Key Points
Pomalidomide with/without dexamethasone has promising activity and manageable toxicity in relapsed and refractory multiple myeloma patients.
Abstract
This phase 1 dose-escalation study determined the maximum tolerated dose (MTD) of oral pomalidomide (4 dose levels) administered on days 1 to 21 of each 28-day cycle in patients with relapsed and refractory multiple myeloma (RRMM). After four cycles, patients who progressed or had not achieved minimal response (serum and urine M-protein reduction of ≥ 25% and ≥ 50%) could receive dexamethasone 40 mg per week. Safety and efficacy were evaluated. Thirty-eight patients who had received both bortezomib and lenalidomide (median 6 prior therapies) were enrolled; 63% were refractory to both lenalidomide and bortezomib. There were four dose-limiting toxicities (grade 4 neutropenia) at 5 mg per day and so the MTD was 4 mg per day. Rates of peripheral neuropathy and venous thromboembolism were low (≤ 5%). Among the 38 patients enrolled (including 22 with added dexamethasone), 42% achieved minimal response or better, 21% achieved partial response or better, and 3% achieved complete response. Median duration of response, progression-free survival, and overall survival were 4.6, 4.6, and 18.3 months, respectively. Pomalidomide 4 mg per day on days 1 to 21 of each 28-day cycle, with or without dexamethasone (40 mg/week), has encouraging activity with manageable toxicity in RRMM, including those refractory to both lenalidomide and bortezomib. This study is registered at http://www.clinicaltrials.gov as #NCT00833833.
Introduction
The introduction of novel agents including thalidomide, bortezomib, and lenalidomide has significantly improved survival outcomes for patients with multiple myeloma (MM)1 ; however, almost all patients with MM eventually relapse and survival times shorten progressively with each subsequent relapse.2-4 The prognosis for patients who are refractory to novel agents is especially poor: patients who are refractory to bortezomib, lenalidomide, and thalidomide have a median overall survival (OS) of 9 months and an event-free survival of 5 months.3 Therefore, effective new treatments that reestablish tumor response are urgently required to improve outcomes for these patients.4,5
Pomalidomide is a new immunomodulatory agent with significant in vitro antiproliferative6-11 and proapoptotic effects.8,12 Recent studies have indicated limited cross-resistance between lenalidomide and pomalidomide.13 Several phase 1 and phase 2 studies evaluating continuous (2 mg/day) or alternate (5 mg/day) dose schedules of pomalidomide in patients with both relapsed and refractory (RR) MM have been reported.5,14-17 Pomalidomide plus low-dose dexamethasone has shown activity in patients with advanced MM who have relapsed after multiple lines of therapy, including those who are refractory to both lenalidomide and bortezomib.5,16
This open-label, phase 1, dose-escalation study was conducted to evaluate the maximum tolerated dose (MTD) of pomalidomide when given for 21 days of each 28-day cycle in patients with RRMM who had previously received multiple lines of treatment, including bortezomib and lenalidomide. The primary objective of the study was to determine the MTD, and the secondary objectives were to evaluate the safety and activity of pomalidomide, with or without dexamethasone, in this population.
Materials and methods
Patient population
Patients aged ≥18 years, with RRMM and an Eastern Cooperative Oncology Group performance status score of <2 were eligible. All patients had to have received prior treatment that included ≥2 cycles of lenalidomide and ≥2 cycles of bortezomib (in separate regimens or within the same combination regimen). Patients must have received ≥2 prior therapies, and have relapsed after having achieved at least stable disease (SD) for a minimum of one treatment cycle of a prior regimen before developing progressive disease (PD). Patients must have progressed on or within 60 days of the last treatment regimen used before enrollment (to define occurrence of refractory disease). Eligible patients had measurable levels of M-protein in the serum (≥0.5 g/dL) or urine (≥0.2 g/day) at study entry.
Patients were excluded if they had an absolute neutrophil count (ANC) of <1000/µL; platelet counts of <75 000/µL (in patients in whom <50% of bone marrow nucleated cells were plasma cells) or <30 000/µL (in patients in whom ≥50% of bone marrow nucleated cells were plasma cells); a serum creatinine level of ≥3.0 mg/dL; serum transaminase levels >3 times the upper limit of normal (ULN); and a serum total bilirubin level of >2.0 mg/dL. Patients were also excluded if they had grade ≥2 peripheral neuropathy (PN) or known hypersensitivity to lenalidomide, thalidomide, or dexamethasone.
The study was approved by the institutional review boards of the participating centers, overseen by a data safety monitoring committee, and conducted according to the Declaration of Helsinki International Conference on Harmonization and the Guidelines for Good Clinical Practice. Written informed consent was obtained from all patients before enrollment.
Study design and treatment
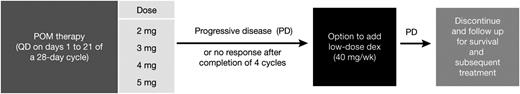
Patients received oral pomalidomide on days 1 to 21 of each 28-day cycle. After completion of the first treatment cycle, patients could choose to continue the study at their assigned pomalidomide dose. Patients who developed PD at any time during pomalidomide treatment or who did not achieve at least minimal response (MR) after completing four cycles—defined as serum and urine M-protein reduction of ≥25% and ≥50%, respectively, compared with baseline—had the option to add oral dexamethasone (40 mg/day on days 1, 8, 15, and 22 of each 28-day cycle) to their current dose level of pomalidomide and could continue treatment until progression. Patients with PD who chose not to add dexamethasone were discontinued from the study.
All patients received aspirin, 81 to 100 mg per day, or another form of thromboprophylaxis. Treatment with bisphosphonates, transfusions of platelets, or red blood cells was permitted at the investigator’s discretion. The use of hematopoietic growth factors was permitted after the completion of cycle 1.
Determination of MTD
The primary end point was to determine the MTD of pomalidomide. The first three patients were enrolled in the lowest dose level (2 mg/day) cohort. Following a standard “3+3” design, if one of the first three patients in the 2-mg cohort, or any subsequent dose-level cohort, experienced a dose-limiting toxicity (DLT) within the first cycle and then an additional three patients were to be enrolled into that dose-level cohort. If ≥2 patients within the expanded cohort experienced a DLT within the first cycle, then the MTD was considered to have been exceeded and no further dose escalations were needed. If ≤1 of the six patients in a dose-level cohort experienced a DLT within the first cycle, then the next cohort of three patients were enrolled at the next highest dose level. Patients who were not evaluable for toxicity were replaced. The MTD was defined as the highest dose level at which ≤1 of six patients experienced a DLT within the first 28-day cycle.
DLT was defined as grade 4 neutropenia (ANC <500/μL); febrile neutropenia (fever ≥38.5°C and ANC <1000/μL); grade 4 thrombocytopenia (platelet count <25 000/μL); or grade 3 or 4 pomalidomide-related nonhematologic toxicity. Grade 3 or 4 nausea, vomiting, or diarrhea that could not be controlled using symptomatic treatment was considered a DLT. Grade 4 transaminitis (serum transaminase >20 × ULN) or grade 3 transaminitis (serum transaminase >5 × ULN) present for ≥7 days was considered a DLT, as was any delay to the start of the second treatment cycle of >7 days as a result of pomalidomide-related adverse events (AEs). For grade 4 neutropenia occurring after cycle 1, pomalidomide therapy was interrupted and granulocyte colony-stimulating factor (G-CSF) was administered. Pomalidomide was resumed at the start of the next treatment cycle if neutropenia was the only DLT; otherwise, the pomalidomide dose was decreased by one dose level. Use of G-CSF during cycle 1 was not allowed, but was permitted thereafter.
Efficacy and safety assessments
Efficacy assessments were performed every 28 days after completion of the first cycle. Tumor responses, including identification of PD, were determined using modified European Group for Bone Marrow Transplantation criteria18,19 and International Myeloma Working Group uniform response criteria.20 Response assessments were based on serum and urine M-protein levels, bone marrow evaluation, and radiographic assessments of lytic bone lesions and/or extramedullary plasmacytoma. Complete blood counts were monitored weekly during cycle 1, biweekly during cycle 2, and then on the first day of each subsequent treatment cycle.
The overall response rate (ORR), time to response, duration of response, and OS were secondary end points. The efficacy analysis was based on both the intent-to-treat (ITT) population, defined as patients who received at least one dose of the study drug, and a subset of efficacy-evaluable population, defined as patients who met the eligibility criteria received at least one dose of the study drug and had a baseline and at least one post-baseline efficacy assessment. The ORR included any patient with at least a confirmed partial response (PR). Time to response was calculated as the time from treatment initiation to the first documented response of at least PR. The duration of response was defined as the time from the response to first evidence of PD. Progression-free survival was also evaluated as part of an exploratory analysis and was defined as the time from treatment initiation to disease progression or death. Overall survival was measured as the time from treatment initiation to death as a result of any cause.
All patients who received at least one dose of pomalidomide were included in the safety analysis. Adverse events were monitored throughout the study and were graded.21 Safety and DLTs were summarized at the completion of each dose level and reviewed by the data safety monitoring committee.
Statistical analysis
The standard “3+3” design for dose escalation anticipated enrollment of 6 to 60 patients. Treatment responses are presented as mean (± SD) or median values with 95% confidence intervals (CIs). Kaplan-Meier methodology was used to analyze duration of response, PFS, and OS.
Results
Patient characteristics
Thirty-eight patients were enrolled into four pomalidomide dose-level cohorts (Figure 1): 2 mg (n = 6); 3 mg (n = 8); 4 mg (n = 14); and 5 mg (n = 10). The baseline characteristics were similar across the dose cohorts (Table 1). Patients had received a median of six prior antimyeloma treatments (range, 2 to 17). Sixty-three percent of the patients were refractory to lenalidomide-bortezomib combination treatment.
