supplemental Figure 5). These findings suggest that IFNα is not directly toxic to Jak2VF stem cells but enforces a preexisting Jak2VF-driven erythroid-lineage differentiation program within Jak2VF HSCs.9
Effect on HSPC populations of 4 weeks of daily IFNα on Jak2VF chimeric mice. (A) Representative flow cytometry plots showing reduction in lineagelowKithighSca1+CD150+CD48− LT-HSCs from the Jak2VF IFNα-treated cells (expressed as percentage of parent LKS+ gate). (B) Reduction in LT-HSCs expressed as absolute number per lower limb cellularity (vehicle-treated WT: 8896 ± 6612 vs Jak2VF 6930 ± 6128; P = .34; n = 14; IFNα-treated WT: 6503 ± 8885 vs Jak2VF 2020 ± 3007; P = .01; n = 15; vehicle vs IFNα: Jak2VF; P = .01; vehicle vs IFNα WT: P = .78). Experimental replicates are shown as circles, squares, and diamonds (experiments 1, 2, and 3, respectively). (C) Representative flow cytometry plots showing that IFNα treatment in WT cells causes a relative reduction in MEPs (lineagelowKithighSca1−CD34−FcGR−), with relative expansion of GMP (lineagelowKithighSca1−CD34+FcGR+). Conversely, IFNα treatment in Jak2VF cells causes further expansion in MEP and relative reduction in GMP. (Percentage of parent lineagelowKithigh, LK+ gate). (D) Two weeks of IFNα treatment in WT cells increases GMP differentiation (WT vehicle: 60.8 ± 2.7% vs IFNα: 80.5 ± 3.3%; P < .01; n = 4-5) and reduces GMP differentiation in Jak2VF cells (Jak2VF vehicle: 36.8 ± 8.1% vs IFNα: 19.6 ± 2.7%; P < .01; n = 4-5). (E) Two weeks of IFNα treatment in WT cells reduces MEP differentiation (WT vehicle: 19.8 ± 2.8% vs IFNα: 12.9 ± 3.2%; P < .01; n = 4-5) and increases MEP differentiation in Jak2VF cells (Jak2VF vehicle: 50.7 ± 9.6% vs IFNα: 76.3 ± 1.9%; P < .01; n = 4-5). Each data point represents an individual mouse. Data shown are representative of at least 2 independent experiments. Similar results were found in each experiment. Results given are mean ± standard deviation.
Effect on HSPC populations of 4 weeks of daily IFNα on Jak2VF chimeric mice. (A) Representative flow cytometry plots showing reduction in lineagelowKithighSca1+CD150+CD48− LT-HSCs from the Jak2VF IFNα-treated cells (expressed as percentage of parent LKS+ gate). (B) Reduction in LT-HSCs expressed as absolute number per lower limb cellularity (vehicle-treated WT: 8896 ± 6612 vs Jak2VF 6930 ± 6128; P = .34; n = 14; IFNα-treated WT: 6503 ± 8885 vs Jak2VF 2020 ± 3007; P = .01; n = 15; vehicle vs IFNα: Jak2VF; P = .01; vehicle vs IFNα WT: P = .78). Experimental replicates are shown as circles, squares, and diamonds (experiments 1, 2, and 3, respectively). (C) Representative flow cytometry plots showing that IFNα treatment in WT cells causes a relative reduction in MEPs (lineagelowKithighSca1−CD34−FcGR−), with relative expansion of GMP (lineagelowKithighSca1−CD34+FcGR+). Conversely, IFNα treatment in Jak2VF cells causes further expansion in MEP and relative reduction in GMP. (Percentage of parent lineagelowKithigh, LK+ gate). (D) Two weeks of IFNα treatment in WT cells increases GMP differentiation (WT vehicle: 60.8 ± 2.7% vs IFNα: 80.5 ± 3.3%; P < .01; n = 4-5) and reduces GMP differentiation in Jak2VF cells (Jak2VF vehicle: 36.8 ± 8.1% vs IFNα: 19.6 ± 2.7%; P < .01; n = 4-5). (E) Two weeks of IFNα treatment in WT cells reduces MEP differentiation (WT vehicle: 19.8 ± 2.8% vs IFNα: 12.9 ± 3.2%; P < .01; n = 4-5) and increases MEP differentiation in Jak2VF cells (Jak2VF vehicle: 50.7 ± 9.6% vs IFNα: 76.3 ± 1.9%; P < .01; n = 4-5). Each data point represents an individual mouse. Data shown are representative of at least 2 independent experiments. Similar results were found in each experiment. Results given are mean ± standard deviation.
