Key Points
Combination of pomalidomide with dexamethasone is highly active and can salvage end stage myeloma refractory to lenalidomide and bortezomib.
Current data suggest pomalidomide 4 mg/day on days 1 to 21 per 28-days cycle with dexmethasone should be studied in future phase 3 trials.
Abstract
The combination of pomalidomide and dexamethasone can be safely administered to patients with multiple myeloma (MM) and has significant efficacy, although the optimal regimen remains to be determined. Patients with MM whose disease progressed after multiple lines of therapy have limited treatment options. We designed a multicenter, phase 2 randomized study assessing two different dose regimens of pomalidomide and dexamethasone in advanced MM. Treatment response was assessed centrally. Pomalidomide (4 mg) was given orally on days 1 to 21 (arm 21/28) or continuously (arm 28/28) over a 28-day cycle, plus dexamethasone given weekly. Eighty-four patients (43, arm 21/28 and 41, arm 28/28) were randomized. The median number of prior lines was 5. Overall response rate was 35% (arm 21/28) and 34% (arm 28/28), independent of the number of prior lines and level of refractoriness. Median duration of response, time to disease progression, and progression-free survival was 7.3, 5.4, and 4.6 months, respectively, which was similar across cohorts. At 23 months follow-up, median overall survival was 14.9 months, with 44% of the patients alive at 18 months. Toxicity consisted primarily of myelosuppression, which was manageable. The efficacy and safety data presented here, along with data from other phase 2 trials, suggest that pomalidomide 4 mg per day on days 1 to 21 of 28 with dexamethasone should be investigated in future trials. This trial is registered at ClinicalTrials.gov (No. NCT01053949).
Introduction
The introduction of immunomodulatory drugs (IMiDs) and proteasome inhibitors has been a major advance in the treatment of multiple myeloma (MM).1 MM remains incurable, however, and patients will ultimately acquire resistance to these agents.2 A retrospective study has recently demonstrated that patients with relapsed MM who were refractory to bortezomib and were relapsed following, or were refractory to or ineligible to receive treatment with an IMiD, had a median overall survival (OS) and event-free survival (EFS) of nine and five months, respectively.2 The partial response (PR) or better response to the first regimen used after T0 was 24%. Although retrospective, this dataset confirmed that these patients are in desperate need for novel therapies.
Pomalidomide (CC4047) is a distinct IMiD with high in vitro potency. A first phase 1b, single-center, ascending dose study was conducted to identify the maximum tolerated dose (MTD) and evaluate the safety and efficacy of CC4047 in relapsed or refractory MM.3,4 The MTD was determined to be 2 mg daily (cohort 1 at doses of 1, 2, 5, and 10 mg) or 5 mg administered every other day (cohort 2) for four weeks. Based on the encouraging data, several phase 2 studies were launched at Mayo Clinic and related centers, where pomalidomide was given continuously over a 28-day cycle.5 Among patients who were refractory to both lenalidomide and bortezomib, the response rate was 25%.6 Lacy et al have shown that 40% of lenalidomide-refractory MM patients, as well as 60% of bortezomib-refractory patients, showed a response (≥PR) using a continuous regimen of pomalidomide (2 mg per day) plus dexamethasone.7 More recently, Lacy et al reported in two phase 2 trials that MM patients were refractory to both bortezomib and lenalidomide when administered pomalidomide at 2 mg and 4 mg daily (continuous regimen) plus weekly dexamethasone.8 Results showed no advantage for 4 mg over the 2-mg dose in these trials with a response rate (≥PR) of 29% and 28%, respectively, for the two doses. In all of the studies, myelosuppression appeared to be the most common toxicity.
On the other hand, a different schedule design was evaluated in a single-center multicenter phase 1/2 study (MM-002) to investigate CC4047 alone and in combination with low-dose dexamethasone in patients with MM who had relapsed after prior treatment that included both bortezomib and lenalidomide.9 The phase 1 portion of the study determined the MTD of oral pomalidomide (4 dose levels) administered on days 1 to 21 of a 28-day cycle at 4 mg/day. The data suggested a dose-response curve and that duration of treatment increases with increasing dose. In phase 2, 221 patients were enrolled with a median of five previous lines of therapy, and a majority of patients had been treated with—and stopped responding to—both revlimid and velcade. The results showed that 34% of patients in the pomalidomide+dexamethasone group achieved at least a partial response (≥PR) compared with 13% in the pomalidomide-only group. The median duration of response (DOR) was 7.7 months and 8.3 months; the median progression free survival (PFS) was 4.7 months and 2.7 months; and the median OS 16.9 months and 14 months, in the two groups respectively.
These data demonstrate that pomalidomide can be safely administered and has significant efficacy in MM, especially in the context of patients who cannot benefit further from the two novel agents bortezomib and lenalidomide. The optimal regimen remains to be determined, however. We have therefore designed a multicenter, phase 2 randomized, open-label study of two treatment regimens of the combination of pomalidomide and dexamethasone in advanced MM.
Materials and methods
Eligibility
Patients were eligible if they had relapsed MM after at least one prior regimen of myeloma treatment. The patients were considered to be nonresponders to the last line of lenalidomide and to the last line of bortezomib—at least two cycles of either drug—if they did not achieve a response as per International Myeloma Working Group (IMWG) criteria10 (stable disease [stable disease and minor response]) within 2 cycles of treatment. Patients were required to have measurable disease using either intact immunoglobulin and/or light chain immunoglobulin, or serum immunoglobulin free light chain.10 Patients were also required to have a platelet count ≥75 × 109/L, a neutrophil count ≥1.0 × 109/L, and a creatinine clearance ≥50 mL/min. The study was approved by the Intergroupe Francophone du Myélome (IFM) and the CHRU of Lille Review Board in accordance with the Declaration of Helsinki.