The design of the phase 1 dose-escalation study in patients with RRMM. dex, dexamethasone; POM, pomalidomide; QD, one a day; wk, week.
The design of the phase 1 dose-escalation study in patients with RRMM. dex, dexamethasone; POM, pomalidomide; QD, one a day; wk, week.
Patient demographics by dose-level cohort
| . | Pomalidomide dose levels . | ||||
|---|---|---|---|---|---|
| . | 2 mg (n = 6) . | 3 mg (n = 8) . | 4 mg (n = 14) . | 5 mg (n = 10) . | Total (N = 38) . |
| Mean age, years (range) | 64.7 (55-72) | 70.4 (61-78) | 67.5 (45-80) | 61.3 (38-83) | 66.0 (38-83) |
| Age ≥75 y, % | 0 | 25 | 21 | 10 | 16 |
| Male, % | 17 | 38 | 71 | 40 | 47 |
| Caucasian, % | 83 | 100 | 100 | 80 | 92 |
| Durie-Salmon stage at enrollment, n (%) | |||||
| I | 1 (17) | 1 (13) | 3 (21) | 2 (20) | 7 (18) |
| II | 0 | 1 (13) | 4 (29) | 1 (10) | 6 (16) |
| III | 5 (83) | 6 (75) | 7 (50) | 7 (70) | 25 (66) |
| Median duration of active multiple myeloma, years (range) | 4.5 (2.8-14.0) | 5.9 (2.6-12.0) | 5.4 (2.0-23.4) | 5.9 (1.4-10.9) | 5.5 (1.4-23.4) |
| Median number of prior therapies, n (range) | 7.0 (5-11) | 6.5 (2-11) | 6.0 (3-17) | 5.5 (3-10) | 6 (2-17) |
| Prior lenalidomide and bortezomib, n (%) | 6 (100) | 8 (100) | 14 (100) | 10 (100) | 38 (100) |
| Refractory to lenalidomide | 4 (67) | 8 (100) | 12 (86) | 7 (70) | 31 (82) |
| Refractory to lenalidomide as last regimen | 1 (17) | 3 (38) | 5 (36) | 2 (20) | 11 (29%) |
| Refractory to bortezomib | 3 (50) | 7 (88) | 10 (71) | 8 (80) | 28 (74) |
| Refractory to bortezomib as last regimen | 2 (33) | 4 (50) | 7 (50) | 7 (70) | 20 (53) |
| Refractory to both | 3 (50) | 7 (88) | 8 (57) | 6 (60) | 24 (63) |
| Prior carfilzomib, n (%) | 3 (50) | 4 (50) | 3 (21) | 2 (20) | 12 (32) |
| Refractory to carfilzomib | 3 (50) | 3 (38) | 4 (29) | 2 (20) | 12 (32) |
| Prior dexamethasone, n (%) | 6 (100) | 8 (100) | 14 (100) | 10 (100) | 38 (100) |
| Prior thalidomide, n (%) | 4 (67) | 6 (75) | 11 (79) | 9 (90) | 30 (79) |
| Prior stem cell transplantation, n (%) | 4 (67) | 4 (50) | 11 (79) | 6 (60) | 25 (66) |
| . | Pomalidomide dose levels . | ||||
|---|---|---|---|---|---|
| . | 2 mg (n = 6) . | 3 mg (n = 8) . | 4 mg (n = 14) . | 5 mg (n = 10) . | Total (N = 38) . |
| Mean age, years (range) | 64.7 (55-72) | 70.4 (61-78) | 67.5 (45-80) | 61.3 (38-83) | 66.0 (38-83) |
| Age ≥75 y, % | 0 | 25 | 21 | 10 | 16 |
| Male, % | 17 | 38 | 71 | 40 | 47 |
| Caucasian, % | 83 | 100 | 100 | 80 | 92 |
| Durie-Salmon stage at enrollment, n (%) | |||||
| I | 1 (17) | 1 (13) | 3 (21) | 2 (20) | 7 (18) |
| II | 0 | 1 (13) | 4 (29) | 1 (10) | 6 (16) |
| III | 5 (83) | 6 (75) | 7 (50) | 7 (70) | 25 (66) |
| Median duration of active multiple myeloma, years (range) | 4.5 (2.8-14.0) | 5.9 (2.6-12.0) | 5.4 (2.0-23.4) | 5.9 (1.4-10.9) | 5.5 (1.4-23.4) |
| Median number of prior therapies, n (range) | 7.0 (5-11) | 6.5 (2-11) | 6.0 (3-17) | 5.5 (3-10) | 6 (2-17) |
| Prior lenalidomide and bortezomib, n (%) | 6 (100) | 8 (100) | 14 (100) | 10 (100) | 38 (100) |
| Refractory to lenalidomide | 4 (67) | 8 (100) | 12 (86) | 7 (70) | 31 (82) |
| Refractory to lenalidomide as last regimen | 1 (17) | 3 (38) | 5 (36) | 2 (20) | 11 (29%) |
| Refractory to bortezomib | 3 (50) | 7 (88) | 10 (71) | 8 (80) | 28 (74) |
| Refractory to bortezomib as last regimen | 2 (33) | 4 (50) | 7 (50) | 7 (70) | 20 (53) |
| Refractory to both | 3 (50) | 7 (88) | 8 (57) | 6 (60) | 24 (63) |
| Prior carfilzomib, n (%) | 3 (50) | 4 (50) | 3 (21) | 2 (20) | 12 (32) |
| Refractory to carfilzomib | 3 (50) | 3 (38) | 4 (29) | 2 (20) | 12 (32) |
| Prior dexamethasone, n (%) | 6 (100) | 8 (100) | 14 (100) | 10 (100) | 38 (100) |
| Prior thalidomide, n (%) | 4 (67) | 6 (75) | 11 (79) | 9 (90) | 30 (79) |