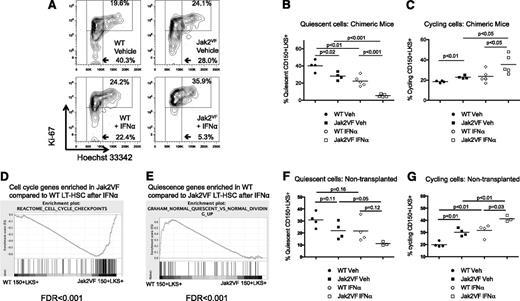
IFNα depletes quiescent Jak2VF disease-inducing long-term stem cells
The maintenance of a quiescent LT-HSC population is essential for long-term self-renewal and this correlates with HSC function in vivo.40,41 To test the hypothesis that IFNα depletes Jak2VF LT-HSCs by activating the stem cell cycle and enforcing differentiation, we determined the cell cycle status of LT-HSC–enriched populations (lineagelowKithighSca1+CD150+). We used Hoechst 33342 and Ki-67 staining to determine the relative contribution of quiescent G0 cells (Ki-67-H333422n), activated G1 cells (Ki-67+H333422n) and actively cycling cells (Ki-67+H333424n) (Figure 4A). In vehicle-treated mice, there was a consistent reduction in G0 Jak2VF LT-HSCs compared with WT cells. Consistent with previous reports, IFNα treatment caused activation of cell cycle in WT and Jak2VF LT-HSCs.27,28 Importantly, Jak2VF LT-HSCs were more sensitive to IFNα treatment compared with WT LT-HSCs, as evidenced by significantly reduced quiescent, but more cycling LT-HSCs in Jak2VF compared with WT cells after IFNα treatment (Figure 4B-C). These findings were confirmed with Hoechst 33342/ Pyronin Y staining on sorted LT-HSC–enriched populations (supplemental Figure 6). We found similar results in primary (nontransplanted) Jak2VF mice (Figure 4F-G).
Effect of IFNα treatment on LT-HSC cell cycle in chimeric transplant recipients and primary mice. (A) Representative flow cytometry plots showing cell cycle in lineagelowKithighSca1+CD150+ WT (CD45.1) or Jak2VF (CD45.2) LT-HSCs from chimeric recipient mice. G0 (quiescent) cells are Ki-67lowH333422n, G1 cells are Ki-67+H333422n, and SG2M cells are Ki67+H333424n. (B) Reduction in quiescent HSCs after IFNα treatment in WT (gated on CD45.1 WT vehicle: 40.3 ± 6.6% vs IFNα: 22.4 ± 6.0%; P < .01; n = 4) and reduction in quiescent HSCs after IFNα treatment in Jak2VF (gated on CD45.2 Jak2VF, vehicle: 28.0 ± 2.3% vs IFNα: 5.4 ± 0.7%; P < .0001; n = 5). There were significantly fewer quiescent Jak2VF compared with WT HSCs (1.4-fold; P = .02). More profound depletion of quiescent Jak2VF HSC compared with WT HSCs (4.2 fold; P = .0003). (C) Increase in actively cycling (Ki67+H333424n) HSCs after IFNα treatment in Jak2VF cells (gated on CD45.1 WT vehicle: 19.6 ± 0.4% vs WT IFNα: 24.2 ± 6.5%; P = .21; gated on CD45.2 Jak2VF vehicle: 24.1 ± 0.9% vs Jak2VF IFNα: 35.9 ± 9.6%; P < .05; n = 4-5). There were significantly more Jak2VF than WT HSCs in active cell cycle in vehicle-treated recipients (1.2-fold; P < .01) and also in IFNα-treated recipients (1.5-fold; P = .05). Each data point represents an individual mouse. Data shown are representative of at least 2 independent experiments using chimeric recipient mice. Similar results were found in each experiment. Results given are mean ± standard deviation. (D) GSEA performed on lineagelowKithighSca1+CD150+ WT (CD45.1) or Jak2VF (CD45.2) cells from chimeric recipient mice revealed that genes regulating cell cycle were enriched in Jak2VF HSCs compared with WT HSCs. (E) Genes regulating quiescence were relatively enriched in WT HSCs compared with Jak2VF HSCs. (F) Reduction in quiescent (Ki67lowH333422n) HSCs in WT and Jak2VF primary mice after IFNα treatment (WT vehicle: 31.0 ± 3.2% vs WT IFNα: 21.7 ± 4.9%; P = .16; Jak2VF vehicle: 22.0 ± 3.6% vs Jak2VF IFNα: 11.1 ± 0.9%; P = .05; n = 3-4/group). (G) Increase in actively cycling (Ki67+H333424n) HSCs in WT and Jak2VF primary mice after IFNα treatment (WT vehicle: 20.0 ± 1.1% vs WT IFNα: 31.7 ± 2.6%; P < .01; Jak2VF vehicle: 30.1 ± 1.6% vs Jak2VF IFNα: 41.3 ± 1.4%; P < .01; n = 3-4/group). There were significantly more Jak2VF than WT HSCs in active cell cycle in vehicle-treated recipients (P < .01) and also in IFNα-treated recipients (P = .03).
