Key Points
Deep clonal responses improve outcomes and can change the natural history of advanced (cardiac stage III) AL amyloidosis.
NT-proBNP >8500 ng/L and SBP <100 mm Hg identify a very poor risk subgroup of stage III AL amyloidosis.
Abstract
Treatment outcomes of patients with cardiac stage III light chain (AL) amyloidosis remain poorly studied. Such cases have been excluded from most clinical studies due to perceived dismal prognosis. We report treatment outcomes of 346 patients with stage III AL amyloidosis from the United Kingdom, Italy, Germany, and Greece. Median overall survival (OS) was 7 months with OS at 3, 6, 12, and 24 months of 73%, 55%, 46%, and 29%, respectively; 42% died before first response evaluation. On an intention-to-treat basis, the overall hematologic response rate was 33%, including a complete response rate of 12%. OS rates at 12 and 24 months, respectively, for 201 response evaluable patients were 88% and 85% for complete responders, 74% and 53% for partial responders, and 39% and 22% for nonresponders. Forty-five percent of responders achieved an organ response. Amino-terminal fragment of brain-type natriuretic peptide (NT-proBNP) >8500 ng/L and systolic blood pressure (SBP) <100 mm Hg were the only factors that independently impacted OS and identified an especially poor prognosis subgroup of patients with a median OS of only 3 months. Outcome and organ function of stage III AL amyloidosis without very elevated NT-proBNP and low SBP is improved by a very good hematologic response to chemotherapy.
Introduction
Systemic light chain (AL) amyloidosis is a rare multisystem disease caused by the deposition of misfolded immunoglobulin light chain protein in various tissues and organs. Patients with AL amyloidosis present with nonspecific symptoms frequently leading to a delay in diagnosis,1 and over a third of patients present with advanced disease. Cardiac involvement is the leading cause of morbidity and mortality in AL amyloidosis.2 Elevated serum cardiac biomarkers (brain-type natriuretic peptide [BNP], its more stable N-terminal fragment [NT-proBNP], and cardiac troponin T [TnT]/troponin I [TnI]) usefully define advanced disease.3-5 Even modest elevations of serum NT-proBNP at presentation may be predictive of developing clinically significant cardiac involvement during the disease course.6 The staging system reported by the Mayo Clinic group, based on the combination of elevated serum levels of NT-proBNP and cardiac TnT or TnI at presentation, has become the standard for staging patients at diagnosis.7,8 The median overall survival (OS) of patients with stage I, II, and III AL amyloidosis was 26.4, 10.5, and 3.5 months, respectively, using NT-proBNP and TnT/TnI staging in the original description of the system in 2004.7 A recent revision that incorporates the difference between involved and uninvolved serum free light chain concentration (dFLC) as a further criteria may be even more discriminatory.9 The Mayo staging system has since become one of the chief criteria for patient selection (or exclusion) in clinical trials.
We and others have reported high response rates with treatment10-14 and improving outcomes15 in AL amyloidosis. Although some recent single-center data suggest better outcomes in stage III patients than previously reported,5,16 the perceived poor prognosis of stage III AL amyloidosis has led to the exclusion of such patients from nearly all prospective treatment studies.
We report the features and treatment outcomes of a large group of patients with stage III AL amyloidosis attending 4 major European amyloidosis centers. Stage III AL amyloidosis encompasses a heterogeneous group of patients among whom the prognosis of a substantial subgroup may be improved through a complete hematologic response (CR) to chemotherapy.
Patients and methods
Study participants and assessments
Three hundred forty-six newly diagnosed patients with systemic AL amyloidosis assessed at the amyloidosis centers in London (United Kingdom) (71 patients), Pavia (Italy) (164 patients), Heidelberg (Germany) (92 patients), and Athens (Greece) (19 patients) between January 2001 and December 2010 with Mayo stage III disease were, retrospectively, included in this study. Stage III disease was defined as NT-proBNP >332 ng/L and cardiac TnT >0.035 µg/L or TnI >0.1 µg/L.7 The presence of amyloid deposition was confirmed by characteristic birefringence after Congo red staining of a tissue biopsy. AL-type amyloidosis was confirmed by immunohistochemical/immunoelectron microscopy staining supported by demonstration of a plasma cell dyscrasia and, where necessary, by exclusion of hereditary amyloidosis by demonstration of wild-type sequence for the genes encoding known hereditary amyloidogenic proteins.17 All patients were treated according to local protocols using cyclical chemotherapy regimens previously described,10,11,18-20 which included oral melphalan dexamethasone (MDex), alkylator-thalidomide-dexamethasone combination, bortezomib combination, or lenalidomide combination regimes. All patients had rigorous protocolized assessments at baseline and after chemotherapy, which included evaluation of clonal disease (including serum free light chains) and detailed assessment of amyloidotic organ function. NT-ProBNP and TnT or TnI concentrations were measured by standard commercially available assays used in the local laboratories.