| Prior stem cell transplantation, n (%) | 4 (67) | 4 (50) | 11 (79) | 6 (60) | 25 (66) |
Determination of MTD and the recommended phase 2 dose
Patients received pomalidomide at dose levels of 2 mg (n = 6), 3 mg (n = 8), 4 mg (n = 14), or 5 mg (n = 10). No DLTs were observed in the first three patients enrolled at the 2-mg dose level. However, one patient discontinued because of thrombocytopenia, and therefore the investigators agreed to enroll three additional patients in this cohort. Only one of the six patients treated with 2 mg experienced a DLT (grade 3 fatigue). No DLTs were initially observed in the 3-mg dose level; however, one patient withdrew because of PD and was replaced by another patient. Of the following three patients enrolled at the 4-mg dose, one had a DLT (grade 4 neutropenia) and the other had renal failure. After four additional patients were enrolled at the 3-mg dose level because of the possibility of two DLTs at the 4-mg dose level, the renal failure experienced by one patient was confirmed to be unrelated to pomalidomide. One of these patients developed a DLT (grade 4 neutropenia). An additional three patients were enrolled at the 4-mg dose level; two of these were not evaluable and were replaced (one withdrew because of PD and one received an incorrect dose of pomalidomide). No additional DLTs were observed at 4 mg. Of the next three patients enrolled at the 5-mg dose level, two were not evaluable (1 received radiation therapy and 1 had the dose of pomalidomide reduced to 4 mg because of development of a rash) and were replaced. One DLT was observed (grade 4 neutropenia), so three additional patients were enrolled, one of whom experienced a DLT (grade 3 neutropenia). Although the MTD had been reached at 5 mg, two additional patients had already been enrolled at the 5-mg level, and both patients experienced DLTs (grade 4 neutropenia) before re-trying the 4-mg dose level. Therefore, six patients were enrolled at the 4-mg dose level. One of these patients experienced a DLT (grade 4 neutropenia) so the MTD was determined to be 4 mg.
The DLTs that occurred during the first cycle of treatment are summarized by dose level in Table 2. Overall, eight (21.1%) patients experienced ≥1 DLT. Except for one case of grade 3 fatigue in a patient in the pomalidomide 2-mg cohort, all of the DLTs were grade 4 neutropenia. DLTs were reported in 1, 1, 2, and 4 patients in the 2-mg, 3-mg, 4-mg, and 5-mg cohorts, respectively. All patients in the 5-mg cohort required dose reduction during study treatment. The MTD recommended for the phase 2 dose was, therefore, pomalidomide 4 mg per day for this dosing schedule (namely days 1 to 21 of each 28-day cycle).
Summary of dose-limiting toxicities reported at each pomalidomide dose level
| Daily dose of pomalidomide . | Median number of completed treatment cycles* (range) . | Number of dose-limiting toxicities (type) . |
|---|---|---|
| 2 mg (n = 6) | 1.5 (1-12) | 1 (grade 3 fatigue) |
| 3 mg (n = 8) | 5.0 (2-13) | 1 (grade 4 neutropenia) |
| 4 mg (n = 14) | 5.5 (1-30) | 2 (grade 4 neutropenia) |
| 5 mg (n = 10) | 8.0 (1-26) | 3 (grade 4 neutropenia) |
| 1 (grade 3 neutropenia) |
| Daily dose of pomalidomide . | Median number of completed treatment cycles* (range) . | Number of dose-limiting toxicities (type) . |
|---|---|---|
| 2 mg (n = 6) | 1.5 (1-12) | 1 (grade 3 fatigue) |
| 3 mg (n = 8) | 5.0 (2-13) | 1 (grade 4 neutropenia) |
| 4 mg (n = 14) | 5.5 (1-30) | 2 (grade 4 neutropenia) |
| 5 mg (n = 10) | 8.0 (1-26) | 3 (grade 4 neutropenia) |
| 1 (grade 3 neutropenia) |
Use of G-CSF was not allowed during the first 28 days (cycle 1) of the phase 1 study.
Drug exposure and safety
Patients received a median of 5 (range, 1 to 30) cycles of pomalidomide (Table 2). The median duration of treatment with pomalidomide, with or without dexamethasone, was 4.9 months (range, 0.5 to 29). In the 2-mg cohort, the median duration of pomalidomide treatment (1 month) was less than that of the other cohorts (4.5, 5, and 8.1 months in the 3-, 4-, and 5-mg cohorts, respectively). Reasons for early discontinuation in this dose cohort were grade 4 thrombocytopenia (0.5 months), withdrawal of consent (0.5 months), and PD (0.9 months; this patient was included in the efficacy analysis). In total, 13 patients (34.2%) completed ≥9.2 months (40 weeks) of pomalidomide treatment (2-mg cohort [n = 1, 16.7%]; 3-mg cohort [n = 2, 25%]; 4-mg cohort [n = 6, 42.9%]; 5-mg cohort [n = 4, 40%]). Dexamethasone was added in 22 of the 38 enrolled patients after a median of 2.8 months. At data cutoff for the phase 1 study (April 1, 2011), four patients were still receiving pomalidomide.
All 38 patients in the phase 1 study experienced ≥1 treatment-emergent AEs during treatment. The most commonly reported treatment-emergent AEs (grade 3 or 4) included neutropenia (20 patients, 53%); anemia (8 patients, 21%); thrombocytopenia (7 patients, 18%); and fatigue (6 patients, 16%) (Table 3). G-CSF was administered in 33%, 38%, 36%, and 70% of patients in the 2-, 3-, 4-, and 5-mg cohorts, respectively. Two patients developed grade 3 deep vein thrombosis (DVT) (1 in the 4-mg cohort was receiving dexamethasone and prophylaxis; the other patient was in the 5-mg cohort and was a protocol violation as the patient was not on prophylaxis); PN was reported in 1 patient as a newly emergent event and in four patients who had prior history of PN (only 1 patient had grade 3 PN, and the remainder had grade 1 or 2). There were no cases of febrile neutropenia. There was no relationship between frequency of AEs and pomalidomide dose, with the notable exception of neutropenia (Table 3). There were no unexpected increases in AE frequency after the addition of dexamethasone (data not shown). Overall, 19 patients (50%) had ≥1 serious AE (SAE). Five patients (13%) had ≥1 SAE attributed to pomalidomide, which included pharyngeal abscess, sepsis, neutropenia, cellulitis, asthenia, musculoskeletal chest pain, atrioventricular block, syncope, and DVT. Dexamethasone-related SAEs occurred in three of the 22 patients (14%) and included infectious arthritis, sepsis, or pharyngeal abscess in two patients and musculoskeletal pain in one patient.
Treatment-emergent AEs grade 3 or 4 occurring in ≥5% of patients across the pomalidomide dosing cohorts
| . | Grade 3 and 4 . | All Grades . | ||||
|---|---|---|---|---|---|---|
| AEs, n (%) . | POM 2 mg (n = 6) . | POM 3 mg (n = 8) . | POM 4 mg (n = 14) . | POM 5 mg (n = 10) . | POM Total (N = 38) . | POM Total (N = 38) . |
| Neutropenia | 1 (17) | 4 (50) | 7 (50) | 8 (80) | 20 (53) | 23 (61) |
| Anemia | 4 (67) | 2 (25) | 2 (14) | 0 | 8 (21) | 17 (45) |
| Thrombocytopenia | 2 (33) | 2 (25) | 1 (7) | 2 (20) | 7 (18) | 10 (26) |
| Sepsis | 1 (17) | 2 (25) | 0 | 1 (10) | 4 (11) | 4 (11) |
| Pneumonia | 1 (17) | 0 | 2 (14) | 0 | 3 (8) | 5 (13) |
| Fatigue | 2 (33) | 1 (13) | 2 (14) | 1 (10) | 6 (16) | 27 (66) |
| Back pain | 1 (17) | 0 | 0 | 1 (10) | 2 (5) | 8 (21) |
| Muscle weakness | 0 | 0 | 2 (14) | 0 | 2 (5) | 2 (5) |
| Renal failure | 1 (17) | 0 | 1 (7) | 0 | 2 (5) | 2 (5) |
