Key Points
A familial form of PMBCL is reported for the first time.
Exome sequencing identifies MLL 5533C>A (His1845Asn) variant segregating with lymphoma in the reported family.
Abstract
Primary mediastinal large B-cell lymphoma (PMBCL) is a subtype of diffuse large B-cell lymphoma (DLBCL) accounting for 2% to 4% of all non-Hodgkin lymphomas. We report a family of 3 siblings with PMBCL and their cousin with extranodal DLBCL. The histopathological characteristics of lymphomas of all 4 patients are similar, implying post–germinal center differentiation and growth deregulation by other mechanisms than BCL2-mediated inhibition of apoptosis and suggesting a shared biological background. We aimed to identify the genetic defect underlying lymphoma susceptibility in this family using exome sequencing and linkage analysis. The only variant segregating in all 4 patients and not reported in genetic databases was 5533C>A (His1845Asn) in the MLL gene. To our knowledge, this is the first time when familial clustering of PMBCL is reported. Although we propose MLL as a candidate predisposition gene for this condition, this finding needs to be validated in additional cases.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL). DLBCL can be classified into distinct subtypes, including germinal-center B-cell–like and activated B-cell–like DLBCL, and primary mediastinal large B-cell lymphoma (PMBCL).1 Single nucleotide polymorphisms have been associated with DLBCL,2 and first-degree relatives are at increased risk.3 Germline mutations predisposing to isolated familial DLBCL have not been described. PMBCL accounts for 2% to 4% of NHLs.1 Typical presentation is an anterior mediastinal mass with supraclavicular lymphadenopathy, infiltration to adjacent structures, and possible extranodal dissemination without bone marrow involvement.1 We report a family with 3 siblings affected by PMBCL and a maternal cousin with extranodal DLBCL. The phenotypes were scrutinized, and tumor biopsies were reviewed to establish diagnoses. Linkage analysis and exome sequencing were used to identify putative predisposing genetic defect(s).
Study design
Patients and samples
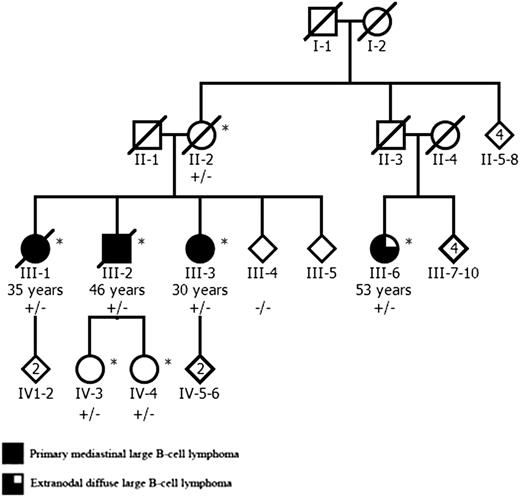
A Finnish family with 4 DLBCL patients was referred to the study through a clinical genetics unit. One sibling (III-1, Figure 1) presented with vena cava syndrome and was diagnosed with localized mediastinal large B-cell lymphoma. Her brother (III-2) presented with lymphadenopathy in cervical, supraclavicular, and axillary lymph nodes and was found to have a mediastinal mass. The third sibling (III-3) complained of fatigue and weight loss. A mediastinal mass and minor liver involvement was found in computer tomography, and biopsy confirmed lymphoma diagnosis. The karyotype of 1 sibling (III-3) was studied and found to be normal. The maternal cousin (III-6) was diagnosed with thickening of the urinary bladder mucosa in cystoscopy. Computer tomography revealed a tumor mass between the urinary bladder and the uterus, and biopsy confirmed extranodal DLBCL. For this study, the medical records of the patients were examined, tumor biopsies from all 4 patients were collected, and hematoxylin and eosin and immunohistochemically stained sections were reviewed by a hematopathologist to establish detailed diagnoses.
The pedigree of the Finnish family with 3 siblings affected by PMBCL and their cousin with extranodal DLBCL. The age of lymphoma onset is shown for all affected individuals. The MLL 5533C>A mutation status is shown for all studied family members: plus indicates mutation, and minus indicates the wild-type allele. Genome-wide genotype data were available from individuals marked with an asterisk. The pedigree has been modified for confidentiality.
The pedigree of the Finnish family with 3 siblings affected by PMBCL and their cousin with extranodal DLBCL. The age of lymphoma onset is shown for all affected individuals. The MLL 5533C>A mutation status is shown for all studied family members: plus indicates mutation, and minus indicates the wild-type allele. Genome-wide genotype data were available from individuals marked with an asterisk. The pedigree has been modified for confidentiality.