Effect of IFNα treatment on LT-HSC cell cycle in chimeric transplant recipients and primary mice. (A) Representative flow cytometry plots showing cell cycle in lineagelowKithighSca1+CD150+ WT (CD45.1) or Jak2VF (CD45.2) LT-HSCs from chimeric recipient mice. G0 (quiescent) cells are Ki-67lowH333422n, G1 cells are Ki-67+H333422n, and SG2M cells are Ki67+H333424n. (B) Reduction in quiescent HSCs after IFNα treatment in WT (gated on CD45.1 WT vehicle: 40.3 ± 6.6% vs IFNα: 22.4 ± 6.0%; P < .01; n = 4) and reduction in quiescent HSCs after IFNα treatment in Jak2VF (gated on CD45.2 Jak2VF, vehicle: 28.0 ± 2.3% vs IFNα: 5.4 ± 0.7%; P < .0001; n = 5). There were significantly fewer quiescent Jak2VF compared with WT HSCs (1.4-fold; P = .02). More profound depletion of quiescent Jak2VF HSC compared with WT HSCs (4.2 fold; P = .0003). (C) Increase in actively cycling (Ki67+H333424n) HSCs after IFNα treatment in Jak2VF cells (gated on CD45.1 WT vehicle: 19.6 ± 0.4% vs WT IFNα: 24.2 ± 6.5%; P = .21; gated on CD45.2 Jak2VF vehicle: 24.1 ± 0.9% vs Jak2VF IFNα: 35.9 ± 9.6%; P < .05; n = 4-5). There were significantly more Jak2VF than WT HSCs in active cell cycle in vehicle-treated recipients (1.2-fold; P < .01) and also in IFNα-treated recipients (1.5-fold; P = .05). Each data point represents an individual mouse. Data shown are representative of at least 2 independent experiments using chimeric recipient mice. Similar results were found in each experiment. Results given are mean ± standard deviation. (D) GSEA performed on lineagelowKithighSca1+CD150+ WT (CD45.1) or Jak2VF (CD45.2) cells from chimeric recipient mice revealed that genes regulating cell cycle were enriched in Jak2VF HSCs compared with WT HSCs. (E) Genes regulating quiescence were relatively enriched in WT HSCs compared with Jak2VF HSCs. (F) Reduction in quiescent (Ki67lowH333422n) HSCs in WT and Jak2VF primary mice after IFNα treatment (WT vehicle: 31.0 ± 3.2% vs WT IFNα: 21.7 ± 4.9%; P = .16; Jak2VF vehicle: 22.0 ± 3.6% vs Jak2VF IFNα: 11.1 ± 0.9%; P = .05; n = 3-4/group). (G) Increase in actively cycling (Ki67+H333424n) HSCs in WT and Jak2VF primary mice after IFNα treatment (WT vehicle: 20.0 ± 1.1% vs WT IFNα: 31.7 ± 2.6%; P < .01; Jak2VF vehicle: 30.1 ± 1.6% vs Jak2VF IFNα: 41.3 ± 1.4%; P < .01; n = 3-4/group). There were significantly more Jak2VF than WT HSCs in active cell cycle in vehicle-treated recipients (P < .01) and also in IFNα-treated recipients (P = .03).
To determine the underlying molecular pathways that were differentially activated by IFNα treatment in Jak2VF HSCs compared with WT HSCs, we performed gene expression profiling on purified HSC populations (lineagelowKithighSca1+CD150+). GSEA revealed significant enrichment of genes regulating cell cycle in IFNα-treated Jak2VF HSCs compared with IFNα-treated WT HSCs (Figure 4D). In contrast, there was a relative enrichment of genes regulating quiescence in WT HSCs compared to Jak2VF HSCs after IFNα treatment (Figure 4E). An enrichment of cell cycle genes in Jak2VF LT-HSCs compared to WT LT-HSCs in vehicle-treated chimeric animals was also observed (supplemental Table 1). Finally, IFNα27 and Stat-1 targets were nondifferentially enriched in IFNα-treated Jak2VF and IFNα-treated WT HSCs compared with vehicle controls (data not shown). Together, these findings demonstrate that Jak2VF LT-HSCs are preferentially sensitive to cell cycle activation by IFNα.
IFNα treatment reduces Jak2VF MPNs in secondary transplant recipients
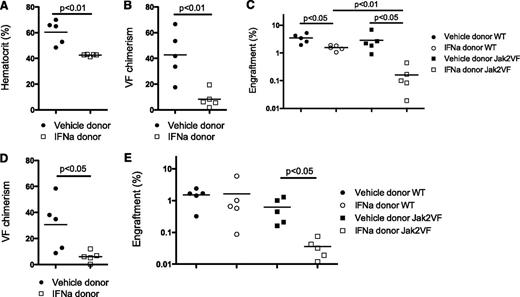
A defining characteristic of disease-initiating stem cells is the ability to transplant or recapitulate the disease in vivo.9,42 To evaluate the effects of IFNα treatment on Jak2VF MPN-initiating cells, we performed serial transplantation experiments. A total of 5 × 105 bone marrow cells from vehicle- or IFNα-treated Jak2VF chimeric mice were transplanted into lethally irradiated congenic recipient mice together with congenic helper bone marrow (1 × 106 cells) and recipient animals were monitored for MPN development. Recipients of bone marrow from vehicle-treated Jak2VF chimeric mice developed elevated HCT that was present by 4 weeks after transplantation; however, recipients of bone marrow from IFNα-treated Jak2VF chimeric mice did not (Figure 5A). In comparison with vehicle-treated Jak2VF chimeric donors, IFNα-treated Jak2VF chimeric donors demonstrated significantly reduced Jak2VF chimerism in secondary recipients, indicating that IFNα selectively targets Jak2VF MPN-initiating cells (Figure 5B). Finally, there was significantly reduced total engraftment in recipient mice that received IFNα-treated bone marrow, with a selective reduction in the engraftment of IFNα-treated Jak2VF bone marrow over IFNα-treated WT bone marrow (Figure 5C). This demonstrates preferential depletion of functional repopulating Jak2VF LT-HSCs compared with WT LT-HSCs after IFNα. The effects on Jak2VF chimerism and total engraftment were sustained up to 16 weeks after transplantation (Figure 5D-E), although persistent, long-term, low-level Jak2VF engraftment was present in all recipient mice. The reduction in secondary transplantation of disease was confirmed at an increased cell dose (2 × 106 donor bone marrow cells). For vehicle-treated chimeric donors, 4 of 4 recipients of the 2 × 106 cell dose demonstrated elevated HCT 4 and 16 weeks after transplantation. In contrast, for IFNα-treated chimeric donors, 1 of 4 recipients of the 2 × 106 cell dose demonstrated elevated HCT 4 weeks after transplantation and 3 of 4 recipients had elevated HCT 16 weeks after transplantation. These findings demonstrate that IFNα treatment depletes functional Jak2VF disease-initiating stem cells and impairs the transmission of Jak2VF MPN in vivo and suggest that in the absence of sustained treatment with IFNα, Jak2VF MPN-propagating stem cells can persist and maintain disease.