| DVT | 0 | 0 | 1 (7) | 1 (10) | 2 (5) | 2 (5) |
| . | Grade 3 and 4 . | All Grades . | ||||
|---|---|---|---|---|---|---|
| AEs, n (%) . | POM 2 mg (n = 6) . | POM 3 mg (n = 8) . | POM 4 mg (n = 14) . | POM 5 mg (n = 10) . | POM Total (N = 38) . | POM Total (N = 38) . |
| Neutropenia | 1 (17) | 4 (50) | 7 (50) | 8 (80) | 20 (53) | 23 (61) |
| Anemia | 4 (67) | 2 (25) | 2 (14) | 0 | 8 (21) | 17 (45) |
| Thrombocytopenia | 2 (33) | 2 (25) | 1 (7) | 2 (20) | 7 (18) | 10 (26) |
| Sepsis | 1 (17) | 2 (25) | 0 | 1 (10) | 4 (11) | 4 (11) |
| Pneumonia | 1 (17) | 0 | 2 (14) | 0 | 3 (8) | 5 (13) |
| Fatigue | 2 (33) | 1 (13) | 2 (14) | 1 (10) | 6 (16) | 27 (66) |
| Back pain | 1 (17) | 0 | 0 | 1 (10) | 2 (5) | 8 (21) |
| Muscle weakness | 0 | 0 | 2 (14) | 0 | 2 (5) | 2 (5) |
| Renal failure | 1 (17) | 0 | 1 (7) | 0 | 2 (5) | 2 (5) |
| DVT | 0 | 0 | 1 (7) | 1 (10) | 2 (5) | 2 (5) |
In total, 14 patients (37%) had ≥1 dose reduction during treatment. The percentage of patients requiring dose reductions was proportionally related to the dosage of pomalidomide and was 100% in the 5-mg cohort (Table 4). As a result, the median average daily dose of pomalidomide was 2.0, 3.0, 4.0, and 4.4 mg per day in the 2-mg, 3-mg, 4-mg, and 5-mg dose cohorts, respectively. The median time to first dose reduction was 1.3 months (range, 0.3 to 13.8) overall and was not evaluable, and was 1.3, 1.0, and 2.7 months in the 2-mg, 3-mg, 4-mg, and 5-mg dose cohorts, respectively. The main reasons for treatment discontinuation were: disease progression (37%), withdrawal of consent (16%), and AEs (11%); one patient discontinued treatment because of an AE that was suspected to be related to pomalidomide (grade 2 rash).
Summary of patient disposition and dose reductions/discontinuations related to pomalidomide treatment
| . | Pomalidomide dose levels . | ||||
|---|---|---|---|---|---|
| n (%) . | 2 mg (n = 6) . | 3 mg (n = 8) . | 4 mg (n = 14) . | 5 mg (n = 10) . | Total (N = 38) . |
| Patients active in the study* | 0 | 0 | 2 (14) | 2 (20) | 4 (11) |
| Patients withdrawn from the study | 6 (100) | 8 (100) | 12 (86) | 8 (80) | 34 (89) |
| Dose reductions and discontinuations | |||||
| POM dose reduction | 0 | 1 (13) | 3 (21) | 10 (100) | 14 (37) |
| Discontinuation | 6 (100) | 8 (100) | 12 (86) | 8 (80) | 34 (89) |
| Disease progression | 2 (33) | 3 (38) | 5 (36) | 4 (40) | 14 (37) |
| AEs§ | 1 (17) | 0 | 2 (14) | 1 (10) | 4 (11) |
| Withdrawn consent | 1 (17) | 1 (13) | 2 (14) | 2 (20) | 6 (16) |
| Death | 0 | 1 (13) | 2 (14) | 0 | 3 (8) |
| . | Pomalidomide dose levels . | ||||
|---|---|---|---|---|---|
| n (%) . | 2 mg (n = 6) . | 3 mg (n = 8) . | 4 mg (n = 14) . | 5 mg (n = 10) . | Total (N = 38) . |
| Patients active in the study* | 0 | 0 | 2 (14) | 2 (20) | 4 (11) |
| Patients withdrawn from the study | 6 (100) | 8 (100) | 12 (86) | 8 (80) | 34 (89) |
| Dose reductions and discontinuations | |||||
| POM dose reduction | 0 | 1 (13) | 3 (21) | 10 (100) | 14 (37) |
| Discontinuation | 6 (100) | 8 (100) | 12 (86) | 8 (80) | 34 (89) |
| Disease progression | 2 (33) | 3 (38) | 5 (36) | 4 (40) | 14 (37) |
| AEs§ | 1 (17) | 0 | 2 (14) | 1 (10) | 4 (11) |
| Withdrawn consent | 1 (17) | 1 (13) | 2 (14) | 2 (20) | 6 (16) |
| Death | 0 | 1 (13) | 2 (14) | 0 | 3 (8) |
As of April 1, 2011.
Includes thrombocytopenia, anemia, gastrointestinal hemorrhage, vomiting, chills, fatigue, pyrexia, metastases to meninges, renal failure, and rash.