Blood-derived DNA was available from cases III-1, III-3, and III-6 and from 4 healthy family members (II-2, III-4, IV-3, and IV-4). Tumor-derived DNA was available from all 4 patients. Fourteen familial NHL cases, 9 sporadic early onset NHL cases, and 83 sporadic DLBCL cases were used as the validation set. Eighty-six Finnish blood donors served as controls. Supplemental Table 1 (see the Blood Web site) describes the available samples. The study was approved by the appropriate Ethics Review Committee. Samples were derived either after a signed informed consent or after authorization from the National Supervisory Authority for Welfare and Health. The study was conducted in accordance with the Declaration of Helsinki.
Linkage analysis
Whole-genome genotype data were obtained from III-1, III-2, III-3, III-6, II-2, IV-3, and IV-4 using HumanOmniExpress-FFPE BeadChip (Illumina) (Figure 1). A parametric linkage analysis with dominant inheritance model was performed using MERLIN.4 Further analyses were focused on segregating genomic regions with a positive logarithm of odds score (>0) and a length of at least 5 cM.
Exome sequencing, data analysis, and direct sequencing
The germline exomes of III-1 and III-3 were sequenced using Agilent Sure Select All Exons Kit v1 and Illumina GAII. A collection of exome data metrics is represented in supplemental Table 2. All nonsilent variants and variants located within 2 bp from the exon-intron boundary were included. Of these, all variants present in the dbSNP132 database, the 1000 Genomes project data set, or 196 in-house control exomes were removed.
All variants found in both patients and located within the linked regions were validated, and their segregation in the family was analyzed by direct sequencing. The effects of the identified variants were predicted in silico using PolyPhen2 (HumDiv).5 MLL 5533C>A (His1845Asn) was screened in all the patients’ tumor samples, the healthy family members, and 86 healthy controls. As the MLL variant resides in a low-complexity region (amino acids 1820-1854; supplemental Figure 1) predicted by SEG6,7 in the Pfam database8 and in an overlapping proline-rich region (PS50099) predicted by the PROSITE database9 (amino acids 1820-1868), the larger region was sequenced in all additional NHL cases (n = 106). See supplemental Methods for more detailed methods.
Results and discussion
The clinical features of the 3 siblings were compatible with PMBCL, and they all lacked bone marrow and infradiaphragmatic lymph node involvement. In reevaluation of the tumor samples, the morphology and immunophenotype of the tumors of III-1 to III-3 were consistent with PMBCL, and the histopathological features of the cousin’s (III-6) tumor were alike. All 4 neoplasms were positive for CD20, CD30, IRF4/MUM1, and BCL6 but negative for CD10 (20% cutoff point), suggesting postgerminal differentiation (supplemental Figure 2). Three out of 4 lymphomas contained a varying proportion of cells with multilobated nuclei resembling Reed-Sternberg cells. The expression of BCL2 was low in all tumors, and apoptotic cells were frequent (supplemental Figure 2). Table 1 summarizes the clinical features of the patients and the immunophenotypes of corresponding lymphomas.
A summary of the clinical features of the lymphoma patients and the corresponding immunophenotypes
| Individual . | Age of onset (y) . | Sites of involvement . | Diagnosis . | Immunophenotype . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD20 . | CD30 . | CD10 . | CD23 . | MIB1 . | IRF4/ MUM1 . | BCL2 . | BCL6 . | PAX5 . | OCT2 . | BOB1 . | LMP-1 . | ||||
| III-1 | 35 | Mediastinal tumor | PMBCL stage IA | + | + | — | — | ∼50% | ± | ± | + | + | + | + | — |
| III-2 | 46 | Mediastinal tumor; cervical, supraclavicular, and axillary lymph nodes | PMBCL stage IIIB | + | + | — | — | ∼50% | + | ± | + | + | + | + | — |
| III-3 | 30 | Mediastinal tumor, minor liver involvement | PMBCL stage IVB | + | + | — | — | ∼50% | + | ± | + | + | + | + | — |
| III-6 | 53 | Tumor between urinary bladder and uterus relapse: intestine and axillary lymph nodes | DLBCL stage IV | + | + | — | — | ∼97% | ++ | — | + | + | + | + | — |
| Individual . | Age of onset (y) . | Sites of involvement . | Diagnosis . | Immunophenotype . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD20 . | CD30 . | CD10 . | CD23 . | MIB1 . | IRF4/ MUM1 . | BCL2 . | BCL6 . | PAX5 . | OCT2 . | BOB1 . | LMP-1 . | ||||
| III-1 | 35 | Mediastinal tumor | PMBCL stage IA | + | + | — | — | ∼50% | ± | ± | + | + | + | + | — |
| III-2 | 46 | Mediastinal tumor; cervical, supraclavicular, and axillary lymph nodes | PMBCL stage IIIB | + | + | — | — | ∼50% | + | ± | + | + | + | + | — |
| III-3 | 30 | Mediastinal tumor, minor liver involvement | PMBCL stage IVB | + | + | — | — | ∼50% | + | ± | + | + | + | + | — |
| III-6 | 53 | Tumor between urinary bladder and uterus relapse: intestine and axillary lymph nodes | DLBCL stage IV | + | + | — | — | ∼97% | ++ | — | + | + | + | + | — |
+, ++, positive staining; ±, moderate staining; −, negative staining.