The study was performed with institutional review board approval, and informed consent was obtained from each patient in accordance with the Declaration of Helsinki.
Outcome measures
Organ involvement and hematologic and amyloidotic organ responses were assessed according to the Consensus Opinion from the 10th International Symposium on Amyloid and Amyloidosis.21 A very good partial response (VGPR) using dFLC was defined as dFLC <40 mg/L. Performance status was assessed as described by the Eastern Cooperative Oncology Group (ECOG) criteria.22 The primary outcome measure was OS and impact of hematologic response to treatment on survival.
Statistics
Statistical analysis was undertaken using the SPSS 20 (SPSS, Chicago, IL) software package. Survival was assessed by the Kaplan-Meier method and compared by log-rank test. Categorical variables were compared with χ2 or Fisher’s tests as appropriate. Continuous variables with a normal distribution were compared with a paired or unpaired t test as appropriate, and those where a normal distribution was not confirmed were compared with a Mann-Whitney test or Wilcoxon rank sum test as appropriate. All P values were 2-sided with a significance level of .05. Receiver operating characteristic analysis with death at 1 year was used to identify the threshold for NT-proBNP and systolic blood pressure (SBP), which were then analyzed as dichotomous variables. Serum free light chain level cutoffs were explored based on previously reported dFLC values (>180 mg/L) and a median for the series. Cox models were fitted to compute hazard ratios and 95% confidence intervals for death for a series of potential predictors. The proportional hazard assumption was tested and satisfied in all cases. All responses to treatment were assessed on an intention-to-treat (ITT) basis. Patients who died prior to response assessment were classified as nonresponders. A landmark analysis was conducted for patients surviving beyond 3 months.
Results
A total of 346 patients were included in this study. The baseline characteristics are given in Table 1. Three hundred thirty-eight (97%) had cardiac involvement according to the echocardiographic criteria. There was renal involvement in 216 (62%) and liver involvement in 77 (22%) patients. This cohort of patients had a median presenting NT-proBNP of 9106 ng/L (range, 379-216 187) and cardiac TnI of 0.18 ng/mL (range, 0.1-12) or cardiac TnT of 0.09 ng/mL (range, 0.04-8.2). The median left ventricular (LV) wall thickness was 15 mm (7-24), and ejection fraction was 53% (range, 12% to 81%). Twenty-two percent of patients had an ejection fraction of <30%. Fifty-seven percent had dyspnea ≥3 (New York Heart Association [NYHA] class), and 28% had ECOG performance status ≥3.
Baseline characteristics
| Parameter (% with missing data) . | Baseline (n = 346) median (range)/number of patients (%) . | Deaths within 3 mo (n = 103) median (range)/number of patients (%) . | P value* . |
|---|---|---|---|
| Age in y (median) (0%) | 66 (37-88) | 68 (42-83) | .02 |
| Monoclonal light chain (0.8%) | |||
| κ | 73 (21) | 20 | .66 |
| λ | 274 (79) | 83 | .75 |
| Serum monoclonal protein (including light chain band only) | 270 (78) | 80 (77) | |
| Serum monoclonal protein >5 g/L | 36 (10) | 13 (12) | .377 |
| dFLC median (range) (mg/L) | 230 (12-8140) | 348 (10-8140) | .002† |
| Serum creatinine (mg/dL) (1%) | 1.3 (0.5-9.5) | 1.3 (0.5-8.7) | .99 |
| 24-h Proteinuria (g/24 h) (7%) | 1.7 (0-16) | 1.5 (0-16) | .74 |
| eGFR (mL/min) (1%) | 49 (ESRD-152) | 48 (ESRD-152) | |
| SBP (mm Hg) (9%) | 109 (65-210) | 103 (70-210) | .0002† |
| SBP ≤100 mm Hg | 93 (27) | 50 (48) | |