Treatment schedule
Pomalidomide 4 mg was given orally either daily on days 1 to 21 of each 28-day cycle (arm 21/28 days) or continuously of each 28-day cycle (arm 28/28 days). Dexamethasone 40 mg was given orally and once weekly to all patients. All patients received thrombo-prophylaxis at the physician’s discretion. We allowed granulocyte colony-stimulating factor starting at cycle 2. We permitted dose adjustments based on grade 3 or 4 adverse events that involved lowering the dose of pomalidomide to 3 or 2 mg per day and the dose of dexamethasone to 20 mg weekly. Patients unable to tolerate the lowest doses of pomalidomide or dexamethasone were to stop therapy permanently.
Objectives of the study
The primary objective was to determine response rate (ORR, ≥PR as per IMWG criteria10 ) to pomalidomide and dexamethasone. Secondary objectives were to separately determine the safety profiles of the two regimens. The time to response, DOR, time to disease progression (TTP), PFS and EFS, and OS were analyzed overall, per arm, and with regards to adverse prognosis. MM response was determined centrally at the CHRU of Lille. The fluorescent in situ hybridization (FISH) cytogenetic analysis was performed at the CHU of Nantes and the cytogenetic abnormalities, deletion 17p (del17p), and translocation t (4;14) were defined on bone marrow plasma cells as published.10
Response and toxicity criteria
Responses and progression were assessed according to IMWG criteria,10 and required two consecutive assessments made at any time. Responses and progression were reviewed and adjudicated by an Independent Review Committee (IRC). The National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0) was used to grade adverse events and to assign perceived attribution of these events to the study treatment regimen.
Protocol outlines
The sample size was calculated according to the two-stage Simon design and based on the primary endpoint of ORR. Two interim analyses were conducted as per protocol, and the Data Monitoring Committee declared the two regimens safe for further enrollment at each step. The first analysis focused on the safety profile of the combination in either arm and was performed when six patients had completed at least cycle 1 in each arm. The second analysis was performed at the end of the first stage when 17 patients had been randomized to either arm. The recruitment of the second stage was to be permitted if at least four responses were observed in each arm and no imbalance was seen in terms of safety. Both arms met the criteria, with five and four responders in the two arms, respectively, with similar safety profiles between the two arms.11
Statistical design and analysis
Primary analysis was performed in the intent-to-treat (ITT) population, including all patients randomized and treated (N = 84). Secondary analysis was also to be performed on the efficacy-evaluable population (EE), which corresponded to patients who had received at least two cycles of pomalidomide and dexamethasone, had at least one post–cycle 2 disease assessment, and had no major deviation. Data are summarized both overall and for each treatment arm, where appropriate. Exact binomial confidence intervals were constructed for survival end points. All time-to-event analyses were estimated using the Kaplan-Meier method except for time to response, for which descriptive statistics were used. A data cutoff was made on February 1, 2012. Statistics analysis was performed using SAS software (SAS Institute, Cary, NC).
Results
Patient population
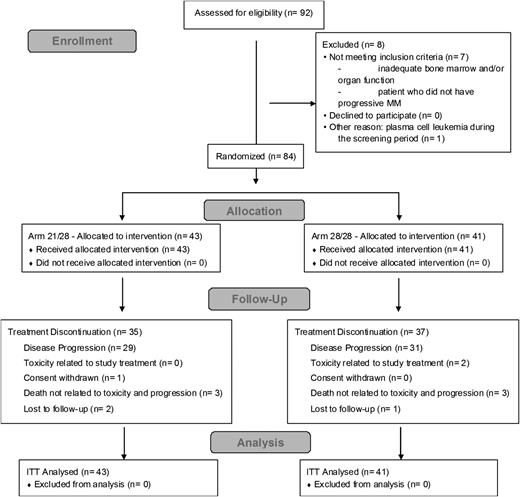
Ninety-two patients were included in the study between October 2009 and August 2010 (Figure 1). Eight patients were screening failures. The ITT population included all randomized patients (N = 84); 43 patients (51%) randomized to arm 21 of 28 days and 41 patients (49%) randomized to arm 28 of 28 days (Table 1). The EE population comprised 66 patients, 34 in arm 21 of 28 days and 32 in arm 28 of 28 days. Overall the median age was 60 years (range, 42 to 83). The median time from diagnosis was 5.9 years (range, 0.8 to 23.1), with 40 patients (48%) older than 6 years (similar in the 2 arms). All patients had received prior treatment with bortezomib and lenalidomide, 73% received alkylating agents with 81% previously exposed to autologous stem cell transplantation, 76% received anthracylines, and 73% received thalidomide. In their last line of therapy, 38% of patients received bortezomib, 34.5% received lenalidomide, and 11% received a combination containing both bortezomib and lenalidomide. The data for patients who matched the criteria of refractoriness as per IMWG criteria10 are summarized in Table 2. Overall, 76% of the patients were refractory to bortezomib and lenalidomide (double refractory) and 31% were refractory to lenalidomide as their last line.10
