Key Points
IGH translocations in myeloma can occur through at least 5 mechanisms.
t(11;14) and t(14;20) DH-JH rearrangement-mediated translocations occur indicating these appear to occur in a pregerminal center cell.
Abstract
Translocations in myeloma are thought to occur solely in mature B cells in the germinal center through class switch recombination (CSR). We used a targeted captured technique followed by massively parallel sequencing to determine the exact breakpoints in both the immunoglobulin heavy chain (IGH) locus and the partner chromosome in 61 presentation multiple myeloma samples. The majority of samples (62%) have a breakpoint within the switch regions upstream of the IGH constant genes and are generated through CSR in a mature B cell. However, the proportion of CSR translocations is not consistent between cytogenetic subgroups. We find that 100% of t(4;14) are CSR-mediated; however, 21% of t(11;14) and 25% of t(14;20) are generated through DH-JH recombination activation gene–mediated mechanisms, indicating they occur earlier in B-cell development at the pro–B-cell stage in the bone marrow. These 2 groups also generate translocations through receptor revision, as determined by the breakpoints and mutation status of the segments used in 10% and 50% of t(11;14) and t(14;20) samples, respectively. The study indicates that in a significant number of cases the translocation-based etiological events underlying myeloma may arise at the pro–B-cell hematological progenitor cell level, much earlier in B-cell development than was previously thought.
Introduction
Chromosomal translocations arise when DNA double-strand breaks at different sites in the genome are brought together and aberrantly rejoined.1 They are common in tumors of the lymphoid lineage because of the “off-target” effects of the normal physiological mechanisms mediating DNA rearrangement at the immunoglobulin (Ig) heavy chain (IGH) locus. Translocations into the IGH locus predominantly occur either during recombination activation gene (RAG) complex-mediated variable, diversity, and joining—V(D)J—rearrangement, such as in mantle cell lymphoma (MCL) (t11;14),2 or during class switch recombination (CSR). In myeloma the primary translocations are thought to be generated via abnormal CSR events mediated by activation-induced cytidine deaminase (AID).3 This concept has been developed and is based on the location of the translocation breakpoints determined in myeloma cell lines and a few primary samples. Added to this, the myeloma clone is derived from a mature plasma cell, which has undergone somatic hypermutation in the germinal center4 and does not express the RAG complex.
In myeloma, primary aberrant rearrangements into the IGH locus are present in up to 60% of cases.5,6 There are 5 main translocation partner chromosomes including the t(4;14), t(6;14), t(11;14), t(14;16), and t(14;20), which result in the overexpression of MMSET and FGFR3, CCND3, CCND1, MAF, and MAFB, respectively, and are thought to confer a selective advantage to the clone.7 Until now, because of the difficulty in characterizing translocations, our understandings of the mechanisms underlying myeloma-specific translocations have been predominantly based on the characterization of immortalized cell lines with only a limited number of primary patient samples having been examined.5,8-12 The development of massively parallel sequencing technology has given us the ability to characterize a significant number of translocations from primary patient material in a high-throughput manner.
Here we have used a targeted capture of the Ig loci combined with massively parallel sequencing to characterize the translocations in 61 cases of presenting myeloma known to have a range of translocations into the Ig loci. The results of this analysis reveal previously unsuspected variability in the site of the breakpoint within the Ig regions, including non-CSR events, which sheds new information on the molecular mechanisms acting early on in the process of myeloma genesis.
Materials and methods
CD138-positive bone marrow plasma cells were selected to a purity >95% using magnetic assisted cell sorting (Miltenyi Biotech). Tumor DNA and RNA were extracted using the AllPrep kit (Qiagen).
All experiments were approved by the National Research Ethics Service Committee London-Surrey Borders under REC Reference 08/H0806/98. This study was conducted in accordance with the Declaration of Helsinki.
We developed a targeted capture using the SureSelect (Agilent) system by tiling RNA baits across the IGH, IGK, and IGL loci. Baits covered the V, D, and J segments as well as being tiled across the entire constant region, including the switch regions in the IGH locus.