| Patients with eGFR <40 mL/min | 128 (37) | ||
| NT-proBNP (ng/L) (0%) | 9106 (379-216 187) | 18 184 (609-179 300) | .0002† |
| NT-proBNP >8500 ng/L | 182 (52) | 69 (67) | |
| Cardiac TnT (ng/mL)‡ (0%) | 0.1 (0.04-8.2) | 0.22 (0.04-1.87) | |
| Cardiac TnI (ng/mL)‡ (0%) | 0.18 (0.1-12) | 0.52 (0.1-8.2) | |
| Organ involvement* | |||
| Liver involvement (0.5%) | 77 (22) | 28 (27) | .15 |
| Renal involvement (0.5%) | 216 (62) | 64 (63) | .99 |
| Mean LV wall thickness (39%) | 15 (5-30) | 16.7 (7-30) | |
| Ejection fraction (39%) | 53 (12-81) | 55 (27-81) | |
| Soft tissue involvement (0.5%) | 66 (19) | 19 (18) | .86 |
| Peripheral neuropathy (0.5%) | 56 (16) | 11 (11) | .88 |
| Autonomic neuropathy (0.5%) | 55 (16) | 18 (17) | .77 |
| Gastrointestinal tract (0.5%) | 52 (15) | 12 (12) | .32 |
| Total number of organs* (0.5%) | |||
| 1 organ | 82 (24) | 28 (27) | .33 |
| 2 organs | 129 (37) | 34 (33) | .38 |
| 3 or more organs | 136 (39) | 40 (39) | .99 |
| ECOG performance status (2%) | |||
| ≤1 | 132 (38) | 20 (19) | .0001† |
| 2 | 114 (33) | 37 (36) | .45 |
| ≥3 | 95 (27) | 46 (45) | .0001† |
| NYHA class (9%) | |||
| NYHA class 1 | 50 (14) | 8 (9) | .02 |
| NYHA class 2 | 84 (24) | 24 (26) | .89 |
| NYHA class 3 or 4 | 182 (52) | 71 (65) | .0001† |
| Parameter (% with missing data) . | Baseline (n = 346) median (range)/number of patients (%) . | Deaths within 3 mo (n = 103) median (range)/number of patients (%) . | P value* . |
|---|---|---|---|
| Age in y (median) (0%) | 66 (37-88) | 68 (42-83) | .02 |
| Monoclonal light chain (0.8%) | |||
| κ | 73 (21) | 20 | .66 |
| λ | 274 (79) | 83 | .75 |
| Serum monoclonal protein (including light chain band only) | 270 (78) | 80 (77) | |
| Serum monoclonal protein >5 g/L | 36 (10) | 13 (12) | .377 |
| dFLC median (range) (mg/L) | 230 (12-8140) | 348 (10-8140) | .002† |
| Serum creatinine (mg/dL) (1%) | 1.3 (0.5-9.5) | 1.3 (0.5-8.7) | .99 |
| 24-h Proteinuria (g/24 h) (7%) | 1.7 (0-16) | 1.5 (0-16) | .74 |
| eGFR (mL/min) (1%) | 49 (ESRD-152) | 48 (ESRD-152) | |
| SBP (mm Hg) (9%) | 109 (65-210) | 103 (70-210) | .0002† |
| SBP ≤100 mm Hg | 93 (27) | 50 (48) | |
| Patients with eGFR <40 mL/min | 128 (37) | ||
| NT-proBNP (ng/L) (0%) | 9106 (379-216 187) | 18 184 (609-179 300) | .0002† |
| NT-proBNP >8500 ng/L | 182 (52) | 69 (67) | |
| Cardiac TnT (ng/mL)‡ (0%) | 0.1 (0.04-8.2) | 0.22 (0.04-1.87) | |
| Cardiac TnI (ng/mL)‡ (0%) | 0.18 (0.1-12) | 0.52 (0.1-8.2) | |
| Organ involvement* | |||
| Liver involvement (0.5%) | 77 (22) | 28 (27) | .15 |
| Renal involvement (0.5%) | 216 (62) | 64 (63) | .99 |
| Mean LV wall thickness (39%) | 15 (5-30) | 16.7 (7-30) | |
| Ejection fraction (39%) | 53 (12-81) | 55 (27-81) | |
| Soft tissue involvement (0.5%) | 66 (19) | 19 (18) | .86 |
| Peripheral neuropathy (0.5%) | 56 (16) | 11 (11) | .88 |
| Autonomic neuropathy (0.5%) | 55 (16) | 18 (17) | .77 |
| Gastrointestinal tract (0.5%) | 52 (15) | 12 (12) | .32 |
| Total number of organs* (0.5%) | |||
| 1 organ | 82 (24) | 28 (27) | .33 |
| 2 organs | 129 (37) | 34 (33) | .38 |
| 3 or more organs | 136 (39) | 40 (39) | .99 |
| ECOG performance status (2%) | |||
| ≤1 | 132 (38) | 20 (19) | .0001† |
| 2 | 114 (33) | 37 (36) | .45 |
| ≥3 | 95 (27) | 46 (45) | .0001† |
| NYHA class (9%) | |||
| NYHA class 1 | 50 (14) | 8 (9) | .02 |
| NYHA class 2 | 84 (24) | 24 (26) | .89 |
| NYHA class 3 or 4 | 182 (52) | 71 (65) | .0001† |
ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate.
P values calculated between patients who died within 3 months and those alive after 3 months.
Highly significant P values (bold).
TnI measured in patients from Pavia (n = 164) and TnT in all other patients.