Functional depletion of Jak2VF disease-initiating stem cells after IFNα treatment. (A) Normal HCT in recipients from IFNα-treated but not vehicle-treated donor Jak2VF chimeric mice bone marrow 4 weeks after transplantation (vehicle: 60.5 ± 9.2% vs IFNα: 42.6 ± 0.8%: P < .01; n = 5). (B) Reduced Jak2VF chimerism in recipients 4 weeks after transplantation from IFNα-treated mice but not from vehicle-treated donor mice (vehicle: 42.6 ± 18.8% vs IFNα: 8.2 ± 6.7%; P < .01; n = 5). (C) Absolute engraftment (total CD45.2+ cells in peripheral blood) 4 weeks after transplantation in secondary transplant recipient (WT vehicle: 3.5 ± 1.3% vs IFNα: 1.5 ± 0.3%; P < .05; n = 5; Jak2VF vehicle: 2.9 ± 2.3% vs IFNα: 0.2 ± 0.2%; P < .05; n = 5; vehicle WT vs Jak2VF: 3.5% vs 2.9%; P = .64; IFNα-treated WT vs Jak2VF: 1.5% vs 0.2%; P < .01). (D) Sixteen weeks of chimerism demonstrating sustained reduction in Jak2VF chimerism in recipients of bone marrow from IFNα-treated mice but not vehicle-treated donor mice (vehicle: 30.6 ± 20.2% vs IFNα: 6.1 ± 4.2%; P < .05; n = 5). (E) Engraftment in secondary transplant recipients 16 weeks after transplantation (WT vehicle: 1.5 ± 0.7% vs IFNα: 1.6 ± 2.4%; P = .90; n = 5; Jak2VF vehicle: 0.6 ± 0.5% vs IFNα: 0.04 ± 0.02%; P < .05; n = 5). Each data point represents an individual mouse. Data shown are representative of at least 2 independent experiments. Similar results were found in each experiment. Results given are mean ± standard deviation.
Functional depletion of Jak2VF disease-initiating stem cells after IFNα treatment. (A) Normal HCT in recipients from IFNα-treated but not vehicle-treated donor Jak2VF chimeric mice bone marrow 4 weeks after transplantation (vehicle: 60.5 ± 9.2% vs IFNα: 42.6 ± 0.8%: P < .01; n = 5). (B) Reduced Jak2VF chimerism in recipients 4 weeks after transplantation from IFNα-treated mice but not from vehicle-treated donor mice (vehicle: 42.6 ± 18.8% vs IFNα: 8.2 ± 6.7%; P < .01; n = 5). (C) Absolute engraftment (total CD45.2+ cells in peripheral blood) 4 weeks after transplantation in secondary transplant recipient (WT vehicle: 3.5 ± 1.3% vs IFNα: 1.5 ± 0.3%; P < .05; n = 5; Jak2VF vehicle: 2.9 ± 2.3% vs IFNα: 0.2 ± 0.2%; P < .05; n = 5; vehicle WT vs Jak2VF: 3.5% vs 2.9%; P = .64; IFNα-treated WT vs Jak2VF: 1.5% vs 0.2%; P < .01). (D) Sixteen weeks of chimerism demonstrating sustained reduction in Jak2VF chimerism in recipients of bone marrow from IFNα-treated mice but not vehicle-treated donor mice (vehicle: 30.6 ± 20.2% vs IFNα: 6.1 ± 4.2%; P < .05; n = 5). (E) Engraftment in secondary transplant recipients 16 weeks after transplantation (WT vehicle: 1.5 ± 0.7% vs IFNα: 1.6 ± 2.4%; P = .90; n = 5; Jak2VF vehicle: 0.6 ± 0.5% vs IFNα: 0.04 ± 0.02%; P < .05; n = 5). Each data point represents an individual mouse. Data shown are representative of at least 2 independent experiments. Similar results were found in each experiment. Results given are mean ± standard deviation.