Efficacy
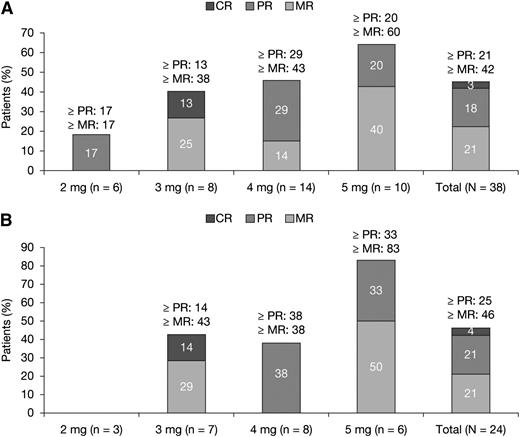
In total, 38 patients were included in the efficacy analysis based on the (ITT) population (Figure 2A).The ORR was 21%, with a confirmed complete response (CR) in one patient (3%) and PR in seven patients (18%). MR or better was observed in 42% of the patients, with a trend toward higher response rates with higher pomalidomide dosage (Figure 2A). The median time to response was four months (range, 2 to 26). The best response (≥PR) to single-agent pomalidomide before the addition of dexamethasone was 13% and an additional eight patients (21%) achieved MR when dexamethasone was added. Overall, 24 patients (63%) were refractory to both lenalidomide and bortezomib, and a confirmed response (at least PR) was seen in six patients (25%; 4% CR) (Figure 2B). In the ITT population, the Kaplan-Meier estimate for the median duration of response was 4.6 months (95% CI, 3.7- 13.8). The Kaplan-Meier survival estimates for median PFS and median OS were 4.6 months (95% CI, 2.8-8.3) and 18.3 months (95% CI, 12.3-25.1), respectively.
Treatment responses to pomalidomide in patients with RRMM. (A) ITT population (N = 38). (B) Dual lenalidomide and bortezomib–refractory patients (N = 24).
Treatment responses to pomalidomide in patients with RRMM. (A) ITT population (N = 38). (B) Dual lenalidomide and bortezomib–refractory patients (N = 24).
Efficacy outcomes were similar when the data were analyzed using the efficacy-evaluable population (28 patients). In total, 10 of the 38 patients were not evaluable for efficacy because they did not fulfill the eligibility criteria (n = 5; because of laboratory abnormalities) or had no post-baseline efficacy assessment (n = 5; 3 patients discontinued because of AEs and 2 withdrew consent). In this patient population, CR was reported in 4% of the patients and PR was achieved in 25% of the patients (ORR, 29%). Fifty percent of the patients achieved MR or better. Overall, 22 patients evaluable for response were refractory to both lenalidomide and bortezomib, and a confirmed response (at least PR) was seen in six patients (27%; 5% CR). In the population eligible for response assessment, the Kaplan-Meier estimate for the median duration of response was 4.6 months (95% CI, 3.7-13.8). The Kaplan-Meier survival estimates for median PFS and median OS were 4.6 months (95% CI, 2.8-8.3) and 18.5 months (95% CI, 12.3-25.1), respectively.
Discussion
This phase 1 study used a classic “3+3” design to determine the MTD of oral pomalidomide, which was found to be 4 mg per day when administered as a single agent on days 1 to 21 of a 28-day treatment cycle. All patients treated had RRMM with advanced disease and had received a median of six prior lines of treatment including lenalidomide, bortezomib, thalidomide, and carfilzomib. The most common grade 3 or 4 treatment-emergent AEs included neutropenia, anemia, thrombocytopenia, and fatigue. The rate of grade 3 or 4 neutropenia in this study was 53%, consistent with a previous study in RRMM, in which pomalidomide 4 mg per day was used (albeit using a different dose schedule),5 and was equivalent to rates reported with lenalidomide monotherapy in a similar population.22,23 The incidence of grade 3 or 4 thromboembolic events in the current study was low (5%) and also comparable with previous studies with single-agent lenalidomide.23 The low incidence of treatment-emergent PN is noteworthy because the patients in this study had received multiple lines of prior therapy, including bortezomib and thalidomide, both of which are commonly associated with neurotoxicity.24-26 Discontinuations because of AEs were uncommon, allowing patients to receive therapy consistently and continuously until disease progression.
In our study, pomalidomide, with or without dexamethasone (40 mg per week) was associated with an ORR of 21% (29% for the efficacy-evaluable population, including 3% CR, 4% for the efficacy-evaluable population) and a ≥MR rate of 42% (50% for the efficacy-evaluable population) in patients with RRMM who had received multiple lines of prior therapy. This promising clinical activity is consistent with previous phase 2 studies of pomalidomide.2,5,16,27 Specifically, pomalidomide plus low-dose dexamethasone has demonstrated overall response rates (CR + very good PR [VGPR] + PR) of 63% in patients with relapsed MM (<3 prior regimens),2 32% in patients refractory to lenalidomide (median of 4 lines of treatment),16 and up to 29% in heavily pretreated patients who had received prior thalidomide and were refractory to both lenalidomide and bortezomib (median of 6 lines of treatment).5 Interestingly, in contrast to the latter study,5 our data suggest a dose-dependent response to pomalidomide, with higher response rates in patients who had received higher doses of pomalidomide. Importantly, in dual-refractory patients in the study of Lacy et al,5 duration of response appeared to be dose dependent, supporting our observation. However, the patient cohorts in both of these studies were small, and therefore evaluation of the dose response in larger numbers of patients is warranted.
Responses to pomalidomide, generally in combination with dexamethasone (40 mg per week), were durable (median response duration 4.6 months in both the ITT and the efficacy-evaluable population). The median PFS was also 4.6 months for both populations, and the median OS was 18.3 months in the ITT population and 18.5 months in the efficacy-evaluable population. The survival outcomes observed in the current study are encouraging and compare favorably with data with another second generation novel agent, carfilzomib, tested in a similar population of RRMM patients.27 However, although very encouraging, these results with pomalidomide (with or without dexamethasone) are still preliminary and require further exploration in larger phase 2 trials.