Treatments given were as follows: bortezomib combinations, 23 (7%); MDex, 154 (44%); thalidomide combinations, 96 (28%); lenalidomide combinations, 13 (4%); and other regimes (including melphalan-prednisolone, dexamethasone alone, and cyclophosphamide-dexamethasone/prednisone) 31 (9%). Twenty-nine (8%) were deemed too ill for treatment or died prior to treatment initiation. One hundred eighteen (34%) patients completed a full planned course of chemotherapy. Fifty (14%) and 32 (9%) stopped after 1 and 2 cycles of chemotherapy, respectively, due to death or toxicity. Two hundred one patients (58%) were evaluable for response. The median time to response evaluation was 5.6 months with 15% evaluated within 3 months and another 24% within 4 months (total 39% within 4 months of starting chemotherapy). On an ITT basis, 114 (32%) achieved a hematologic response; the hematologic responses are given in Table 2. Fifty-seven percent of the evaluable patients achieved a hematologic response. The response rates, on an ITT basis, with MDex, cyclophosphamide-thalidomide-dexamethasone, bortezomib combinations, or lenalidomide combinations were all low, from 32% to 43% (Table 2).
Hematologic responses
| Regime . | n . | ORR (ITT) . | CR (ITT) . | PR (ITT) . | ORR (evaluable) . |
|---|---|---|---|---|---|
| MDex | 154 | 40% | 15% | 25% | 60% |
| Thalidomide combination | 96 | 32% | 11% | 21% | 64% |
| Bortezomib combination | 23 | 43% | 26% | 13% | 62% |
| Lenalidomide combination | 13 | 38% | 0% | 38% | 41% |
| Regime . | n . | ORR (ITT) . | CR (ITT) . | PR (ITT) . | ORR (evaluable) . |
|---|---|---|---|---|---|
| MDex | 154 | 40% | 15% | 25% | 60% |
| Thalidomide combination | 96 | 32% | 11% | 21% | 64% |
| Bortezomib combination | 23 | 43% | 26% | 13% | 62% |
| Lenalidomide combination | 13 | 38% | 0% | 38% | 41% |
ORR, overall response rate.
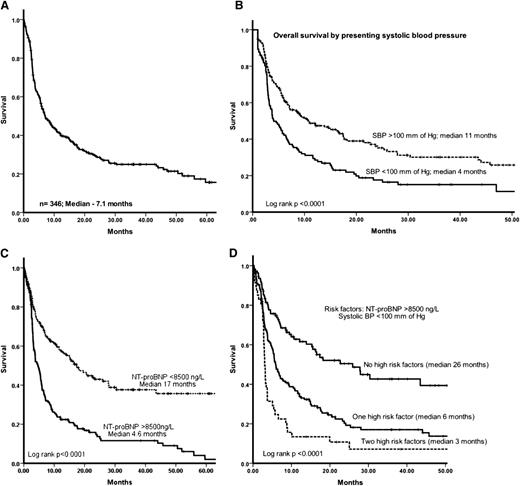
The median OS for the cohort was 7.1 months (Figure 1A). The estimated OS at 3, 6, 12, and 24 months was 73%, 55%, 46%, and 29%, respectively. Patients who died within 3 months of diagnosis had significantly higher NT-proBNP (median 11 794 ng/L vs 7957 ng/L; P = .0002), lower SBP (median 100 mm vs 110 mm Hg; P = .002), and higher presenting serum dFLC (median 348 mg/L vs 218 mg/L; P = .002), and a higher proportion of patients had NYHA grade 3 to 4 dyspnea (65% vs 45%; P = .0001) and ECOG performance status ≥3 (45% vs 20%; P = .0001).
(A) OS of the whole cohort. The median follow-up of the whole cohort was 6.5 months and of live patients was 21 months. (B) OS stratified by presenting SBP (mm Hg) (ie, significantly poorer OS for patients with SBP <100 mm Hg at presentation). (C) OS stratified by presenting NT-proBNP (ie, significantly poorer OS for patients with presenting NT-proBNP >8500 ng/L). (D) OS stratified by high risk factors (presenting NT-proBNP >8500 ng/L and low SBP <100 mm Hg): presence of none, 1, or 2 high risk factors identified 3 groups with median OS of 26 months vs 6 months vs 3 months, respectively (P < .0001).
(A) OS of the whole cohort. The median follow-up of the whole cohort was 6.5 months and of live patients was 21 months. (B) OS stratified by presenting SBP (mm Hg) (ie, significantly poorer OS for patients with SBP <100 mm Hg at presentation). (C) OS stratified by presenting NT-proBNP (ie, significantly poorer OS for patients with presenting NT-proBNP >8500 ng/L). (D) OS stratified by high risk factors (presenting NT-proBNP >8500 ng/L and low SBP <100 mm Hg): presence of none, 1, or 2 high risk factors identified 3 groups with median OS of 26 months vs 6 months vs 3 months, respectively (P < .0001).