Patient demographics and baseline characteristics at entry into IFM 2009-02, unless specified (N = 84)
| Characteristic . | 21/28 (N = 43) . | 28/28 (N = 41) . |
|---|---|---|
| Median time from diagnosis to randomization, years (range) | 5.1 (0.9- 8.7) | 6.5 (0.8-23.1) |
| ≤3 years, n (%) | 13 (30) | 4 (10) |
| Median age, years (range) | 60 (45-81) | 60 (42-83) |
| Age ≥65 years, n (%) | 11 (26) | 15 (37) |
| ISS stage, %* | ||
| II/III | 32 / 24 | 42 / 17 |
| Myeloma type, n (%) | ||
| Intact Ig | 36 (84) | 34 (83) |
| Light chain | 4 (9) | 5 (12) |
| Freelite® measurable only† | 3 (7) | 2 (5) |
| Lytic bone lesions, n (%) | 37 (86) | 37 (90) |
| Yes | 31 (86) | 32 (86.5) |
| Number of lytic lesions: [3-6] | 9 (25) | 8 (22) |
| Number of lytic lesions: > 6 | 11 (31) | 17 (46) |
| Osseous Fracture | 10 (29) | 11 (32) |
| Medullary compression | 0 | 3 (9) |
| Plasmacytoma | 9 (21) | 5 (12) |
| Median β2-microglobulin, mg/L (range) | 3.9 (1.0-13.2) | 3.7 (1.6-12.0) |
| (3.5, 5.5) mg/L, n (%) | 19 (44) | 11 (27.5) |
| >5.5 mg/L, n (%) | 10 (23) | 11 (27.5) |
| Median albumin, g/L (range) | 37.1 (24.6-49.5) | 38.2 (28.1-46.6) |
| Median serum creatinine, (µmol/l) (range) | 80.0 (44.0-193.0) | 87.0 (42.0-196.0) |
| >115 µmol/L, n (%) | 6 (14) | 4 (10) |
| Median hemoglobin, g/dL (range) | 10.5 (7.2-14.1) | 10.5 (6.4-14.1) |
| <10 g/dL, n (%) | 18 (42) | 15 (37.5) |
| Median neutrophils, ×109/L (range) | 2.6 (0.04-10.4) | 2.3 (0.9-8.3) |
| Median platelets, x109/L (range) | 161 (51-366) | 147 (33-269) |
| <100 G/L, n (%) | 8 (19) | 10 (25) |
| Median number of lines | 5 | 5 |
| (min-max) | (1-13) | (2-10) |
| Characteristic . | 21/28 (N = 43) . | 28/28 (N = 41) . |
|---|---|---|
| Median time from diagnosis to randomization, years (range) | 5.1 (0.9- 8.7) | 6.5 (0.8-23.1) |
| ≤3 years, n (%) | 13 (30) | 4 (10) |
| Median age, years (range) | 60 (45-81) | 60 (42-83) |
| Age ≥65 years, n (%) | 11 (26) | 15 (37) |
| ISS stage, %* | ||
| II/III | 32 / 24 | 42 / 17 |
| Myeloma type, n (%) | ||
| Intact Ig | 36 (84) | 34 (83) |
| Light chain | 4 (9) | 5 (12) |
| Freelite® measurable only† | 3 (7) | 2 (5) |
| Lytic bone lesions, n (%) | 37 (86) | 37 (90) |
| Yes | 31 (86) | 32 (86.5) |
| Number of lytic lesions: [3-6] | 9 (25) | 8 (22) |
| Number of lytic lesions: > 6 | 11 (31) | 17 (46) |
| Osseous Fracture | 10 (29) | 11 (32) |
| Medullary compression | 0 | 3 (9) |
| Plasmacytoma | 9 (21) | 5 (12) |
| Median β2-microglobulin, mg/L (range) | 3.9 (1.0-13.2) | 3.7 (1.6-12.0) |
| (3.5, 5.5) mg/L, n (%) | 19 (44) | 11 (27.5) |
| >5.5 mg/L, n (%) | 10 (23) | 11 (27.5) |
| Median albumin, g/L (range) | 37.1 (24.6-49.5) | 38.2 (28.1-46.6) |
| Median serum creatinine, (µmol/l) (range) | 80.0 (44.0-193.0) | 87.0 (42.0-196.0) |
| >115 µmol/L, n (%) | 6 (14) | 4 (10) |
| Median hemoglobin, g/dL (range) | 10.5 (7.2-14.1) | 10.5 (6.4-14.1) |
| <10 g/dL, n (%) | 18 (42) | 15 (37.5) |
| Median neutrophils, ×109/L (range) | 2.6 (0.04-10.4) | 2.3 (0.9-8.3) |
| Median platelets, x109/L (range) | 161 (51-366) | 147 (33-269) |
| <100 G/L, n (%) | 8 (19) | 10 (25) |
| Median number of lines | 5 | 5 |
| (min-max) | (1-13) | (2-10) |
ISS classification at diagnosis.
Patients considered not measurable based on serum intact immunoglobulin and urine light-chain excretion were measurable if they had serum immunoglobulin free light chain more than 100 mg/L and an abnormal free light chain ratio.
Incidence rate (number and percentage) of patients with various adverse prognostic factors by arm (N = 84)
| . | 21/28 . | 28/28 . | . |
|---|---|---|---|
| . | (N = 43) . | (N = 41) . | Total . |
| Patients >6 lines of therapy, n (%) | 12 (28) | 7 (17) | 19 (23) |
| Refractory to*, n (%) | |||
| Lenalidomide | 36 (84) | 39 (95) | 75 (89) |
| Lenalidomide last prior therapy | 15 (35) | 11 (27) | 26 (31) |
| Bortezomib | 34 (79) | 34 (83) | 68 (81) |
| Both lenalidomide and bortezomib | 32 (74) | 32 (78) | 64 (76) |
| Last prior therapy | 36 (70) | 35 (68) | 71 (84.5) |
| FISH cytogenetics, n (%)† | N = 33 | N = 32 | |
| Deletion 17p | 6 (21) | 9 (33) | — |
| Translocation (4;14) | 2 (7) | 4 (17) | — |
| . | 21/28 . | 28/28 . | . |
|---|---|---|---|
| . | (N = 43) . | (N = 41) . | Total . |
| Patients >6 lines of therapy, n (%) | 12 (28) | 7 (17) | 19 (23) |
| Refractory to*, n (%) | |||
| Lenalidomide | 36 (84) | 39 (95) | 75 (89) |
| Lenalidomide last prior therapy | 15 (35) | 11 (27) | 26 (31) |
| Bortezomib | 34 (79) | 34 (83) | 68 (81) |
| Both lenalidomide and bortezomib | 32 (74) | 32 (78) | 64 (76) |
| Last prior therapy | 36 (70) | 35 (68) | 71 (84.5) |
| FISH cytogenetics, n (%)† | N = 33 | N = 32 | |
| Deletion 17p | 6 (21) | 9 (33) | — |
| Translocation (4;14) | 2 (7) | 4 (17) | — |
Refractory to as per IMWG criteria.10
High-risk cytogenetics by FISH consisted of deletion 17p or t(4;14) at diagnosis and/or at entry in the IFM 2009-02 trial.