DNA from samples (n = 61) were assayed using 150 ng DNA and a modified capture protocol13 with 8 cycles of prehybridization polymerase chain reaction (PCR) and 11 cycles of posthybridization PCR. Samples were barcoded using Illumina indexes and up to 27 samples were sequenced per lane on a HiSeq2000 generating 76-base pair (bp) paired-end reads. After base calling and quality control metrics, the raw fastq reads were aligned to the reference human genome (build GRCh37), resulting in a median depth of 289× per sample after deduplication for the captured region.
The translocation partner had previously been identified by fluorescence in situ hybridization in 36 samples.14,15 The remaining 25 samples were assayed by real-time quantitative PCR for overexpression of the partner oncogenes to determine the translocation. Samples assayed comprised t(4;14), n = 14; t(6;14), n = 5; t(11;14), n = 29; t(16;14), n = 9; t(14;20), n = 4. Translocation breakpoints were identified in the sequencing data using both a visual scanning of the alignments in integrative genome viewer and using SVDetect. Derivative chromosome breakpoints were reconstructed using Velvet or by manually aligning reads. Functionally rearranged V(D)J heavy chain alleles were manually aligned and mutation and segment usage determined using IMGT/V-QUEST.16 Once derivative chromosome breakpoints were reconstructed, some examples were chosen for validation by PCR.
Results
The site of breaks in the non-Ig loci
Chromosome 4 breakpoints.
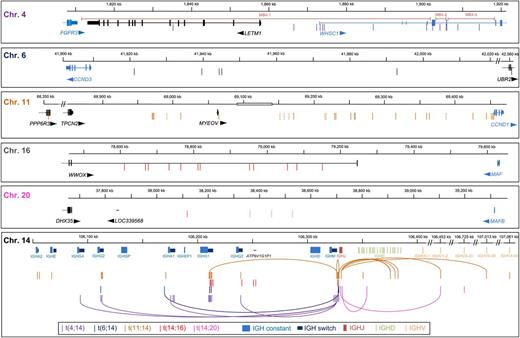
Fourteen samples were identified with a breakpoint on chromosome 14 within the vicinity of FGFR3 and MMSET. Of these, 8 were present between FGFR3 and exon 4 of MMSET in the region that generates MB4-1 IGH-MMSET hybrid transcripts, 4 breakpoints were in the region that generates MB4-2 hybrid transcripts, and 2 breakpoints were in the region to generate MB4-3 hybrid transcripts (Figure 1). Two samples had deletion of der(14), containing the overexpressed FGFR3 allele, and the breakpoints in these samples are centromeric of FGFR3, indicating that FGFR3 would initially have been highly expressed and deletion of der(14) occurred later.
Summary of translocation breakpoints in multiple myeloma presentation samples. Genes with exon/intron boundaries and the location on the chromosome are marked and arrows indicate the direction of transcription. Breakpoints are indicated with vertical lines of color corresponding to the partner chromosome (eg, t(4;14) are shown as purple lines and t(6;14) as dark blue lines). Oncogenes overexpressed because of the translocation are shown in blue. On chromosome 14, the IGH constant regions are indicated by light blue boxes and the upstream switch regions as dark blue boxes. DH segments and the IGHJ segment region are also annotated, as are VH segments in which translocations were detected. Arcs on chromosome 14 indicate an unbalanced translocation in which 1 sample has 2 breakpoints, 1 from each derivative chromosome. On chromosomes 11 and 20, dashed vertical lines indicate breakpoints in which the partner breakpoint on chromosome 14 is located in the V or D segments. On chromosome 11, the open box indicates a 50-kb gap in the genomic sequence.