Factors affecting OS on univariate and multivariate analysis are given in Table 3. Using receiver operating characteristic analysis, the NT-proBNP cutoff identified for death at 1 year was 8500 ng/L (area under the curve, 0.68; P < .0001) and the SBP cutoff was 100 mm Hg (area under the curve, 0.69; P < .0001). On univariate analysis, factors significantly associated with poorer OS were NTproBNP >8500 ng/L, SBP <100 mm Hg, NYHA grade 4 dyspnea, presence of congestive heart failure, and presence of liver involvement. The mean LV wall thickness did not significantly impact survival. Presenting dFLC level was significant on univariate analysis using the previously reported9 cutoff of dFLC >180 mg/L; the median for the series, dFLC >230 mg/L; or the threshold of >500 mg/L. On a multivariate analysis, presenting NT-proBNP >8500 ng/L and SBP <100 mm Hg were the only 2 independent factors impacting survival (Figure 1B-D). Rather surprisingly, dFLC, which has been identified as a significant factor impacting OS in other studies, was not significant on multivariate analysis using the previously reported cutoff value of 180 mg/L or using the median for the current series of 230 mg/L or >500 mg/L. A landmark analysis was conducted for patients alive at 3 months. In the multivariate model for the patients in the landmark analysis, NT-proBNP >8500 ng/L, SBP <100 mm Hg, and lack of hematologic response to treatment were significant independent factors associated with especially poor OS.
Univariate and multivariate analysis of factors affecting OS
| Factor . | P value; hazard ratio (95% confidence interval) . | ||
|---|---|---|---|
| Univariate . | Multivariate at baseline* . | Multivariate for 3 mo landmark analysis* . | |
| Renal involvement | .89; 1.01 (0.78-1.91) | ||
| Liver involvement | .034; 1.3 (1.02-1.8) | .79; 1.04 (0.73-1.5) | .33; 0.6 (0.6-52.3) |
| Presence of CHF | .031; 1.7 (1.05-2.9) | .90; 1.09 (0.26-4.6) | |
| NYHA class | |||
| NYHA 1-2 | .39; 1.2 (0.7-1.9) | .98; 0.98 (0.24-4.0) | .14; 0.53 (0.23-1.2) |
| NYHA 3-4 | .06; 1.4 (0.9-2.2) | .86; 0.89 (0.22-3.4) | .28; 0.69 (0.35-1.3) |
| dFLC | |||
| dFLC (> or ≤180)† | .002; 1.5 (1.17-2.0) | .28; 1.2 (0.85-1.6) | |
| dFLC (< or ≥289 mg/L)† | .17; 1.1 (1.07-3.1) | .26; 1.2 (0.98-2.4) | |
| dFLC (< or ≥500 mg/L) | .003; 1.15 (1.1-1.9) | .21; 1.1 (0.8-1.9) | |
| SBP (as continuous variable) | <.0001; 0.98 (0.98-0.99) | ||
| SBP (>100 mm or ≤100 mm) | <.0001; 1.6 (1.2-2.1) | .001; 1.7 (1.6-3.05) | .017; 1.9 (1.1-3.2) |
| NT-proBNP (< or ≥8500 ng/L) | <.0001; 2.4 (1.8-3.1) | <.001; 2.2 (1.2-2.3) | .001; 2.3 (1.4-3.8) |
| Mean LV wall thickness | .66; 1.06 (0.096-1.05) | ||
| Hematologic response | |||
| CR | Ref | ||
| PR | .025; 2.6 (1.1-6.4) | ||
| NR | <.0001; 7.3 (3.1-16) | ||
| Factor . | P value; hazard ratio (95% confidence interval) . | ||
|---|---|---|---|
| Univariate . | Multivariate at baseline* . | Multivariate for 3 mo landmark analysis* . | |
| Renal involvement | .89; 1.01 (0.78-1.91) | ||
| Liver involvement | .034; 1.3 (1.02-1.8) | .79; 1.04 (0.73-1.5) | .33; 0.6 (0.6-52.3) |
| Presence of CHF | .031; 1.7 (1.05-2.9) | .90; 1.09 (0.26-4.6) | |
| NYHA class | |||
| NYHA 1-2 | .39; 1.2 (0.7-1.9) | .98; 0.98 (0.24-4.0) | .14; 0.53 (0.23-1.2) |
| NYHA 3-4 | .06; 1.4 (0.9-2.2) | .86; 0.89 (0.22-3.4) | .28; 0.69 (0.35-1.3) |
| dFLC | |||
| dFLC (> or ≤180)† | .002; 1.5 (1.17-2.0) | .28; 1.2 (0.85-1.6) | |
| dFLC (< or ≥289 mg/L)† | .17; 1.1 (1.07-3.1) | .26; 1.2 (0.98-2.4) | |
| dFLC (< or ≥500 mg/L) | .003; 1.15 (1.1-1.9) | .21; 1.1 (0.8-1.9) | |
| SBP (as continuous variable) | <.0001; 0.98 (0.98-0.99) | ||
| SBP (>100 mm or ≤100 mm) | <.0001; 1.6 (1.2-2.1) | .001; 1.7 (1.6-3.05) | .017; 1.9 (1.1-3.2) |
| NT-proBNP (< or ≥8500 ng/L) | <.0001; 2.4 (1.8-3.1) | <.001; 2.2 (1.2-2.3) | .001; 2.3 (1.4-3.8) |
| Mean LV wall thickness | .66; 1.06 (0.096-1.05) | ||
| Hematologic response | |||
| CR | Ref | ||
| PR | .025; 2.6 (1.1-6.4) | ||
| NR | <.0001; 7.3 (3.1-16) | ||
CHF, congestive heart failure; Ref, reference.
Highly significant P values are in bold.
Separate multivariate models were generated for all patients at baseline, and a landmark analysis was done at 3 months for those patients who were assessable for response to treatment at/after 3 months.
Separate multivariate models were generated using dFLC > or ≤180 mg/L, dFLC < or ≥289 mg/L, and dFLC < or ≥500 mg/L.
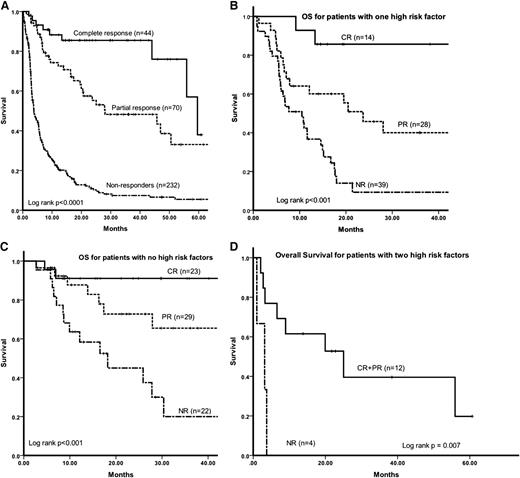
The OS, at 12, 24, and 48 months, respectively, for patients evaluable for a hematologic response was as follows (Figure 2A): for those patients in a CR, 88%, 85%, and 76%; for patients in a partial hematologic response (PR), 74%, 53%, and 33%; and for nonresponders, 39%, 22%, and 14%. The estimated OS at 12, 24, and 48 months for patients achieving a dFLC-VGPR (but not a CR) was 81%, 68%, and 54%, respectively, and was significantly better than patients achieving a PR (log rank P < .0001). Based on the presence or absence of NT-proBNP >8500 ng/L or SBP <100 mm Hg (labeled hence as high risk factors), the 2 parameters identified in the multivariate analysis, it was possible to stratify the stage III AL patients into 3 groups. Patients with none, either, or both of these factors present had an OS of 25 months, 6 months, and 3 months, respectively (Figure 1D). The impact of depth of hematologic response on survival was assessed in these 3 patient subgroups. On an ITT basis, the OS for patients with neither of the 2 high risk factors was as follows (Figure 2B): CR, median not reached; PR, 69 months; and no response (NR), 7 months. The OS for patients with only 1 of the 2 high risk factors was as follows (Figure 2C): CR, 59 months; PR, 23 months; and NR, 4 months. Only 34% (16/47) of patients with 2 high risk factors were assessable for treatment response, and, although there was a suggestion of better outcomes among responders, numbers are too few to make any significant conclusions (Figure 2D).
(A) OS stratified by hematologic response on an ITT basis. (B) OS by hematologic response in patients with either NT-proBNP >8500 ng/L or SBP <100 mm Hg. (C) OS by hematologic response in patients with NT-proBNP <8500 ng/L and SBP >100 mm Hg. (D) OS by hematologic response in patients with both NT-proBNP >8500 ng/L and SBP <100 mm Hg.
(A) OS stratified by hematologic response on an ITT basis. (B) OS by hematologic response in patients with either NT-proBNP >8500 ng/L or SBP <100 mm Hg. (C) OS by hematologic response in patients with NT-proBNP <8500 ng/L and SBP >100 mm Hg. (D) OS by hematologic response in patients with both NT-proBNP >8500 ng/L and SBP <100 mm Hg.
On an ITT basis, organ responses were seen in 52 (15%) patients. This accounted for 26% of patients evaluable for a hematologic response and 45% of patients achieving at least a partial hematologic response. Using NT-proBNP to define cardiac response (>30% and 300 ng/L decrease over baseline), on an ITT basis, 43 (12%) achieved a cardiac response. Additionally, 17 (5%) had a renal response, and 11 (3%) had a liver response. Of the patients evaluable for a hematologic response, 21% had a cardiac response. The patients who achieved a cardiac response had a median 94% decrease in dFLC over the baseline. The median involved free light chain value at the time of response assessment in cardiac responders was 25 mg/L and dFLC 9.5 mg/L. Sixteen out of 52 (30%) patients achieving a cardiac response had NT-proBNP >8500 ng/L. Five (9%) with a cardiac response had NT-proBNP >8500 ng/L and SBP <100 mm Hg; the median survival of these 5 patients was 19 months.