The effects of IFNα are cell autonomous and mediated through type 1 interferon signaling
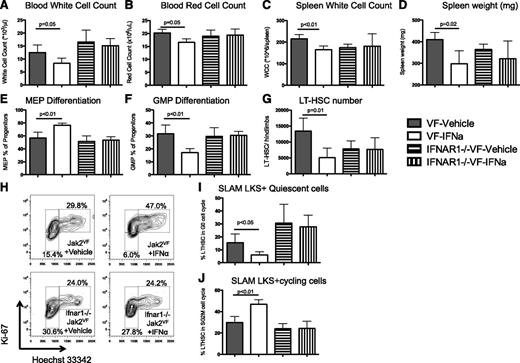
To determine whether the effects on Jak2VF LT-HSCs were cell autonomous and specific to type 1 interferon signaling, we established chimeric recipient mice from Jak2VF or Jak2VF IFNα receptor null (Ifnar1−/−) donors.31 These chimeric recipients were ∼70% Jak2VF CD45.2 and 30% WT CD45.1. Chimeric recipient mice were treated with 10 000 U/d IFNα for 4 weeks. Consistent with the previous data, IFNα treatment caused a significant reduction in the WCC and RCC in the peripheral blood of chimeric mice, whereas no changes were seen in Jak2VFIfnar1−/− chimeras (Figure 6A-B). Furthermore, extramedullary hematopoiesis, as evidenced by spleen size and spleen WCC, was reduced in Jak2VF chimeras after IFNα treatment and these changes were not seen in Jak2VFIfnar1−/− chimeras (Figure 6C-D). Treatment with IFNα caused increased MEP and suppressed GMP differentiation in Jak2VF cells; however, this was not observed in Jak2VFIfnar1−/− chimeras (Figure 6E-F). There was a reduction in total LT-HSC numbers in Jak2VF chimeras after treatment with IFNα; however, there was no change in total LT-HSC numbers in Jak2VFIfnar1−/− (Figure 6G). Finally, treatment with IFNα led to a reduction in the G0 (quiescent) HSC population and an increase in the actively cycling HSC population in Jak2VF cells. However, there was no change to cell cycle in Jak2VFIfnar1−/− chimeras after treatment with IFNα (Figure 6H-J). Together, these data demonstrate that the effects of IFNα on Jak2VF HSCs are specific to type 1 interferon signaling and are cell autonomous. Moreover, these data confirm the effects of IFNα treatment in a system that is biased toward a higher Jak2VF chimeric burden.
The effects of IFNα are cell autonomous and specific to type 1 interferon signaling. (A) Peripheral blood WCC was reduced in IFNα-treated Jak2VF chimeric mice compared with vehicle controls (12.5 ± 2.9 × 106/L vs 8.3 ± 2.0 × 106/L, respectively; P = .05) but not in IFNα-treated Ifnar1−/−Jak2VF chimeric mice compared with vehicle controls (16.5 ± 4.6 × 106/L vs 15.1 ± 2.8 × 106/L, respectively; P = .62). (B) Peripheral blood RCC was reduced in IFNα-treated Jak2VF chimeric mice compared with vehicle controls (20.2 ± 0.8 × 109/L vs 16.6 ± 0.7 × 109/L, respectively; P = .02) but not in IFNα-treated Ifnar1−/−Jak2VF chimeric mice compared with vehicle controls (18.9 ± 1.2 × 109/L vs 19.4 ± 1.2 × 109/L, respectively; P = .81). (C) Spleen WCC was reduced in IFNα-treated Jak2VF chimeric mice compared with vehicle controls (165 ± 8 × 106 vs 215 ± 10 × 106, respectively; P < .01) but not in IFNα-treated Ifnar1−/−Jak2VF chimeric mice compared with vehicle controls (181 ± 29 × 106 vs 174 ± 8 × 106, respectively; P = .83). (D) Spleen weight was reduced in IFNα-treated Jak2VF chimeric mice compared with vehicle controls (298 ± 30 mg vs 410 ± 17 mg, respectively; P = .02); however, there was no difference in IFNα-treated Ifnar1−/−Jak2VF chimeric mice compared with vehicle controls (321 ± 41 mg vs 364 ± 12 mg, respectively; P = .36). (E) MEP differentiation was potentiated by IFNα treatment in Jak2VF chimeric mice compared with vehicle controls (76.4 ± 1.7% vs 56.9 ± 4.4%, respectively; P < .01), but there was no effect in Ifnar1−/−Jak2VF chimeric mice compared with vehicle controls (53.4 ± 2.7% vs 51.3 ± 4.4%, respectively; P = .69). (F) GMP differentiation was impaired in IFNα-treated Jak2VF chimeric mice compared with vehicle controls (17.1 ± 1.5% vs 31.7 ± 3.3%, respectively; P < .01), but there was no effect in IFNα-treated Ifnar1−/−Jak2VF chimeric mice compared with vehicle controls (30.4 ± 1.6% vs 29.6 ± 3.4%, respectively; P = .85). (G) Reduced total LT-HSC numbers in IFNα-treated Jak2VF chimeric mice compared with vehicle controls (5040 ± 1502 vs 13 430 ± 2026, respectively; P = .01) but no effect in IFNα-treated Ifnar1−/−Jak2VF chimeric mice compared with vehicle controls (7662 ± 1843 vs 7849 ± 1237, respectively; P = .94). (H) Representative flow cytometry plots showing cell cycle in lineagelowKithighSca1+CD150+ Jak2VF or Ifnar1−/−Jak2VF LT-HSCs from chimeric recipient mice. G0 (quiescent) cells are Ki-67lowDNA2n, G1 cells are Ki-67+DNA2n, and SG2M cells are Ki67+DNA4n. (I) Quiescence in Jak2VF HSCs was reduced after IFNα treatment compared with vehicle control (6.0 ± 1.2% vs 15.4 ± 3.4%, respectively; P = .04), but there was no difference in Ifnar1−/−Jak2VF HSCs with IFNα treatment or vehicle control (27.8 ± 4.5% vs 30.6 ± 7.3%, respectively; P = .75). (J) Actively cycling Jak2VF HSCs increased after IFNα treatment compared with vehicle control (47.0 ± 2.1% vs 29.8 ± 2.9%, respectively; P < .01), but there was no difference in Ifnar1−/−Jak2VF HSCs after IFNα treatment compared with vehicle control (24.2 ± 3.4% vs 24.0 ± 2.4%, respectively; P = .96) (n = 4/group for each experiment, mean +/− standard deviation).
The effects of IFNα are cell autonomous and specific to type 1 interferon signaling. (A) Peripheral blood WCC was reduced in IFNα-treated Jak2VF chimeric mice compared with vehicle controls (12.5 ± 2.9 × 106/L vs 8.3 ± 2.0 × 106/L, respectively; P = .05) but not in IFNα-treated Ifnar1−/−Jak2VF chimeric mice compared with vehicle controls (16.5 ± 4.6 × 106/L vs 15.1 ± 2.8 × 106/L, respectively; P = .62). (B) Peripheral blood RCC was reduced in IFNα-treated Jak2VF chimeric mice compared with vehicle controls (20.2 ± 0.8 × 109/L vs 16.6 ± 0.7 × 109/L, respectively; P = .02) but not in IFNα-treated Ifnar1−/−Jak2VF chimeric mice compared with vehicle controls (18.9 ± 1.2 × 109/L vs 19.4 ± 1.2 × 109/L, respectively; P = .81). (C) Spleen WCC was reduced in IFNα-treated Jak2VF chimeric mice compared with vehicle controls (165 ± 8 × 106 vs 215 ± 10 × 106, respectively; P < .01) but not in IFNα-treated Ifnar1−/−Jak2VF chimeric mice compared with vehicle controls (181 ± 29 × 106 vs 174 ± 8 × 106, respectively; P = .83). (D) Spleen weight was reduced in IFNα-treated Jak2VF chimeric mice compared with vehicle controls (298 ± 30 mg vs 410 ± 17 mg, respectively; P = .02); however, there was no difference in IFNα-treated Ifnar1−/−Jak2VF chimeric mice compared with vehicle controls (321 ± 41 mg vs 364 ± 12 mg, respectively; P = .36). (E) MEP differentiation was potentiated by IFNα treatment in Jak2VF chimeric mice compared with vehicle controls (76.4 ± 1.7% vs 56.9 ± 4.4%, respectively; P < .01), but there was no effect in Ifnar1−/−Jak2VF chimeric mice compared with vehicle controls (53.4 ± 2.7% vs 51.3 ± 4.4%, respectively; P = .69). (F) GMP differentiation was impaired in IFNα-treated Jak2VF chimeric mice compared with vehicle controls (17.1 ± 1.5% vs 31.7 ± 3.3%, respectively; P < .01), but there was no effect in IFNα-treated Ifnar1−/−Jak2VF chimeric mice compared with vehicle controls (30.4 ± 1.6% vs 29.6 ± 3.4%, respectively; P = .85). (G) Reduced total LT-HSC numbers in IFNα-treated Jak2VF chimeric mice compared with vehicle controls (5040 ± 1502 vs 13 430 ± 2026, respectively; P = .01) but no effect in IFNα-treated Ifnar1−/−Jak2VF chimeric mice compared with vehicle controls (7662 ± 1843 vs 7849 ± 1237, respectively; P = .94). (H) Representative flow cytometry plots showing cell cycle in lineagelowKithighSca1+CD150+ Jak2VF or Ifnar1−/−Jak2VF LT-HSCs from chimeric recipient mice. G0 (quiescent) cells are Ki-67lowDNA2n, G1 cells are Ki-67+DNA2n, and SG2M cells are Ki67+DNA4n. (I) Quiescence in Jak2VF HSCs was reduced after IFNα treatment compared with vehicle control (6.0 ± 1.2% vs 15.4 ± 3.4%, respectively; P = .04), but there was no difference in Ifnar1−/−Jak2VF HSCs with IFNα treatment or vehicle control (27.8 ± 4.5% vs 30.6 ± 7.3%, respectively; P = .75). (J) Actively cycling Jak2VF HSCs increased after IFNα treatment compared with vehicle control (47.0 ± 2.1% vs 29.8 ± 2.9%, respectively; P < .01), but there was no difference in Ifnar1−/−Jak2VF HSCs after IFNα treatment compared with vehicle control (24.2 ± 3.4% vs 24.0 ± 2.4%, respectively; P = .96) (n = 4/group for each experiment, mean +/− standard deviation).
Discussion
IFNα treatment achieves molecular remissions in MPN patients,5,6,22 and in murine models, IFNα has been shown to exhaust long-term WT HSC function.27,28 In aggregate, these results suggest that IFNα may eliminate the JAK2V617F mutant clone in MPN patients by preferentially targeting JAK2V617F MPN-propagating stem cells and depleting them over time. However, because the JAK2V617F allelic burden is typically quantified in peripheral blood granulocytes and because the majority of clonal expansion in MPN occurs in progenitor cells, it is plausible that patients that have no detectable JAK2V617F in granulocytes could still have a reservoir of JAK2V617F mutant HSC that persists. Indeed, JAK2V617F mutant colonies can be detected at a low level in some MPN patients in CMR.43 Intriguingly, several groups have reported long-term molecular responses after discontinuation of IFNα therapy, suggesting that JAK2V617F mutant HSCs are being eradicated by IFNα,23,25,39 although molecular relapse after cessation of IFNα has also been observed.44 In this study, we use a conditional knockin murine model of Jak2V617F-induced MPN to investigate the effects of IFNα on Jak2VF mutant and WT LT-HSC in vivo and to evaluate the means by which IFNα eradicates Jak2VF mutant cells in MPN.
Given the clinical IFNα data as outlined and the experimental evidence demonstrating the effects of IFNα on HSC homeostasis,27,28,45,46 we focused on the HSC compartment. We have not observed differences in LT-HSC numbers at steady state in primary Jak2VF compared with WT mice.9 We find that Jak2VF MPN stem cells are preferentially sensitive to IFNα therapy with depletion of Jak2VF LT-HSC over time. We show that IFNα treatment promotes the exit from quiescence of Jak2VF LT-HSC and Jak2VF-induced terminal erythroid differentiation. Using flow cytometry and gene expression profiling performed on populations enriched for LT-HSC activity (CD150+LKS+), we show that Jak2VF LT-HSC demonstrate enhanced cell cycle activation compared with WT LT-HSC, in response to treatment with IFNα. Using Ifnar1 knockout mice, we demonstrate that the effects of IFNα on cell cycle are cell autonomous and specifically mediated through type 1 interferon signaling. In vehicle-treated mice, we find a more activated cell cycle in Jak2VF LT-HSCs compared with WT LT-HSCs, and consistent with this, GSEA indicated an enrichment of cell cycle genes in Jak2VF LT-HSCs compared with WT LT-HSCs in vehicle-treated chimeric animals. We previously reported no difference in cell cycle between Jak2VF and WT cells, but this earlier analysis was performed on less purified total LKS+ cells from nontransplanted primary mice.9 In aggregate, these results indicate that Jak2VF LT-HSCs demonstrate enhanced cell cycle activity at baseline and preferential sensitivity to cell cycle activation in response to IFNα treatment.
The apparently paradoxical finding of enhanced LT-HSC cell cycling and enhanced self-renewal has been demonstrated in other MPN models. We previously reported a subtle self-renewal advantage for Jak2VF LT-HSC,18 and in the present study, we find enhanced cell cycle activity in Jak2VF LT-HSC compared with WT LT-HSC in vehicle-treated chimeric mice. Interestingly, Reynaud et al47 recently reported, using a murine transgenic BCR-ABL model, that CML LT-HSC also demonstrate enhanced cycling that occurs together with a gain in LT-HSC self-renewal and similar findings have been reported in a transgenic JAK2V617F MPN model.48 The exact mechanisms underlying these findings remain unclear; however, altered regulation of key self-renewal pathways (eg, Notch) may play a role.47,49 Our data evaluating the effects of IFNα indicate that Jak2VF LT-HSCs are preferentially sensitive to IFNα treatment by showing a more marked increase in cycling, which results in a preferential depletion in response to IFNα. This sensitivity may occur as a result of baseline cell cycle activation in Jak2VF MPN stem cells. These data also suggest that increased cell cycle may not be the sole mechanism by which IFNα depletes Jak2VF LT-HSCs, because vehicle-treated Jak2VF LT-HSCs also have increased cell cycling but do not undergo depletion during long-term BMT assays. Additional studies aimed at identifying key cell cycle and self-renewal modulators differentially regulated at baseline and in response to IFNα treatment in Jak2VF and WT LT-HSCs remain an ongoing focus of our work. Finally, we also note that the Jak2VF LT-HSC competitive repopulation advantage we previously found became manifest with prolonged follow-up (>1 year)18 and our findings in the present study, where Jak2VF LT-HSCs have no significant repopulating advantage at 8 weeks (Figure 3B), are consistent with this. In the present study, we find no significant difference in the engraftment of Jak2VF vehicle-treated cells compared with WT vehicle-treated cells at 16 weeks in a secondary BMT assay (Figure 5E). It is not clear why a self-renewal advantage is not seen in this secondary BMT; however, it is possible that a change in competitive repopulation ability is too subtle to be observed after only 16 weeks posttransplantation. Alternatively, we cannot exclude that the late clonal advantage observed could occur as a result of the acquisition of secondary mutations.
Notably, depletion of Jak2VF LT-HSC was observed only in IFNα-treated mice, and importantly, depletion of the MPN-propagating stem cell population was associated with reduced MPN transplantation efficiency in serially transplanted recipient mice. Serial transplantation is the most stringent functional assay to assess effects on the disease-initiating stem cell population, and these experiments indicate that sustained IFNα treatment targets MPN-propagating HSC in vivo. The presence of persistent low-level Jak2VF chimerism after IFNα treatment in serially transplanted mice suggests that IFNα may not be sufficient to completely eradicate Jak2VF LT-HSCs or that a significantly prolonged IFNα treatment course may be required for definitive disease eradication. We do not find any evidence to indicate that IFNα treatment in vivo has proapoptotic effects on LT-HSCs and the absence of a reduction in Ifnar1−/−Jak2VF HSCs in response to 4 weeks of continuous IFNα treatment argues strongly against an immune-mediated depletion of Jak2VF cells. These results are also consistent with human clinical trials in MPN, in which at least 1 year of IFNα therapy was required for CMR to be achieved.5,6 In aggregate, our results indicate that IFNα has differential effects on Jak2VF and normal WT LT-HSCs in vivo and suggest that the durable molecular remissions induced by IFNα in patients with MPN occur as a result of MPN stem cell exhaustion through activated cell cycling and enforced differentiation.
The Jak2VF model we use in this study closely recapitulates the features of human PV and we have exploited this to evaluate the effects of IFNα on Jak2VF erythropoiesis in vivo. We find that IFNα treatment selectively expands the Jak2VF MEP compartment in the bone marrow by driving Jak2VF LT-HSCs out of quiescence and down a predetermined erythroid-lineage differentiation program. IFNα treatment induces apoptosis in Jak2VF erythroid precursor cells, with immature Jak2VF erythroid precursors (CD71highTer119mid-high) demonstrating enhanced sensitivity to IFNα inhibition compared with mature Jak2VF erythroid precursors (CD71mid-lowTer119high cells). These effects account for the majority of the reduction in Jak2VF-mediated splenomegaly that we find in response to IFNα treatment and are consistent with the known sensitivity of JAK2V617F erythroid colonies to IFNα treatment in vitro.50 We find that the reduction in HCT is modest after 4 weeks of IFNα treatment but that the HCT is normalized after 8 weeks of IFNα treatment, a finding likely related to the prolonged life span of a mature RBC. In aggregate, these results indicate that IFNα exerts distinct effects on different Jak2VF hematopoietic cellular compartments, activating Jak2VF LT-HSCs to induce differentiation while inducing apoptosis in Jak2VF erythroid precursor cells to normalize RBC counts.
Although IFNα clearly has therapeutic efficacy in MPN, it is far from an ideal treatment and remains an experimental MPN therapy at present.25 One of the major challenges in the clinical use of IFNα is poor patient tolerability due to the multitude of side effects IFNα causes. Although these have improved with the use of lower dosing and pegylated forms of the drug, many patients remain intolerant of IFNα. Future studies aimed at identifying unique IFNα effector pathways in Jak2VF MPN stem cells have the potential to facilitate the development of more specific, less toxic IFNα derivatives for the treatment of MPN. This work also provides support for the rational combination of IFNα with new agents with promising but incomplete activity in MPN, including the JAK2 inhibitors. Combinatorial IFNα/JAK2 inhibitor treatment may be therapeutically synergistic as a result of IFNα-mediated induction of cell cycle in quiescent Jak2VF LT-HSCs coupled with the antiproliferative effects of JAK2 kinase inhibition in progenitor cells. Finally, it is important to note that only a minority of MPN patients achieves a CMR in response to IFNα treatment. Part of the heterogeneity in response may be related to the presence of other genetic abnormalities, in addition to JAK2V617F, in some patients. Emerging evidence suggests that TET2 and DNMT3A mutations may contribute to IFNα resistance in MPN.43,51,52 Given the key roles of TET253,54 and DNMT3A55 in HSC homeostasis, it will be important to investigate the mechanisms of this resistance as a means to further delineate the specific effects of IFNα on MPN-propagating stem cells.
In conclusion, we report the therapeutic efficacy of IFNα on Jak2VF disease-initiating LT-HSCs in vivo, a finding consistent with that previously reported in abstract form by Marty et al.56 We demonstrate that IFNα preferentially depletes Jak2VF MPN-propagating stem cells by activating cell cycle and promoting Jak2VF-driven erythroid-lineage differentiation. Through this work, we offer insights into the mechanism by which IFNα achieves CMR in MPN patients and provide a biological rationale in support of combinatorial IFNα/JAK2 inhibitor clinical trials in MPN patients.
The online version of this article contains a data supplement.
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE44961).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of the Ebert, Armstrong, Hill, and Williams laboratories for technical assistance, helpful discussion, and insights. In particular, the authors acknowledge the expert advice from Fatima Al-Shahrour, Amit Sinha, and Glen Boyle with the gene expression data analyses. The authors thank Grace Chojnowski and Paula Hall for flow cytometry and sorting.
This work was supported by the MPN Foundation (B.L.E.) and the National Institutes of Health, National Cancer Institute (P01 CA108631 and P01 CA066996 to B.L.E.) and the National Institutes of Health, National Heart, Lung, and Blood Institute (K08 HL109734 to A.M.). G.R.H. is a NHMRC Australia Fellow and Queensland Health Senior Clinical Research Fellow. F.H.H. was supported by a Mildred-Scheel grant of the German Cancer Aid (DKH D/08/00661, Deutsche Krebshilfe). D.H. was supported by the German Cancer Foundation, Mildred-Scheel Fellowship. D.K. was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K01DK092300. A.M. has received support from the Jeanne D. Housman Fund for Research on Myeloproliferative Disorders and is an ASH Scholar recipient. S.W.L. has received funding from the Leukaemia Foundation of Australia, National Health and Medical Research Council project grant 1026594, J.J.Richards/ In Vitro Technologies Rhys Pengelly Fellowship in Leukaemia Research and private philanthropic donations.
Authorship
Contribution: A.M. and S.W.L. provided the concept, designed experiments, performed experiments, analyzed data, and wrote the manuscript; C.B., L.P., F.H.H., A.P., T.V., R.A., D.H., L.J.B., C.P.K., and D.K. performed experiments and analyzed data; S.A.A., D.A.W., G.R.H., and B.L.E. designed experiments and provided supervision for the project. All authors provided critical review for the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven Lane, Queensland Institute of Medical Research, 300 Herston Rd, Herston, Brisbane, Australia, 4006; e-mail: steven.lane@qimr.edu.au; and Ann Mullally, 1 Blackfan Circle, Karp 5.007, Boston, MA 02115; e-mail: amullally@partners.org.