Outcomes with the dosing schedule studied here are also comparable with other previously reported outcomes for pomalidomide, with or without dexamethasone, where median PFS ranged from 3.2 to 11.6 months and median OS ranged from 13.9 to 20.7 months.2,5,14,16 Interestingly, the IFM 2009-02 phase 2 trial compared two schedules of pomalidomide plus low-dose dexamethasone in patients refractory to lenalidomide and bortezomib, either 4 mg per day on days 1 to 21 of each 28-day cycle (21/28) or 4 mg per day each day of the 28-day cycle (28/28). PR or better was reported in 42% and 39% of patients, for the 21of 28-day and 28 of 28-day dosing regimens, respectively, with a longer duration of response and less toxicity for the 21 of 28-day schedule, but comparable 6-month survival rates of 88% and 85%, respectively.17 A similar, encouraging 2-year survival rate of 76% has recently been reported in patients with RRMM treated with pomalidomide and low-dose dexamethasone.28 The safety profiles of pomalidomide and low-dose dexamethasone combination therapy in three other phase 1 and 2 clinical trials were also comparable with the current study.5,15,16
The results of this phase 1 study show that pomalidomide, given at 4 mg per day on days 1 to 21 of each 28-day cycle in combination with dexamethasone 40 mg per week, is associated with encouraging response rates and manageable safety in patients with RRMM previously treated with lenalidomide and bortezomib. Pomalidomide, administered at a dose of 4 mg per day on days 1 to 21 of each 28-day cycle is being evaluated in ongoing phase 2 and 3 trials. Larger studies are required to further confirm the role of pomalidomide in patients with RRMM, both in combination with dexamethasone and with other novel agents, such as bortezomib.29,30
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the following institutions, which collaborated and supported this study: Dana-Farber Cancer Institute, John Theuer Cancer Center–Hackensack University Medical Center, H. Lee Moffitt Cancer and Research Institute, Massachusetts General Hospital, Mayo Clinic Arizona, Mayo Clinic Minnesota, Roswell Park Cancer Institute, The Ohio State University–James Cancer Hospital, University of Michigan Comprehensive Cancer Center, Washington University–Siteman Cancer Center, St Vincent’s Comprehensive Cancer Center, University of Pittsburgh Cancer Institute, Emory University, Princess Margaret Hospital–UHN, Cross Cancer Center, University of Calgary–Tom Baker Cancer Center, Vancouver General Hospital, Diamond Health Care Centre, Royal Victoria Hospital McGill University, Multiple Myeloma Research Consortium, Clinical Research Staff, Celgene Corporation. We also thank our patients and their families.
The authors received editorial support from Adriana Stan, PhD (Excerpta Medica) in the preparation of this manuscript, funded by Celgene Corporation.
Authorship
Contribution: P.G.R., M.H.Z., C.J., K.C.A., S.L.K., S.W., D. Siegel, and R.B. conceived and designed the study; P.G.R., D. Siegel, R.B., N.C.M., J.L., D. Sullivan, M.A., R.S., I.M.G., D.D., N.L., L.M., E.B., P.A., L.N., and G.L. collected and interpreted the data; P.G.R., M.C., M.H.Z., and C.J. performed the statistical analysis; P.G.R., R.B., N.L., M.H.Z., C.J., and K.C.A. wrote the manuscript; P.G.R., D.Siegel., R.B., S.L.K., N.C.M., J.L., D. Sullivan, M.A., R.S., I.M.G., D.D., N.L., L.M., E.B., P.A., L.N., S.W., G.L., M.C., M.H.Z., C.J., and K.C.A. gave final approval of the manuscript.
Conflict-of-interest disclosure: G.L., M.C., M.H.Z., and C.J. are employed by or hold leadership positions with Celgene. D. Siegel, D.D., and E.B. received honoraria from Celgene. K.C.A. owns stock in Acetylon and Oncopep. The following authors hold consultant or advisory roles with the indicated companies: P.G.R.: Millennium, Celgene, Bristol-Myers Squibb, Johnson & Johnson, Novartis. D. Siegel, R.B., E.B.: Celgene. N.C.M.: Celgene, Millennium. M.A.: Millennium. I.M.G.: Genzyme, Novartis, Millennium. K.C.A.: Celgene, Millennium, Onyx, Bristol-Myers Squibb, Merck. The following authors received research funding from the indicated companies: D. Siegel: Celgene. R.B.: Celgene, Millennium, Novartis, Ortho Biotech, Bristol-Myers Squibb. M.A.: Millennium, Ortho Biotech, Celgene. The remaining authors declare no competing financial interests.
Corresponding author: Paul G. Richardson, MD, Division of Hematologic Malignancies, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: Paul_Richardson@dfci.harvard.edu.