Discussion
This study reports the treatment outcomes of a large cohort of patients with advanced AL amyloidosis (Mayo stage III disease) from 4 major European amyloidosis centers and illustrates the complexity of AL amyloidosis. This study shows that a good hematologic response will translate into improved survival in many patients with stage III AL amyloidosis, and identifying these patients early is critical. However, nearly half of all patients with very advanced stage III AL amyloidosis still die within 6 months of diagnosis either due to disease progression or possibly decompensation of organ function from treatment toxicity. This group remains a major unmet medical need for treatment strategies using agents targeting the amyloid deposits.
The cardiac biomarker–based staging system originally reported by the Mayo group, using TnT/TnI and NT-proBNP, is the current standard for staging patients with AL amyloidosis. The outcomes of patients with Mayo stage III disease is poor, and the median survival reported in the initial Mayo series was just 3 months. Recent studies report improved outcomes of patients with AL amyloidosis with median survival in unselected cases improving to nearly 4 years23 and in stage III disease to 8 to 9 months.5,16 Although this large cohort confirms the earlier reports that overall the survival of stage III patients has improved (median OS 7 months), the outcomes are still dismal with 27% of deaths within 3 months of diagnosis and 2-year estimated survival of only 29%.
The current findings show that patients defined as having stage III AL amyloidosis encompass a heterogeneous spectrum. The recent update from the Mayo group reported a strong prognostic impact of dFLC on survival in unselected patients with AL amyloidosis,9 which may help to refine disease classification, but the cardiac biomarker thresholds used to define disease stages were different from the previous Mayo report.7 This study, which included only stage III patients as per the 2004 definition,7 was not designed to replicate those findings. In this cohort, patients with high presenting dFLC had worse outcomes, but dFLC was not an independent predictor of survival. NT-proBNP >8500 ng/L and presenting supine SBP <100 mm Hg were independent markers of poor prognosis. The data in this study are unable to assess the utility of increasing the troponin value for prognosis as different centers used either TnT or TnI measurements. If patients had either NT-proBNP >8500 ng/L or SBP <100 mm Hg, or both, the OS was 6 months and 3 months, respectively, compared with 25 months in the absence of these 2 markers of poor prognosis. NT-proBNP is well established in prognosis of AL amyloidosis, and this study suggests that a very high absolute presenting NT-proBNP value is a useful marker to identify patients at risk of early death. Supine SBP was a simple and interesting marker of poor outcome identified in this study. We acknowledge its limitations: optimal blood pressure measurement needs to be standardized (supine vs standing; averaged over a few days vs a single reading) and has the potential to be influenced by diuretics and other cardiac drugs. However, given it universal applicability and simplicity, it should be considered for further prospective validation.
Patients with systemic AL amyloidosis diagnosed and treated in the era before availability of novel plasma cell therapeutic agents had poor hematologic responses (and consequently worse outcomes).24 We and others have reported improving treatment outcomes in AL amyloidosis in recent years: hematologic response rates of nearly 65% for MDex10 or cyclophosphamide-thalidomide-dexamethasone25 , 74% for bortezomib-dexamethasone,11 and more than 90% for bortezomib-alkylator-dexamethasone combinations.12,13 Prospective studies in AL also report similar response rates with MDex26 and bortezomib.27 We have previously reported, in a small case series, that thalidomide-28 or melphalan-based regimes29 fail to improve outcomes in stage III patients. Most prospective clinical trials in AL amyloidosis have excluded patients with stage III disease. In the current series, 146 (42%) patients died before response assessment, and on an ITT basis, just over one-third of all patients achieve a hematologic response. The very high response rates previously reported with bortezomib (or indeed other regimes) by us11,12 and others13 are not replicated in this cohort. There is a suggestion of higher CR rates for patients treated with bortezomib in the current cohort (26% with bortezomib vs 11% and 15% for thalidomide combinations or MDex, respectively) (Table 2), but the retrospective nature, the potential selection bias, and the small numbers treated with bortezomib remain major confounders. The overall hematologic response in this cohort remains disappointing. The main factor is a very high proportion of early deaths. There was a suggestion that CR rates were lower in patients with 1 or 2 of the high risk factors identified in this study. A possible factor, which is difficult to analyze in this retrospective series, is the treatment intensity and delays, which are frequent due to marked toxicity in this fragile patient group and may also account for lower CR rates in advanced stage III disease.