Summary of translocation breakpoints in multiple myeloma presentation samples. Genes with exon/intron boundaries and the location on the chromosome are marked and arrows indicate the direction of transcription. Breakpoints are indicated with vertical lines of color corresponding to the partner chromosome (eg, t(4;14) are shown as purple lines and t(6;14) as dark blue lines). Oncogenes overexpressed because of the translocation are shown in blue. On chromosome 14, the IGH constant regions are indicated by light blue boxes and the upstream switch regions as dark blue boxes. DH segments and the IGHJ segment region are also annotated, as are VH segments in which translocations were detected. Arcs on chromosome 14 indicate an unbalanced translocation in which 1 sample has 2 breakpoints, 1 from each derivative chromosome. On chromosomes 11 and 20, dashed vertical lines indicate breakpoints in which the partner breakpoint on chromosome 14 is located in the V or D segments. On chromosome 11, the open box indicates a 50-kb gap in the genomic sequence.
Chromosome 6 breakpoints.
Six t(6;14) samples were analyzed, revealing that the majority of breakpoints (5/6) are located within the intergenic space between CCND3 and TAF8, the remaining breakpoint being located >600 kb upstream of CCND3 within UBR2 (Figure 1).
Chromosome 11 breakpoints.
All 29 samples with a t(11;14) had the breakpoint on chromosome 11 upstream of CCND1 (Figure 1). The breakpoint in 22 of the 29 samples was within the intergenic space between CCND1 and the next centromeric gene, MYEOV. Six of the remaining samples were between MYEOV and TPCN2 and the last breakpoint was 1.1 Mb centromeric of CCND1 within PPP6R3. Therefore, breakpoints on chromosome 11 vary from 1 kb upstream to CCND1 to over 1 Mb upstream. It is known that the t(11;14) translocation is also found in MCL and breakpoints are clustered in the region known as the major translocation cluster. No myeloma breakpoints were located within this region.
Chromosome 16 breakpoints.
All samples (n = 12) with a t(14;16) had the breakpoints on chromosome 16 located within the last intron of WWOX, which is centromeric of MAF (Figure 1). This intron is 1 Mb in size and contains the fragile site FRA16D. The t(14;16) breakpoints have a dual impact of positioning the IGH μ enhancer near MAF and disrupting the WWOX gene, a known tumor suppressor.
Chromosome 20 breakpoints.
Of the 4 t(14;20) samples, all had their breakpoints in the large intergenic space downstream of MAFB (Figure 1). This intergenic space between MAFB and DHX35 is 1.6 Mb and the 4 breakpoints were clustered within 400 kb in the center of the region.
The breakpoints determined on the IGH partner chromosomes are shown in Figure 1 and described in the supplemental data on the Blood website.
Chromosome 14 breakpoints.
The breakpoints on chromosome 14 reveal at least 5 mechanisms for translocation generation in myeloma, including those mediated via aberrant CSR, homologous recombination, somatic hypermutation, aberrant V(D)J rearrangement, and receptor revision-mediated.
CSR translocations.
All 14 t(4;14) and the 5 t(6;14) samples have their breakpoints within switch regions (Figure 1). For t(4;14), the most common breakpoints were within the IGHM switch (n = 7), followed by IGHG1 switch (n = 5). All but 3 of the t(14;16) samples have their breakpoints within switch regions and comprised IGHG1 (n = 4) and IGHG3 (n = 1) switch regions. Fourteen of 29 of the t(11;14) samples have breakpoints within switch regions, mostly being IGHG1 (n = 5) or IGHG2 (n = 3). Both derivative chromosome breakpoints were captured in 1 t(11;14) and 4 t(4;14) samples that covered 2 different switch regions, consistent with the translocation having developed during CSR and the intervening region on chromosome 14 having been deleted as an excision product (supplemental Figure 1). The only translocation group to have all breakpoints occurring between the IGH μ enhancer and the 3′ enhancer, centromeric of the IGH locus, is the t(4;14). This may be due to the essential oncogenic activation of both MMSET and FGFR3 on chromosome 4, which relies on both derivative chromosomes receiving 1 of the enhancers such that all breakpoints must fall between FGFR3 and MMSET on chromosome 4 and the μ and 3′ enhancers on chromosome 14. However, in total, only 37/59 (62%) samples with a breakpoint within the IGH locus are in switch regions.
Non-CSR translocations
Homologous recombination.
Of particular interest in respect of the etiology of myeloma are the locations of breakpoints within the IGH locus that are out with the switch regions. In the t(14;16) group, 3 breakpoints were out with the switch regions. Two of these breakpoints straddle ATP6V1G1P1, an adenosine triphosphatase pseudogene located between IGHG3 and IGHD constant regions, with the third lying centromeric of JH6. One t(6;14) breakpoint was located 3′ (centromeric) of the IGHA1 constant region outside a switch region. The breakpoints straddling ATP6V1G1P1 have up to 249 bp (93%) identity with a region upstream of the IGHD constant gene and so may be mediated by homologous recombination (supplemental Figure 2).
Somatic hypermutation.
A striking finding of this study is the large number of breakpoints found 5′ (telomeric) of the IGHM switch region (defined by accession number X54713.1) (Figure 1). When analyzing the t(11;14) breakpoints, 15 (52%) were found 5′ of the IGHM switch region, of which 9 were between the switch region and the JH6 segment. Also located within this region are 2 t(6;14) and 1 t(14;16) breakpoint. These breakpoints are out with the expected IGHM switch region and encompass some of the μ enhancer sequence. The reason for translocations occurring in the enhancer region is unclear, but has been speculated to be due to somatic hypermutation that extends past the JH6 segment.17
V(D)J recombination.
Evidence for involvement of aberrant V(D)J recombination being important comes from several breakpoints that occur near or within the J segments and include 6 t(11;14), 1 t(14;16), and 2 t(14;20) samples, of which all but the t(14;16) sample have an additional breakpoint from the other derivative chromosome within the VH or DH segments. Both the breakpoints on chromosome 14, within 1 sample, map to 1 location on the partner chromosome, indicating 1 translocation event that occurred during VH-DH-JH rearrangements. In addition to these 9 samples, there are 3 samples with single breakpoints within VH or DH segments, giving a total of 12 samples (20%) with breakpoints in the VH or DH segments (Table 1).
V(D)J usage in samples with a translocation occurring in the V, D, or J segments
| Sample . | Functional allele . | Translocated allele . | Additional rearrangement . | Translocation . | Mechanism . | ||||
|---|---|---|---|---|---|---|---|---|---|
| VH (identity) . | DH . | JH (identity) . | VH (identity) . | DH . | JH (identity) . | ||||
| 90992 | 3-30 (93.75) | 5-18 | 4 (85.42) | 6-6 | 6 (100) | t(11;14) | D-J | ||
| 11/1030 | 3-21 (94.79) | 3-16 | 6 (95.16) | 6-6 | 4 (100) | t(11;14) | D-J | ||
| 91514 | 3-30 (94.79) | 6-25 | 4 (100) | 3-3 | 4 (100) | t(11;14) | D-J | ||
| 11/1286 | 3-74 (87.85) | 3-22 | 4 (91.67) | 6-6 | 4 (100) | t(11;14) | D-J | ||
| 11/1066 | 4-39 (93.81) | 1-14 | 6 (82.26) | 4-23 | DH4-23/JH4* | t(11;14) | D-J | ||
| 90003 | 3-53 (87.37) | 3-10 | 3 (92) | 2-2 | 4 (100) | t(11;14) | D-J | ||
| 90108 | 3-23 (95.83) | 2-2 | 4 (87.5) | 2-21 | 4 (100) | t(14;20) | D-J | ||
| 90857 | 1-46 (91.32) | 3-22 | 5 (92.16) | 1-2 (96) | 3′ of 6 | t(11;14) | Revision/SHM | ||
| 11/808 | 3-43 (95.83) | 2-8 | 4 (97.92) | 3-65 (100) | 4 (100) | t(11;14) | Revision | ||
| 12/0213 | 1-69 (94.79) | 2-21 | 4 (83.3) | 3′ to 4-55 (100) | t(11;14) | Revision | |||
| 90280 | 4-39 (93.13) | 2-15 | 4 (89.58) | 1-2 (94.27) | 5 (94.17) | t(14;20) | Revision/SHM | ||
| 90191 | 1-2 (95.49) | 3-10 | 6 (90.32) | 3-23† (99.31) | 2-21 | 4 (64.51) | t(14;20) | Revision/SHM | |
| Sample . | Functional allele . | Translocated allele . | Additional rearrangement . | Translocation . | Mechanism . | ||||
|---|---|---|---|---|---|---|---|---|---|
| VH (identity) . | DH . | JH (identity) . | VH (identity) . | DH . | JH (identity) . | ||||
| 90992 | 3-30 (93.75) | 5-18 | 4 (85.42) | 6-6 | 6 (100) | t(11;14) | D-J | ||
| 11/1030 | 3-21 (94.79) | 3-16 | 6 (95.16) | 6-6 | 4 (100) | t(11;14) | D-J | ||
| 91514 | 3-30 (94.79) | 6-25 | 4 (100) | 3-3 | 4 (100) | t(11;14) | D-J | ||
| 11/1286 | 3-74 (87.85) | 3-22 | 4 (91.67) | 6-6 | 4 (100) | t(11;14) | D-J | ||
| 11/1066 | 4-39 (93.81) | 1-14 | 6 (82.26) | 4-23 | DH4-23/JH4* | t(11;14) | D-J | ||
| 90003 | 3-53 (87.37) | 3-10 | 3 (92) | 2-2 | 4 (100) | t(11;14) | D-J | ||
| 90108 | 3-23 (95.83) | 2-2 | 4 (87.5) | 2-21 | 4 (100) | t(14;20) | D-J | ||
| 90857 | 1-46 (91.32) | 3-22 | 5 (92.16) | 1-2 (96) | 3′ of 6 | t(11;14) | Revision/SHM | ||
| 11/808 | 3-43 (95.83) | 2-8 | 4 (97.92) | 3-65 (100) | 4 (100) | t(11;14) | Revision | ||
| 12/0213 | 1-69 (94.79) | 2-21 | 4 (83.3) | 3′ to 4-55 (100) | t(11;14) | Revision | |||
| 90280 | 4-39 (93.13) | 2-15 | 4 (89.58) | 1-2 (94.27) | 5 (94.17) | t(14;20) | Revision/SHM | ||
| 90191 | 1-2 (95.49) | 3-10 | 6 (90.32) | 3-23† (99.31) | 2-21 | 4 (64.51) | t(14;20) | Revision/SHM | |
Nonfunctional rearrangement.
Functional rearrangement but with translocation in VH segment.
SHM, somatic hypermutation.
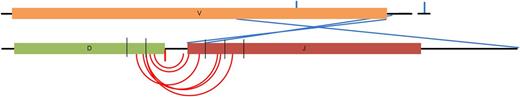
Analysis of the breakpoints in these 12 non–CSR-mediated translocations shows 2 different mechanisms involved in the generation of the translocations. First, 7 samples have the 2 breakpoints on the derivative chromosomes spanning DH-JH junctions, indicating the intervening DNA in the IGH locus has been excised during the translocation process (Figure 2). These breakpoints are within 15 bp of the DH or JH segment exon boundaries and are typical of DH-JH rearrangements that occur during heavy chain rearrangements in a pro-B cell (supplemental Figure 3). Of these 7 samples, 3 use DH6-6, indicating a possible common mechanism, and the remaining use DH2-2, DH2-21, DH3-3, and DH4-23. JH segment usage is predominantly JH4 (n = 5), with 1 JH6 and 1 sample with no JH segment translocation partner detected (Table 1). All but 1 of these DH-JH translocations is a t(11;14), with the last being a t(14;20).
Schematic representation of generic V-D-J segments indicating the position of breakpoints within them. Samples can be broken down into groups based on the location of the breakpoint; D-J–mediated translocations (red) have breakpoints within 15 bp of the end of the D or J segments, whereas receptor revision–mediated translocations (blue) have breakpoints within, or near, the V and J segments with no D segment detected. Arcs indicate the positions of the breakpoints on both derivative chromosomes from the same sample. In some cases, both derivative chromosomes were not detected (solitary lines). Thin black lines on the D and J segments indicate 5-bp gaps.
Schematic representation of generic V-D-J segments indicating the position of breakpoints within them. Samples can be broken down into groups based on the location of the breakpoint; D-J–mediated translocations (red) have breakpoints within 15 bp of the end of the D or J segments, whereas receptor revision–mediated translocations (blue) have breakpoints within, or near, the V and J segments with no D segment detected. Arcs indicate the positions of the breakpoints on both derivative chromosomes from the same sample. In some cases, both derivative chromosomes were not detected (solitary lines). Thin black lines on the D and J segments indicate 5-bp gaps.
The second group of samples differs from the DH-JH translocated samples in 2 respects. First, the breakpoints on the derivative chromosomes are found at VH and JH segments, with no DH segment involved (Figure 2). Second, the breakpoints in the VH and JH segments are not always located at the segment boundaries and often occur within the VH segment or out with the JH segment. These breakpoints are not typical of D-JH or V-DJH rearrangements, but may be representative of receptor editing or receptor revision events or somatic hypermutation (supplemental Figure 4). Receptor editing and revision differ in the location at which they occur, with editing occurring in the bone marrow at the pro–B-cell stage and revision occurring in the germinal center at a mature B-cell stage. To delineate the stage at which these events occur, we looked at the VH and JH segments on the translocated allele for hypermutation. In 3 of the 5 samples with evidence of receptor editing/revision, there was evidence of hypermutation (Table 1), indicating that these translocations most likely occur in the germinal center and are a byproduct of aberrant receptor revision or somatic hypermutation. Of the 5 samples falling into this category, VH1-2 was used twice and JH4 was used twice and consisted of 3 t(11;14) and 2 t(14;20) samples. The 2 remaining samples had no somatic hypermutation and are therefore more likely to occur through receptor revision.
Because we detected DJH translocations in these myeloma samples, we compared the breakpoints to that of MCL, which also has DJH translocations involving CCND1 on chromosome 11. Most MCL translocations are found at the major translocation cluster on chromosome 11. However, several unclustered samples have also been analyzed18 and the breakpoints occur within 234 kb centromeric of CCND1 (supplemental Figure 5). All unclustered MCL translocations have predominantly D-JH breakpoints but can also have V-JH or D-DH breakpoints, similar to the breakpoints detected in the myeloma samples. However, the D-JH and V-JH breakpoints in the majority of myeloma samples cluster together in a 78-kb region 228-306 kb centromeric from CCND1, with little overlap with the MCL breakpoints. This would indicate a molecular mechanism within this region, which makes these samples susceptible to non-CSR translocations in the myeloma t(11;14). Because MCL samples have been shown to be AID-mediated, localized near CpG or WGCW motifs, we looked at the incidence of these in myeloma and show that 59% of breakpoints are near these motifs, providing evidence that AID is active in creating these breakpoints (see supplemental data and supplemental Table 1).
Peri-breakpoint sequence motifs.
We analyzed the breakpoints for motifs involved in double-strand breaks mediated by AID. In our dataset, 15 samples (25%) have a breakpoint within 4 bp of a CpG site (supplemental Table 1). Of these 15 samples, 4 have V(D)J breakpoints, 8 have switch region breakpoints, and the remaining 3 are in the IGH μ enhancer. An additional 21 samples have a WGCW motif (where W= A or T) within 20 bp of the breakpoint comprising 17 switch, 2 V(D)J, and 2 enhancer region breakpoints. As such, 50% of V(D)J, 67% of switch, and 62% of enhancer region breakpoints are near a CpG dinucleotide or WGCW motif, providing strong evidence that AID is active in creating these breakpoints.
Discussion
Few translocation breakpoints have been determined to high accuracy in primary myeloma samples, mainly because of the time-consuming process of identifying breakpoints through Southern blotting and cloning. Here we use a custom capture method followed by massively parallel sequencing to comprehensively characterize the breakpoints in a large set of primary myeloma patients consisting of all 5 major translocation partners to the IGH locus.