Duration of treatment
The median follow-up was 22.8 months (similar in the 2 arms). At the cutoff of February 1, 2012, 72 patients had discontinued treatment. The most common reason for treatment discontinuation was disease progression (84% of patients, similar in the 2 arms) (Figure 1). The duration of treatment is summarized in supplemental Table 1, and the dose reduction and dose interruption are summarized in supplemental Table 2. A majority of patients had some dose reduction and/or interruption of pomalidomide plus dexamethasone. However, pomalidomide at a dose of 4 mg daily remained manageable because >80% of the patients received the intended initial daily dose of pomalidomide and of dexamethasone. The median treatment duration appeared superior in the 21 of 28-day arm compared with the 28 of 28-day arm (supplemental Table 1), with a greater median number of cycles of eight compared with six in the 2 arms, respectively. Interestingly, 10 patients (12%) remain on treatment after 30 months.
Efficacy
The ORR (IRC review) was 34.5% for the ITT population, similar across arms, and 40 patients (47%) had stable disease (including minimal response) (Table 3). The ORR based on investigators’ initial assessment was 41.5% (Table 3). Overall, the median (95% CI) duration of response was 7 months (5, 15), and 44% were responders at 12 months (hazard ratio [HR], 0.91; 95% CI, 0.4, 2.0; P = .817) and appeared greater in the 28 of 28-day arm (Table 3). In the EE population, 41% and 50% of patients responded, respectively, and 37.7% maintained their response after one year (Table 3). There was no significant difference in terms of ORR and duration of response in the 2 arms.
Summary of response to treatment and survival end points by arm based on IRC assessment (N = 84)
| . | 21/28 (N = 43) . | 28/28 (N = 41) . | Total . |
|---|---|---|---|
| Response rate, n (%) | ITT (N = 84) | ||
| ORR (≥PR) | 15 (35) | 14 (34) | 29 (34.5) |
| CR* | 1 (2) | 2 (5) | 3 (4) |
| VGPR | 1 (2) | 1 (2) | 2 (2) |
| PR | 13 (30) | 11 (27) | 24 (27) |
| Stable disease | 19 (44) | 21 (51) | 40 (48) |
| Progressive disease | 5 (12) | 3 (7) | 8 (9.5) |
| Not evaluable | 4 (9) | 3 (7) | 7 (8) |
| Median time to first response (95% CI), months | 2.7 (0.8, 9.5) | 1.1 (0.6, 8) | 1.8 (0.6, 9.5) |
| Median duration of response (95% CI), months | 6.4 (4, —) | 8.3 (6.5, 16) | 7.3 (5, 15) |
| One year free of relapse, % | 42 | 47 | 44 |
| EE (N = 66) | |||
| ORR (CR + PR) | 15 (44) | 12 (37.5) | 27 (41) |
| ≥VGPR | 2 (6) | 3 (9) | 5 (7.5) |
| Median survival (95% CI), months | 21/28 (N = 43) | 28/28 (N = 41) | Total |
| Time to progression | 5.8 (3, 10) | 4.8 (3, 7) | 5.4 (4, 8) |
| At 1 y, % | 31 | 25 | 28 |
| Progression-free survival | 5.4 (3, 9) | 3.7 (2, 7) | 4.6 (4, 7) |
| At 1 y, % | 29 | 22 | 25.5 |
| Overall survival | 14.9 (9, —) | 14.8 (9, 20) | 14.9 (11, 20) |
| At 12 mo, % | 58 | 56 | 57 |
| At 18 mo, % | 49 | 39 | 44 |
| . | 21/28 (N = 43) . | 28/28 (N = 41) . | Total . |
|---|---|---|---|
| Response rate, n (%) | ITT (N = 84) | ||
| ORR (≥PR) | 15 (35) | 14 (34) | 29 (34.5) |
| CR* | 1 (2) | 2 (5) | 3 (4) |
| VGPR | 1 (2) | 1 (2) | 2 (2) |
| PR | 13 (30) | 11 (27) | 24 (27) |
| Stable disease | 19 (44) | 21 (51) | 40 (48) |
| Progressive disease | 5 (12) | 3 (7) | 8 (9.5) |
| Not evaluable | 4 (9) | 3 (7) | 7 (8) |
| Median time to first response (95% CI), months | 2.7 (0.8, 9.5) | 1.1 (0.6, 8) | 1.8 (0.6, 9.5) |
| Median duration of response (95% CI), months | 6.4 (4, —) | 8.3 (6.5, 16) | 7.3 (5, 15) |
| One year free of relapse, % | 42 | 47 | 44 |
| EE (N = 66) | |||
| ORR (CR + PR) | 15 (44) | 12 (37.5) | 27 (41) |
| ≥VGPR | 2 (6) | 3 (9) | 5 (7.5) |
| Median survival (95% CI), months | 21/28 (N = 43) | 28/28 (N = 41) | Total |
| Time to progression | 5.8 (3, 10) | 4.8 (3, 7) | 5.4 (4, 8) |
| At 1 y, % | 31 | 25 | 28 |
| Progression-free survival | 5.4 (3, 9) | 3.7 (2, 7) | 4.6 (4, 7) |
| At 1 y, % | 29 | 22 | 25.5 |
| Overall survival | 14.9 (9, —) | 14.8 (9, 20) | 14.9 (11, 20) |
| At 12 mo, % | 58 | 56 | 57 |
| At 18 mo, % | 49 | 39 | 44 |
CR confirmed by bone marrow assessment.
Survival
At data cutoff, 53 ITT patients (63%) had died: 25 (58%) in arm 21 of 28 days and 28 (68%) in arm 28 of 28 days. Death was considered to be related to the studied combination for one patient in the 28 of 28-day arm. For 95% of patients, death was considered to be related to myeloma. Death occurred during treatment in 32% of cases, and >30 days after the last study treatment intake for the remaining 68%. All time-to-event end points were similar across both arms (Table 3). Figure 2A to C shows the updated TTP, PFS, and OS per arm, respectively. Importantly, 28% (31% in arm 21/28 days and 25% in arm 28/28 days) were free of progression at 12 months (HR, 1.25; 95% CI, 0.8, 2.0; P = .35). Furthermore, the median OS (95% CI) was similar in the two arms (HR, 1.23; 95% CI, 0.7, 2.0; P = .45); 57% (similar in the 2 arms) and 44% (49% arm 21/28 days and 39% arm 28/28 days) of patients were alive after 12 months and 18 months of treatment, respectively.
(A-C) Survival end points per 21 of 28-day arm (N = 43) and 28 of 28-day arm (N = 41); Kaplan-Meier estimates (ITT). (A) Time to progression. (B) Progression-free survival. (C) Overall survival. (D-F) Survival end points in the entire population (N = 84); Kaplan-Meier estimates (ITT). (D) Comparison between TTP on protocol and TTP on last prior line for all patients included in the study and for responders only (patients with either CR or VGPR or PR as per IMWG). (E) TTP In responders versus nonresponders as per IMWG criteria (eg, patients with stable disease including minimal response [SD]). (F) Overall survival in responders versus SD. O/N, number of events/number of patients in the group.
(A-C) Survival end points per 21 of 28-day arm (N = 43) and 28 of 28-day arm (N = 41); Kaplan-Meier estimates (ITT). (A) Time to progression. (B) Progression-free survival. (C) Overall survival. (D-F) Survival end points in the entire population (N = 84); Kaplan-Meier estimates (ITT). (D) Comparison between TTP on protocol and TTP on last prior line for all patients included in the study and for responders only (patients with either CR or VGPR or PR as per IMWG). (E) TTP In responders versus nonresponders as per IMWG criteria (eg, patients with stable disease including minimal response [SD]). (F) Overall survival in responders versus SD. O/N, number of events/number of patients in the group.
We have compared the median TTP on protocol to the TTP on the last prior line that patients were exposed to before entry into the trial. The median TTP was 5.8 months (4, 8) and 4.7 months (3, 5) in the two groups. The difference between TTP in the two groups was even more striking when studying responders, 11.6 (8, —) and 5.7 (3, 9) months in the two groups, respectively (Figure 2D). The EFS is 25% and 15% at one year in the overall studied population (entire study), and the EFS at one year is 47% and 23% in patients that obtained a response. We found that TTP was greater with pomalidomide than any other line of therapy that patients had access to in France at the time of the study.
Elderly patients
Twenty-six (31%) patients were aged ≥65 years: 26% of patients in arm 21 of 28 days and 37% in arm 28 of 28 days. The ORR of pomalidomide and dexamethasone was similar in elderly patients (27%, similar across arms) compared with the overall population. However, all time-to-event end points appeared lower in elderly patients (similar in the 2 arms), with a median TTP of 4.6 months (95% CI, 3.6, 8.4) and 23% of patients being progression free after one year (vs 5.5 [3.2, 9.2] months and 30% for patients <65, respectively) (P = ns). The median PFS was 3.8 months (3.0, 7.3) versus 5.0 (2.9, 9.1) (P = ns). The survival rate at 12 and 18 months was 50% and 31% for elderly patients versus 60% and 50% for patients <65, respectively (P = ns). The elderly patients had a shorter treatment benefit, although they displayed similar response rates—a fragile population that may benefit from an effective and safe drug, but with shorter survival late in the course of the disease.
Patients who responded to treatment benefited with improved TTP, PFS, and OS (similar in the 2 arms). The median TTP was longer for responders compared with patients who achieved no better than a SD (HR, 0.28; 95% CI, 0.2, 0.5; P = .0001) (Figure 2E). At one year, 49% of the responders had not progressed versus 21% of SD patients. Similar results were observed for PFS (median 11.3 and 3.8 months, and 48% and 14% one year PFS rate, respectively) and for OS (HR, 0.45; 95% CI, 0.2, 0.9; P = .018) (Figure 2F). At 12 months, the OS rates were 82% and 54.5%, and at 18 months they were 69% and 36%, respectively. Patients who reached a response had a considerably improved outcome in this study, and this may be a good surrogate marker for improved long-term outcome in patients with very advanced myeloma.
Patients with poor cytogenetic abnormalities, del17p and t(4;14)
Using FISH analysis, 21 patients (37.5%) had either del17p (15 [27%]) or t(4;14) (6 [11%]) (Table 2). The response rates and median PFS and OS of this subgroup are presented in Table 4 (similar across arms). The response rate in del17p (5/15% to 33%) was similar to that of the overall population, whereas t(4;14) might display a lower ORR (1/6% to 17%). However, all survival end points, including the PFS (44% vs 95%; [HR, 0.35; 95% CI, 0.2-0.6; P = .0005]) and OS (27% vs 67% at 1 year [HR, 0.30; 95% CI, 0.1-0.6; P = .0002]) appeared shorter compared with patients who did not harbor either of these two cytogenetic abnormalities. Future studies will need to confirm these data, because there is a clear unmet medical need for del17p and/or t(4;14), and there will need to be studies on the benefits of a triplet pomalidomide and dexamethasone–based regimen.
Response rates (ORR, ITT, IRC-based), PFS, and OS in subgroups with adverse prognosis (N = 84)
| . | . | . | PFS (mo) . | OS (mo) . |
|---|---|---|---|---|
| Patients . | N . | ORR, N (%) . | Median (95%CI)/% at 1 y . | Median (95%CI)/% at 1 y . |
| ITT | 84 | 29 (34.5) | 4.6 (4, 7)/25.5 | 14.9 (11, 20)/57 |
| Refractory to lenalidomide | 75 | 27 (36) | 4.2 (3, 6)/23 | 13.9 (9, 18)/54.5 |
| Refractory to lenalidomide as last line * | 26 | 6 (23) | 4.4 (2, 9)/22 | 19.2 (9, —)/63 |
| Refractory to bortezomib | 68 | 20 (29) | 3.8 (3, 6)/24 | 13.8 (9, 18)/54 |
| Refractory to last line * | 71 | 23 (32) | 3.9 (3, 7)/25 | 13.9 (8.5, 19)/55 |
| Double refractory† | 64 | 20 (31) | 3.8 (3, 5)/24 | 13.8 (9, 16)/53 |
| More than 6 lines of therapy* | 19 | 4 (21) | 3.2 (2, 5)/16 | 9.2 (3, —)/47 |
| Del17p and/or t(4;14) | 21 | 6 (27) | 2.6 (2, 4)/5 | 5.4 (3, 9)/33 |
| . | . | . | PFS (mo) . | OS (mo) . |
|---|---|---|---|---|
| Patients . | N . | ORR, N (%) . | Median (95%CI)/% at 1 y . | Median (95%CI)/% at 1 y . |
| ITT | 84 | 29 (34.5) | 4.6 (4, 7)/25.5 | 14.9 (11, 20)/57 |
| Refractory to lenalidomide | 75 | 27 (36) | 4.2 (3, 6)/23 | 13.9 (9, 18)/54.5 |
| Refractory to lenalidomide as last line * | 26 | 6 (23) | 4.4 (2, 9)/22 | 19.2 (9, —)/63 |
| Refractory to bortezomib | 68 | 20 (29) | 3.8 (3, 6)/24 | 13.8 (9, 18)/54 |
| Refractory to last line * | 71 | 23 (32) | 3.9 (3, 7)/25 | 13.9 (8.5, 19)/55 |
| Double refractory† | 64 | 20 (31) | 3.8 (3, 5)/24 | 13.8 (9, 16)/53 |
| More than 6 lines of therapy* | 19 | 4 (21) | 3.2 (2, 5)/16 | 9.2 (3, —)/47 |
| Del17p and/or t(4;14) | 21 | 6 (27) | 2.6 (2, 4)/5 | 5.4 (3, 9)/33 |
Before entry into IFM 2009-02
Double refractory, refractory to bortezomib and lenalidomide. ORR ≥PR.
Other subgroup with adverse prognostic factors
In general, patients with advanced myeloma exposed to most existing agents with multiple lines of therapy respond poorly to treatment and display a poor prognosis, similar to patients who are refractory to bortezomib and lenalidomide (double refractory) or to their last line of therapy (Table 2). The response rates and median PFS and OS for these patients are presented in Table 4 and were similar across arms and in most of the subgroups tested compared with the ITT population. For some subgroups, however, PFS seemed slightly shorter than that determined in the ITT population as a whole. The response rates and median PFS and OS were 31%, 3.8 months with 24% at one year, and 13.8 months with 53% at one year for patients who were double refractory. For patients who were refractory to lenalidomide as their last line of therapy, these results are 23%, 4.4 months with 22% at one year, and 19.2 months with 63% at one year.
These results further demonstrate that the combination of pomalidomide and dexamethasone could be of benefit to patients who have failed current standard treatment regimens, including for patients who were refractory to lenalidomide and bortezomib and those who were refractory to lenalidomide before entry into IFM 2009-02.
Tolerability profile
The toxicity profile of pomalidomide-dexamethasone consisted primarily of myelosuppression. All of the patients in this study experienced adverse events (AEs). Seventy-five patients (89%) had at least one grade 3 or 4 AEs (40 [93%] in arm 21/28 days and 35 [85%] in arm 28/28 days), and 65 (77%) had a serious adverse event (SAE) (32 [74%] in arm 21/28 days and 33 [80.5%] in arm 28/28 days). Only two patients discontinued the treatment because of an AE related to the studied drug (both arm 28/28 days). The most common hematologic and nonhematologic grade 3 and 4 AEs are summarized in Table 5. Overall, grade 3 and 4 AEs that occurred in >10% of cases were neutropenia in 62%, anemia in 36%, thrombocytopenia in 27%, pneumonia in 13%, bone pain in 11%, renal failure in 11%, and dyspnea in 12%. The most common hematologic and nonhematologic study drug-related AEs are summarized in supplemental Table 3. The use of a thrombo-prophylatic treatment was mandatory in this study; 70% received platelet aggregation inhibitors, 40.5% received low-molecular-weight heparin, and 14% received vitamin K antagonists. Only 3 patients (4%) experienced a deep vein thrombosis event while taking aspirin.
Summary of grade 3 or 4 (NCI CTC) AEs that occurred in >5% of cases, according to system organ class (N = 84)
| MedDRA system organ class and AEs, n (%) . | 21/28 (N = 43) . | 28/28 (N = 41) . | Total . |
|---|---|---|---|
| SAEs | 32 (74) | 33 (80) | 65 (77) |
| Study drug related SAEs | 14 (33) | 18 (44) | 32 (38) |
| Any grade 3 and 4 AEs | 40 (93) | 35 (85) | 75 (89) |
| Blood and lymphatic system disorders | 32 (74) | 30 (73) | 62 (74) |
| Anemia | 16 (37) | 14 (34) | 30 (36) |
| Neutropenia | 28 (65) | 24 (58.5) | 52 (62) |
| Thrombocytopenia | 12 (28) | 11 (27) | 23 (27) |
| General disorders and administration site conditions | 10 (23) | 10 (24) | 20 (24) |
| Asthenia | 6 (14) | 2 (5) | 8 (9.5) |
| Infections and infestations | 8 (19) | 11 (27) | 19 (23) |
| Pneumonia | 3 (7) | 8 (19.5) | 11 (13) |
| Musculoskeletal and connective tissue disorders | 9 (21) | 8 (19.5) | 17 (20) |
| Bone pain | 6 (14) | 3 (7) | 9 (11) |
| Renal and urinary disorders | 7 (16) | 2 (5) | 9 (11) |
| Renal failure | 7 (16) | 2 (5) | 9 (11) |
| Respiratory disorders | 8 (19) | 2 (5) | 10 (12) |
| Dyspnea | 5 (12) | 0 | 5 (6) |
| MedDRA system organ class and AEs, n (%) . | 21/28 (N = 43) . | 28/28 (N = 41) . | Total . |
|---|---|---|---|
| SAEs | 32 (74) | 33 (80) | 65 (77) |
| Study drug related SAEs | 14 (33) | 18 (44) | 32 (38) |
| Any grade 3 and 4 AEs | 40 (93) | 35 (85) | 75 (89) |
| Blood and lymphatic system disorders | 32 (74) | 30 (73) | 62 (74) |
| Anemia | 16 (37) | 14 (34) | 30 (36) |
| Neutropenia | 28 (65) | 24 (58.5) | 52 (62) |
| Thrombocytopenia | 12 (28) | 11 (27) | 23 (27) |
| General disorders and administration site conditions | 10 (23) | 10 (24) | 20 (24) |
| Asthenia | 6 (14) | 2 (5) | 8 (9.5) |
| Infections and infestations | 8 (19) | 11 (27) | 19 (23) |
| Pneumonia | 3 (7) | 8 (19.5) | 11 (13) |
| Musculoskeletal and connective tissue disorders | 9 (21) | 8 (19.5) | 17 (20) |
| Bone pain | 6 (14) | 3 (7) | 9 (11) |
| Renal and urinary disorders | 7 (16) | 2 (5) | 9 (11) |
| Renal failure | 7 (16) | 2 (5) | 9 (11) |
| Respiratory disorders | 8 (19) | 2 (5) | 10 (12) |
| Dyspnea | 5 (12) | 0 | 5 (6) |
The combination of pomalidomide plus dexamethasone appeared to be safe in elderly patients, with no significant difference noted between the two arms of the trial. Ninety-two percent of elderly patients had at least one grade 3 or 4 AE (100% and 87% in the 2 arms, respectively) and 88.5% had a SAE (91% and 87%, respectively). One patient discontinued the treatment because of an AE related to the studied drug in the 28 of 28-day arm. The most common grade 3 and 4 AEs were hematologic, with 61.5% as neutropenia, 38.5% as anemia, and 23% as thrombopenia. The most common grade 3 and 4 nonhematologic AEs that occurred in >10% of cases were asthenia in 19%, general physical health deterioration in 11.5%, and bronchitis and pneumonia in 19%.
Discussion
This randomized phase 2 study further demonstrates that pomalidomide plus dexamethasone is a safe and effective combination for the treatment of very advanced MM. Patients were included in the study with a median time from diagnosis of six years, having received a median of five lines of prior treatment. All patients had previously been exposed to both lenalidomide and bortezomib and two-thirds had received prior treatment with thalidomide or an alkylating agent. In this context, the ORR was 34.5% (very similar between both arms and independently assessed) and the incidence of stable disease (including minimal responses) was 48%. Median OS was 14.9 months and the one-year survival rate was 57%, far superior to what would be expected at this advanced stage of MM.2 Kumar et al reported on a lower ORR at 6% in the IMWG study (median 4 lines of therapy compared with 5 in our study), similar median EFS at five months, and a lower median OS at nine months. The difference in terms of OS, although similar to EFS, might come from the higher response rate with pomalidomide plus dexamethasone, that turned into a prolonged DOR, and ultimately a prolonged OS.
The IFM 2009-02 study thus pointed out the survival difference in responders compared with patients with stable disease related to a prolonged DOR. In patients with advanced disease who were exposed to most existing agents, or in patients with adverse prognosis as a whole, the treatment is based on a fragile equilibrium between tolerance of drugs and the importance of obtaining a response. The former helps maintain patients on treatment to avoid rapid progression and escape of tumoral cells in the case that treatment is stopped early, whereas the latter is a necessary step for an optimal control of mechanisms of resistance and less sensitive subclones.12 One might then propose to start with a two drug–based, active antimyeloma regimen such as pomalidomide plus dexamethasone in these patients, knowing that approximately 30% to 40% of the patients will respond. However, in patients who may not reach a rapid response to pomalidomide plus dexamethasone, one might suggest implementing a three drug–based regimen to optimize the control of mechanisms of resistance in tumor cells and in the various subclones. In support of this hypothesis, Palumbo et al have reported an ORR of 73% on a phase 1/2 study of the combination of pomalidomide-cyclophosphamide plus prednisone at American Society of Hematology 2011.13 Similarly, Dr Mark and colleagues reported a 60% ORR with pomalidomide-biaxin and dexamethasone in MM.14
The results in patients who were refractory to lenalidomide or bortezomib, or who were double refractory, also compared favorably with other studies.5,6,8 Lacy et al reported on a double-refractory study population (N = 35) treated with pomalidomide 4 mg per day and dexamethasone 40 mg per week over a 28-day cycle, comparable with that described here: patients having received a median of six lines of prior treatment with a median time from diagnosis of six years.8 The ORR was 29%, with a median duration of response of 3.9 months (1, —; median follow-up 6 months). The median PFS was 3.2 months (95% CI, 2, 9) with 34% of patients free of progression at six months (21, 55). OS at 6 months was 67% (52, 86). These results appear identical to those in our study, where the response rate for double-refractory patients treated in the 28 of 28-day arm was 28%. The median PFS for this group was 5.2 months (3, 8), with 50% of patients free of progression at five months and the one-year survival rate of 31%.
Interestingly, in our study a lower response rate was observed among patients who were refractory to lenalidomide when this was part of their last line of therapy before study entry (ORR, 23%) compared with patients who were refractory to lenalidomide received as a whole (ORR, 36%). However, the median PFS and OS were similar between these two subgroups (PFS 4 months [range, 2-9] vs 4 months [range, 3-6]; OS at 1 year was 63% vs 54.5%, respectively). The lower response rate in the former did not have an impact in terms of survival benefit.
More than two-thirds of patients had AEs of grade 3 intensity or higher in our study. Toxicity consisted mainly of myelosuppression-related events, all of which were manageable by dose reductions or transfusions or through the use of growth factors as allowed by the protocol. The toxicity profile of the pomalidomide and dexamethasone combination was similar to previously reported data.5,6,8 Myeloma is now considered as the leading hematologic malignancy in terms of venous thrombosis risk.14 Thrombotic events have also been established as the most frequent and serious side effect linked to treatment with IMiDs.15 A thrombo-prophylaxis treatment was imposed in our study,16 and as a result of this policy, only three venous thrombotic events were reported in this study. This compares very favorably with the incidence of such events reported in previous trials of the pomalidomide and dexamethasone combination.5,6,8
Although this study was not powered to make any formal comparison between the two treatment arms, the efficacy and safety data presented here, along with data from other phase 2 trials, suggest an advantage for the 21 of 28-days regimen in which treatment is given for three weeks on, with a one-week period of rest. Thus, from a clinical point of view, it appears reasonable to use the less intensive pomalidomide-dexamethasone regimen to minimize the acute and cumulative toxicity. Therefore, on the basis of these results and others, it seems reasonable to propose that a regimen of pomalidomide 4 mg per day on days 1 to 21 of a 28-day cycle combined with dexamethasone 40 mg weekly should be investigated in future phase 3 trials. Furthermore, it would be rational to test the combination of pomalidomide and dexamethasone with other novel agents, including proteasome inhibitors, to further improve response rates and survival outcomes.
In conclusion, the results presented here show that the combination of pomalidomide and low-dose dexamethasone is highly active and well tolerated in the treatment of relapsed and refractory MM, especially in this heavily pretreated population of lenalidomide and bortezomib–refractory MM patients. This study provides further evidence that pomalidomide has no cross-resistance with lenalidomide and suggests that the pomalidomide+dexamethasone combination can salvage patients who have relapsed after other therapy. The data therefore suggest that pomalidomide 4 mg per day on days 1 to 21 per 28-day cycle with dexamethasone should be investigated in future phase 3 trials.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Celgene for their financial support and for providing investigational products. The authors also thank Dr. Brigitte Onraed, Mr. Jean Luc Faucompret. and Mr. Jaime Gutierrez for the central laboratory analysis in CHRU of Lille; Sylvie Brice from the central pharmacy; Sofia Mengual, Christine Cotton, Maud Clouseau, Vandana Pommerais, and Xavier Mengual from Statitec (Toulouse, France) for data management and statistical analysis; Alain Duhamel, CHRU of Lille, for statistical analysis; and Elise Gers, Tatiana Pham, Wassila Bendaoudi, Aurélie Tessier, and Mariam Petrosyan for the DRC of CHRU of Lille.
Authorship
Contribution: X.L. and T.F. conceived and designed the study; X.L., M.A., B.A., P.M., C.T., M.M., G.M., M.M., M.R., B.P., B.K., A.M.S., S.B., L.G., B.R., C.H., L.B., O.D., N.M., D.C., M.E.-B., J.P.F., and T.F. collected and assembled the data; C.M. and M.O.P. offered study and CRF conception, administrative support, and study coordination; H.A.-L. handled cytogenetic data; B.T. and B.B.D. were the central pharmacists; B.H. worked at the central laboratory; X.L., A.D. analyzed and interpreted the data; X.L. wrote the manuscript.
Conflict-of-interest disclosure: X.L. discloses research support and lecture fees from Celgene; M.A. speaker bureau and lecture fees from Celgene; B.A. lecture fees from Celgene; P.M. speaker bureau and lecture fees; G.M. lecture fees from Celgene; M.M. lecture fees from Celgene; M.R. lecture fees from Celgene; B.K. lecture fees from Celgene; A.M.S. lecture fees from Celgene; L.G. lecture fees from Celgene; B.R. lecture fees from Celgene; C.H. lecture fees from Celgene; L.B. lecture fees from Celgene; H.A.-L. speaker bureau from Celgene; T.F. speaker bureau and lecture fees. The remaining authors declare no competing financial interests.
Correspondence: Xavier Leleu, MD, PhD, Service des maladies du sang, Hôpital Huriez, CHRU, Lille, France; e-mail: xavier.leleu@chru-lille.fr.

![Figure 2. (A-C) Survival end points per 21 of 28-day arm (N = 43) and 28 of 28-day arm (N = 41); Kaplan-Meier estimates (ITT). (A) Time to progression. (B) Progression-free survival. (C) Overall survival. (D-F) Survival end points in the entire population (N = 84); Kaplan-Meier estimates (ITT). (D) Comparison between TTP on protocol and TTP on last prior line for all patients included in the study and for responders only (patients with either CR or VGPR or PR as per IMWG). (E) TTP In responders versus nonresponders as per IMWG criteria (eg, patients with stable disease including minimal response [SD]). (F) Overall survival in responders versus SD. O/N, number of events/number of patients in the group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/11/10.1182_blood-2012-09-452375/4/m_1968f2.jpeg?Expires=1767721508&Signature=yk7szAyyMuj5T5x8WTfWBFv7XT2MePO~IEDggG0wsokun6vv7vafAO0TBwtbeEmAbQcGIdJ8zZ4N5pAk-YIvthcyeTF7-VtIRyWxab3FOqahbciNR8uxGauBpvl1pNUf0LoGiBP1zeXN5BKhko8p9ykr2TqmCFDpj2lUABgRY63T10ynAEQyy-O8er9Awie1QvykMfMgoH9FBMLR8fOSRVYgUzXkE2dKBWpcwfUe8PS3qyHyXmfz5trrTKvCWmyG0VwSEtfcSe7Ejkdq3tOnO2ygc-G4SjiZE~Fua9SGVcLMB1GQPisFM~OqOSe~EMzezybtWX~YLa8L3UULwWwINw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)