The analysis of hematologic response impacting OS in AL amyloidosis is difficult to interpret because patients who tolerate chemotherapy and reach the point of response assessment are a self-selecting “better risk group” compared with the early deaths, making interpretation of even ITT analysis difficult. Prospective studies with cycle-by-cycle data analyzed using a time-updated response model may provide more valid results, another limitation of the current cohort. With these caveats, on an ITT basis, in this series, patients who achieve a CR had significantly better outcomes (median OS, 59 months) compared with partial responders (median OS, 28 months). Patients who achieved a dFLC-VGPR also had significantly better outcomes compared with partial responders, suggesting that dFLC-VGPR is a valid treatment end point in this patient group. Using NTproBNP >8500 ng/L and SBP <100 mm Hg as markers of more advanced stage III disease, among patients with neither high risk marker, a hematologic response (CR or PR) to first treatment significantly improved survival. In the group with the presence of either 1 of the 2 high risk factors, a CR significantly improved survival, but patients who only achieved a PR still had a high proportion of early deaths, which was not significantly different from those who did not respond to chemotherapy. This suggests that a profound (and early) clonal response is critical to improving the survival of these patients. Achieving this aim remains difficult, and prospectively evaluating novel combinations with synergistic mechanisms of action, thereby allowing lower doses of each individual agent to be used and thus engendering tolerance, are urgently needed. In the United Kingdom, one such study is testing 2 bortezomib combinations using subcutaneous reduced-dose bortezomib in stage III AL amyloidosis. Other international studies are planned. Autologous stem cell transplant (ASCT) has been reported to be feasible (and safe) in selected patients with cardiac amyloidosis.30 Because of highly conservative patient selection criteria for ASCT in our centers, only 1 patient had ASCT in the current series. The role of ASCT in selected stage III patients, especially in the era of highly effective combination chemotherapy, needs further clarification.
The final goal of therapy in AL amyloidosis is translation of the hematologic responses into an organ response. In this series, only 15% of all patients achieved an organ response. Of the 32% of total patients who were able to receive enough therapy and live long enough to achieve a hematologic response, 45% had a cardiac response as defined by the cardiac biomarkers, and all patients who had a cardiac response had achieved dFLC-VGPR and >90% decrease in dFLC over baseline. Striving for a very good hematologic response is critical for improvement in organ function, which appears to be possible even in patients with advanced presenting disease.
The most advanced stage III patients pose a particular challenge, and accurate identification of such patients is important. In this cohort, the patients with stage III disease who had both NT-proBNP >8500 ng/L and SBP <100 mm Hg had very poor outcomes, and only 34% of these patients were assessable for hematologic response. Although there was a suggestion that responders had better outcomes, the small numbers make it unclear whether chemotherapy really improved survival in this patient subgroup. There is often substantial toxicity due to chemotherapy in these very ill patients, which will invariably have a negative impact on the quality of life. In this subgroup, especially in the elderly, the difficult issue for discussion with the patient and family of using only the best supportive care approach, aimed at giving the maximum quality of life, has to be considered. Strategies directed at targeting the amyloid deposits such as immunotherapy31 or drugs like doxycycline,32 if proved to be clinically useful, may be lifesaving in this patient group.
In summary, stage III AL amyloidosis is a heterogeneous disease. Responses to chemotherapy appear to change the natural course of the disease in patients with less advanced stage III AL amyloidosis. This highlights the critical importance of early diagnosis in AL amyloidosis; routine adoption of simple strategies like checking NT-proBNP levels and urine for the presence of albumin during monitoring of patients with monoclonal gammopathy may help early diagnosis. The outcomes of patients who achieve an excellent hematologic response (CR/VGPR) are significantly better than a lesser degree of clonal response and can translate into improved organ function. High NT-proBNP and low SBP at presentation identify a very poor risk subgroup within the stage III patients; this needs further prospective validation. Studies assessing novel combination regimes using proteasome inhibitors are in progress, but a persistent high early death rate identifies an urgent unmet medical need for treatment strategies directly targeting the amyloid fibrils.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the clinical staff and the histopathology and genetics laboratories at the respective hospitals, as well as the hematologists in the United Kingdom, Italy, Greece, and Germany who treated patients reported in this study.
Authorship
Contribution: A.D.W. designed the study, performed research, analyzed the data, and wrote the manuscript; S.O.S. and E.K. performed research and wrote the manuscript; J.D.G. performed research; M.A.D. performed research and wrote the manuscript; T.L., A.F., D.F., P.M., and L.R. performed research; U.H. performed research and wrote the manuscript; P.N.H. and G.M. designed the study, performed research, and wrote the manuscript; G.P. designed the study, performed research, analyzed the data, and wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: A.D.W. received an honorarium from Janssen Cilag. S.O.S. received honoraria from Celgene and Janssen Cilag. M.A.D. received honoraria from Celgene and Orthobiotech. U.H. received honoraria from Celgene and Janssen Cilag. G.M. is a member of the advisory board of Millennium and Neotope and has received honoraria from Neotope and Pfizer. G.P. has received honoraria from Celgene and Janssen Cilag. The remaining authors declare no competing financial interests.
Correspondence: Ashutosh D. Wechalekar, National Amyloidosis Centre, University College London Medical School (Royal Free Campus), Rowland Hill St, London NW32PF, United Kingdom; e-mail: a.wechalekar@ucl.ac.uk.