As expected, we were able to detect CSR-mediated translocation breakpoints that are present within the switch regions of the heavy chain constant genes. In fact, we detected unbalanced translocations where the breakpoints on the 2 derivative chromosomes occur at different switch regions, but the same point on the partner chromosome, indicating the translocation occurs during CSR and the excision product is lost. All t(4;14) translocation breakpoints are located within the switch regions of the constant genes (Figure 3), and conversely all breakpoints on chromosome 4 are located between FGFR3 and MMSET/WHSC1. The consistency in breakpoints indicates that both FGFR3 and MMSET are necessary for transformation. For both oncogenes to be overexpressed, the breakpoint on chromosome 14 must occur between the μ enhancer and the 3′ IGH enhancers and between FGFR3 and MMSET, resulting in both derivative chromosomes containing an enhancer juxtaposed to an oncogene. In 25% to 30% of t(4;14) samples the der(14) chromosome containing FGFR3 is deleted.19,20 This, together with the absence of non-CSR translocations, would indicate that FGFR3 is essential for initial transformation but sustained expression is not required.
Summary of translocation events in myeloma. Translocations occur between the IGH locus and all main partner chromosomes through CSR in the germinal center. t(11;14) and t(14;20) subgroups also have other mechanisms of developing translocations through receptor revision in the germinal center or through DH-JH recombination at the early pro–B-cell stage.
Summary of translocation events in myeloma. Translocations occur between the IGH locus and all main partner chromosomes through CSR in the germinal center. t(11;14) and t(14;20) subgroups also have other mechanisms of developing translocations through receptor revision in the germinal center or through DH-JH recombination at the early pro–B-cell stage.
Unexpectedly, we find that CSR-mediated translocations are only present in 62% of samples examined, with the remainder of translocations being present outside switch regions, indicating different and independent mechanisms of translocation generation. Here we have determined that IGH translocations in myeloma can occur through homologous recombination, DH-JH rearrangements, receptor revision, somatic hypermutation, and the classic CSR mechanism (Figure 3 and supplemental Figures 1-4). Of these mechanisms, the most striking is the discovery of DH-JH rearrangement–mediated translocations. Myeloma is recognized as a malignancy of mature postgerminal center plasma cells because of their clonal V(D)J rearrangements, which have undergone somatic hypermutation, and the presence of isotype-switched IGH genes. DH-JH rearrangements occur at the pro–B-cell stage in the bone marrow, and the presence of DH-JH–mediated translocations in a subset of myeloma samples indicates an alternative initiating mechanism for a myeloma translocation. The DH-JH translocation events have only been detected in a subset of t(11;14) (21%) and t(14;20) (25%) samples, indicating a possible etiological mechanism at work in these cytogenetic groups.
In addition to DH-JH–mediated translocations, the t(11;14) and t(14;20) cytogenetic groups also have additional VH-DH-JH–related translocations. In these samples, the VH and JH segments are frequently mutated in the translocated allele and do not involve the DH segments (Table 1). We hypothesize that these translocations are receptor revision–mediated and account for 10% of t(11;14) and 50% of t(14;20) samples. Given that the V or J segments involved in the receptor revision–mediated translocations are mutated, it follows that the translocations probably occurred in the germinal center of a mature B cell. Therefore, there must have been aberrant RAG complex expression in these cells to mediate the recombining of VH-DH-JH segments.
Myeloma is not the sole B-cell malignancy to harbor a t(11;14). MCL is derived from a B cell that acquires a translocation upstream of CCND1 during V(D)J rearrangements as a pro-B cell. However, MCL is also a tumor of mature B lymphocytes, indicating that the translocation alone is not sufficient for the full neoplastic phenotype.21 Up to 55% of MCL t(11;14) breakpoints translocate to a region known as the major translocation cluster (at approximately 69.33 Mb on chromosome 11).22 However, those not in the major translocation cluster spread up to 234 kb centromeric of CCND1.18 Comparison of these V(D)JH-mediated breakpoints with those seen in t(11;14) myeloma show a clustering of V(D)JH-mediated breakpoints in myeloma that is distinct from those seen in MCL (supplemental Figure 5). It is not clear what mechanisms or additional genetic events decide whether a pro-B cell with a t(11;14) transforms into a classic mantle cell, a nonnodal, leukemic or splenic mantle cell, or perhaps even into a myeloma cell. However, several subtypes of MCL exist, some of which enter the germinal center and become hypermutated.23 It may be that a genetic mechanism also exists to transform a subset of B cells with a t(11;14) into a myeloma cell (supplemental Figure 6).
In addition to the t(11;14) the t(14;20) also develops DH-JH–mediated translocations. Interestingly, the t(14;20) is known to overexpress MAFB and have a poor prognostic outcome in myeloma,8,24-26 so the occurrence of this translocation at an early stage in B-cell development is unexpected. However, we have previously reported that the t(14;20) is present at a higher frequency in monoclonal gammopathy of undetermined significance (5%) compared with myeloma (1.5%) and that these monoclonal gammopathy of undetermined significance patients have a long stable disease.25 Similar to the t(11;14), these early B-cell translocations may require additional genetic events to progress the disease.
The breakpoints on the IGH partner chromosomes are also of interest. As stated previously, the t(4;14) breakpoints all reside between FGFR3 and MMSET, resulting in overexpression of both oncogenes. In the t(11;14) and t(6;14), the breakpoints are all 5′ of CCND1 and CCND3, the respective oncogenes. Conversely, in the t(14;16) and t(14;20), the breakpoints are 3′ of MAF and MAFB, the respective oncogenes. In addition, the t(14;16) breakpoints are located within the last exon of WWOX, a known tumor suppressor, which we have previously identified as a target gene in myeloma.27,28 This is the location of the fragile site, FRA16D, which may mediate the translocation.29
Given that the oncogenes are always telomeric of the breakpoints results in them being translocated to the der(14) under the control of the 3′ IGH enhancer. The distances from the start of transcription to the breakpoint vary greatly, extending up to >1 Mb in t(11;14) and t(14;20) samples to less than 1 kb in t(11;14) and within MMSET itself in the t(4;14). The breakpoints are characterized by either CpG or WGCW motifs, known AID recognition sites, in up to 61% of samples and are therefore mediated by double-strand break repair mechanisms. In the t(11;14), there are 6 samples with the translocation breakpoint centromeric of MYEOV, so that CCND1 is not the gene adjacent to the enhancer. In these samples, CCND1 was still overexpressed compared with non-t(11;14) samples, and sample 90468, which has a breakpoint over 1 Mb centromeric of CCND1, had the highest expression of CCND1 measured by a real-time quantitative PCR assay30 (data not shown). These data indicate that the breakpoint is not required to be adjacent to the oncogene. This is similar to the other translocations such as in t(4;14) myeloma where the breakpoint is more frequently between MMSET and LETM1, and not next to FGFR3 or the breakpoint can also be downstream of the oncogene, as is the case in t(14;16) and t(14;20) myeloma.
The findings of this study have important implications for the etiological mechanisms leading to the development of myeloma and suggest that in a significant number of non-t(4;14) cases translocations arise before passing through a germinal center reaction, at the time of physiological IGH gene rearrangements in a B-precursor cell.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the Institute of Cancer Research Tumour Profiling Unit for their support and technical expertise in this study. This work was supported by a Myeloma UK program grant (B.A.W., C.P.W., and D.C.J.). F.E.D. is a Cancer Research UK Senior Clinical Fellow.
Authorship
Contribution: B.A.W. designed and carried out research, analyzed data, and wrote the paper; C.P.W. analyzed data; D.B.B. and N.B.D. prepared samples; M.F.K. and F.M.R. carried out research and analyzed data; D.C.J., D.G., and F.E.D. provided important intellectual input; and G.J.M. obtained funding and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gareth Morgan, Haemato-Oncology Research Unit, Division of Molecular Pathology, The Institute of Cancer Research, 15 Cotswold Rd, Sutton, UK, SM2 5NG; e-mail: gareth.morgan@icr.ac.uk.