In genetic analyses, 16 genomic regions with a positive logarithm of odds score and a minimum length of 5 cM segregated in the family (supplemental Table 3). The number of exome variants after each filtering step is shown in supplemental Figure 3. Exome sequencing revealed 2 heterozygous variants that resided in the linked regions and were present only in the studied patients: 1870C>T (Pro624Ser) in SORL1 (complementary DNA, NM_003105.5) and 5533C>A (His1845Asn) in MLL (complementary DNA, NM_001197104.1). Direct sequencing showed that only the MLL alteration (supplemental Figure 4) was present in all 4 lymphoma patients in the family, whereas the SORL1 variant was found only in cases III-1 to III-3. According to PolyPhen2, the MLL variant was predicted as possibly damaging based on conservation. Screening of MLL 5533C>A from the tumor samples of III-1 to III-3 and III-6 did not reveal loss of heterozygosity. The MLL variant was found in 3 healthy family members (Figure 1) but in none of the healthy controls. The predicted domains surrounding MLL 5533C>A were screened in 92 sporadic NHL (mainly DLBCL) and 14 familial NHL cases, but no additional nonsilent variants were observed.
We describe a family of 3 siblings with PMBCL and a cousin with extranodal DLBCL. To our knowledge, this is the first time that familial PMBCL has been reported. The similar histopathological features of all the lymphomas, including post–germinal center phenotype and low BCL2 expression with susceptibility to apoptosis, suggest a shared biological background.
The identified MLL missense change resides in a predicted low-complexity/proline-rich region. The functions of low-complexity regions are still poorly understood, whereas proline-rich regions have been suggested to play a role in many protein-protein interactions.10 This predicted domain region is located within a broader region spanning the MLL bromodomain and PHD-like zinc-binding/PHD4 domain (supplemental Figure 1), which is deleted from oncogenic MLL fusion proteins and which was recently reported to interact with ASB2, the mediator of MLL degradation during hematopoietic differentiation.11 Although the specific functions of the predicted domain region surrounding MLL 5533C>A have not been characterized, it is possible that variations in this region affect protein-protein interactions and/or the regulation of MLL, thus contributing to increased lymphoma susceptibility.
MLL is an intriguing candidate to harbor germline variants predisposing to lymphoid neoplasms because rearrangements of MLL are frequently detected in leukemias, and somatic mutations in MLL2 and other histone-modifying genes have been reported in DLBCL.12-14 Although we propose MLL as a candidate predisposition gene for PMBCL, the results need to be validated in further studies, preferably in additional familial cases of PMBCL. Indeed, this first description of familial predisposition to PMBCL should raise awareness to detect other similar related individuals for robust clinical and molecular characterization of this new form of lymphoma susceptibility.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sini Nieminen, Marjo Rajalaakso, Mairi Kuris, Iina Vuoristo, and Inga-Lill Svedberg for excellent technical assistance. The Institute for Molecular Medicine Finland Genome and Technology Centre and the Biomedicum Imaging Unit are acknowledged for service.
This work was supported by the Academy of Finland (Center of Excellence in Cancer Genetics, 250345), the European Research Council (268648-NGG), the Cancer Society of Finland (L.A.A., S.L., S.S.), the Sigrid Juselius Foundation (L.A.A., S.L.), the Finnish Medical Foundation (S.S.), the Orion-Farmos Research Foundation (S.S.), the Maud Kuistila Memorial Foundation (S.S.), the Emil Aaltonen Foundation (S.S.), and the K. Albin Johansson Foundation (S.S.).
Authorship
Contribution: S.S., P.V., and L.A.A. designed the study; S.S. and E.K. performed research, analyzed the data, and wrote the manuscript; M.-L.K.-L. provided immunophenotypic data and performed pathological evaluation; K.V. and M.A. performed research; R.K. performed data analysis; M.T., S.K., S.L., and M.H. acquired patients and collected data; and P.V. and L.A.A. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lauri A. Aaltonen, Department of Medical Genetics, PO Box 63, 00014 University of Helsinki, Helsinki, Finland; e-mail: lauri.aaltonen@helsinki.fi.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal