Key Points
TMEM16F is not essential for apoptosis-induced phosphatidylserine exposure in platelets.
Collagen plus thrombin-induced phosphatidylserine exposure in platelets results from 2 distinct pathways, one being TMEM16F dependent.
Abstract
Scott syndrome, a bleeding disorder caused by defective phospholipid scrambling, has been associated with mutations in the TMEM16F gene. The role of TMEM16F in apoptosis- or agonist-induced phosphatidylserine (PS) exposure was studied in platelets from a Scott syndrome patient and control subjects. Whereas stimulation of control platelets with the BH3-mimetic ABT737 resulted in 2 distinct fractions with moderate and high PS exposure, the high PS-exposing fraction was markedly delayed in Scott platelets. High, but not moderate, PS exposure in platelets was suppressed by chelation of intracellular Ca2+, whereas caspase inhibition completely abolished ABT737-induced PS exposure in both Scott and control platelets. On the other hand, high PS exposure induced by the Ca2+-mobilizing agonists convulxin/thrombin fully relied on mitochondrial depolarization and was virtually absent in Scott platelets. Finally, PS exposure induced by collagen/thrombin was partly affected in Scott platelets, and the residual PS positive fraction was insensitive to inhibition of caspases or mitochondrial depolarization. In conclusion, TMEM16F is not required for, but enhances, caspase-dependent PS exposure; convulxin-/thrombin-induced PS exposure is entirely dependent on TMEM16F, whereas collagen/thrombin-induced PS exposure results from 2 distinct pathways, one of which involves mitochondrial depolarization and is mediated by TMEM16F.
Introduction
Transbilayer lipid asymmetry in platelets and other cells is maintained by an aminophospholipid translocase, an active transporter pumping aminophospholipids phosphatidylserine (PS) and phosphatidylethanolamine from the outer to the inner leaflet of the plasma membrane. Under particular conditions, phospholipid asymmetry is disrupted by a “scrambling” process, in which the phospholipids become increasingly randomized over both plasma membrane leaflets.1 As a consequence, PS, which in resting cells is exclusively located in the inner leaflet, becomes exposed at the membrane outer leaflet and can be probed with the PS-binding proteins annexin A5 or lactadherin. For apoptotic cells, externalized PS provides a signal for removal by the mononuclear phagocytic system.2 In blood cells, PS-exposing membranes strongly promote the process of thrombin generation and fibrin clot formation.3,4 In platelets, phospholipid scrambling and PS externalization are induced by activation with strong agonists like convulxin/thrombin and collagen/thrombin, stimulating glycoprotein VI, and the platelet thrombin receptors.5,6 Known requirements for agonist-induced phospholipid scrambling are a sustained high intracellular Ca2+ concentration1,3 and loss of the mitochondrial membrane potential.7,8 Hence, platelets lacking cyclophilin D, a positive modulator of the mitochondrial permeability transition pore (MPTP), are protected against PS externalization upon convulxin/thrombin stimulation.9
Scott syndrome is a rare bleeding disorder in which the Ca2+-dependent phospholipid scrambling of platelets and other blood cells is impaired.10 Although agonist-induced Ca2+ responses were found to be unchanged, Scott syndrome platelets show diminished PS exposure upon stimulation with convulxin/thrombin, collagen/thrombin, or Ca2+-ionophore ionomycin compared with healthy subjects.11 Recently, TMEM16F was recognized as a protein critically involved in Ca2+-induced phospholipid scrambling. A homozygous mutation in TMEM16F (IVS12–1G→T, causing premature termination of translation) was found in the genome of the propositus Scott patient.12 Another Scott syndrome patient appeared to be compound heterozygous in TMEM16F,13 suggesting that a defective TMEM16F is the cause of the Scott syndrome phenotype.
Besides the agonist-induced pathway, platelets possess a distinct pathway to PS exposure, which was shown to be elicited with ABT737 or ABT263 (Navitoclax), both of which are BH-3 mimetics that activate caspase-dependent apoptosis in a Bax/Bak-dependent manner.14,15 This apoptotic pathway may play a role in the clearance of aging platelets.16 As Bcl-xL is gradually degraded in such platelets, its restraint of the proapoptotic function of Bax and Bak will gradually diminish in time and hence prime platelets for apoptosis.17,18 This explains why BH-3 mimetics like ABT737 and ABT263 induce thrombocytopenia and affect the platelet hemostatic function.19,20 Whether TMEM16F is involved in PS exposure upon apoptosis has not been investigated.
In this study, we aimed to elucidate the roles of TMEM16F in agonist- and apoptosis-induced PS exposure using platelets from a Scott patient. We provide evidence for TMEM16F-independent phospholipid scrambling in the apoptotic pathway that leads to moderate PS exposure in a caspase-dependent way. The data furthermore point to a TMEM16F-independent process of caspase/mitochondrial-independent PS exposure in response to collagen/thrombin.
Methods
Materials
ABT737 and Ac-DEVD-AFC were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), ABT-263 from Selleckchem (Houston, TX), and annexin A5-labeled with fluorescein isothiocyanate (FITC) from PharmaTarget (Maastricht, The Netherlands). Acetylsalicylic acid (aspirin) was from Genfarma (Maarssen, The Netherlands). Fibrillar type I collagen (Horm) was from Nycomed (Munich, Germany). Fluo-4 acetoxymethyl ester, Alexa Fluor-647 (AF647)-labeled annexin A5, dimethyl BAPTA acetoxymethyl ester, ionomycin, and pluronic F-127 were from Invitrogen (Carlsbad, CA). Apyrase (grade V), dimethylsulfoxide, bovine serum albumin, human fibrinogen, MRS2179, 2-APB, and thrombin were from Sigma-Aldrich (St. Louis, MO). FITC-lactadherin was from Haematologic Technologies (Essex Junction, VT). Tetramethyl rhodamine methyl ester (TMRE) was from Anaspec (San Jose, CA). Q-VD-Oph and cyclosporin A were from Calbiochem (San Diego, CA). A fluorometric assay for caspase 3 activity (BF1100) came from R&D Systems (Minneapolis, MN). Convulxin was purified as described before.21 Cangrelor was kindly provided by AstraZeneca (Mölndahl, Sweden).
Blood collection and platelet preparation
Human blood was obtained from healthy volunteers and a patient from the UK with Scott syndrome after they provided informed consent in accordance with the Declaration of Helsinki under protocols reviewed by the local ethics committees. The UK Scott patient was described earlier as compound heterozygous in TMEM16F with one mutation, IVS6 + 1G→A, resulting in exon 6 skipping and another mutation (c.1219insT) leading to a premature stop of translation, causing defective TMEM16F expression.13 Blood was collected into acid citrate dextrose to prepare washed platelets in N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES) buffer, pH 7.45 (136 mM NaCl, 10 mM HEPES, 2.7 mM KCl, 2 mM MgCl2, 0.1% glucose, and 0.1% bovine serum albumin) as described.22 Platelet counts were determined using a Coulter counter (Beckman).
Measurement of PS exposure
Washed platelets (1 × 108/mL) were preincubated with inhibitor or vehicle control (dimethylsulfoxide) and activated with indicated agents under nonstirring conditions at 37°C. For aging experiments, platelets were stored at room temperature in sterile HEPES buffer, pH 7.45, supplemented with 0.2% penicillin/streptomycin. For experiments performed in the absence of extracellular Ca2+, HEPES buffer was supplied with 1 mM EGTA. Where indicated, intracellular Ca2+ was chelated by platelet treatment with 20 μM dimethyl BAPTA acetoxymethyl ester.
Phospholipid scrambling was measured as PS exposure by labeling in the presence of 2 mM CaCl2 with fluorescent annexin A5 (0.25 μg/mL) or fluorescent lactadherin (16 nM). Labeling was done by transferring 2 μL platelet suspension into 60 μL measurement buffer with 2 mM CaCl2. After 2 min of labeling, samples were analyzed with an Accuri C6 flow cytometer and Flow C6 software. Threshold forward scatter and side scatter gates were set to exclude microparticles. The range M3 was defined as the annexin A5 fluorescence comprising 95% of maximally activated platelets using ionomycin. The range M1 was set to include 95% of all unstimulated platelets, and M2 was the range between M1 and M3.
Calcium measurements
For measurements of [Ca2+]i by flow cytometry, washed platelets (3 × 108/mL) were loaded for 45 min with 8 μM Fluo-4 AM in the presence of 0.4 mg/mL pluronic F-127. Following a wash step, the platelets were resuspended in HEPES buffer, pH 7.45, containing 2 mM CaCl2 and activated by convulxin/thrombin (70 ng/mL, 4 nM) at 2 × 106 platelets/mL.
Detection of mitochondrial depolarization
Platelets were loaded with TMRE (50 nM) for 30 min prior to stimulation for assessment of mitochondrial membrane depolarization by flow cytometry. In combination with confocal microscopy, platelets were allowed to adhere to fibrinogen-coated coverslips before stimulation with indicated agonist. Slides were precoated with 500 μg/mL fibrinogen for 10 min. After stimulation with the indicated agonists, confocal images were taken with a Live-7 Zeiss line-scanning microscope system.
Caspase activity assay
Washed platelets (1 × 108/mL) were preincubated and activated as indicated. At fixed time points, 100-μL samples were centrifuged (2 min at 2300 g), and platelet pellets were resolved into 100 μL lysis buffer (caspase-3 fluorometric assay, R&D Systems). Samples in lysis buffer were mixed 1:1 with 50 µL caspase substrate mix containing 9.8 µM dithiothreitol and 49 µM Ac-DEVD-AFC. Fluorescence was measured in time using a Spectramax M2. Caspase-3 activity was assessed as fluorescence units per minute.
Statistical analysis
Data are presented as means ± SEM. As appropriate, the significance of differences was determined by analysis of variance analysis with Bonferroni posttesting, paired t test, or the independent samples t test, using GraphPad Prism software.
Results
BH3-mimetics induce delayed PS exposure in Scott syndrome platelets
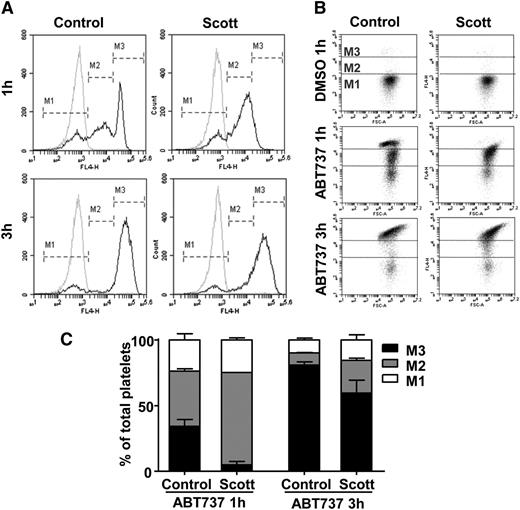
To investigate the role of TMEM16F in apoptosis-induced PS exposure, platelets from a patient with Scott syndrome and from healthy control subjects were treated with the BH3-mimetic ABT737. As shown in Figure 1A, a 1-h treatment with 10 μM ABT737 caused ∼70% of the platelets to expose PS for both control and Scott platelets. However, whereas for control platelets the histogram of the annexin A5-positive fraction gave a bimodal distribution with moderate (marker region M2) and high (marker region M3) PS exposure, the Scott platelets exhibited a fraction with mainly moderate PS exposure. Similar results were found at lower concentrations of ABT737, ranging from 3.3 to 0.33 μM (supplemental Figure 1A). Note that the extent of annexin A5 binding in the M3 fraction of the ABT737-treated control platelets was comparable with that of platelets stimulated with ionomycin or convulxin/thrombin (see below). A similar bimodal labeling pattern was found with the PS-selective probe, FITC-lactadherin (data not shown). Prolonged incubation with ABT737 up to 3 h increased the fraction with the highest (M3) annexin A5 binding at the expense of the fraction with moderate (M2) annexin A5 binding for both Scott and control platelets (Figure 1A-C). Similar results were obtained with the related compound ABT263 (data not shown).
Proapoptotic agent ABT737 causes PS exposure in platelets from a patient with Scott syndrome. Washed platelets from healthy control subjects or a patient with Scott syndrome were treated with ABT737 (10 μM) in the presence of 1 mM CaCl2. (A) Representative histograms (black lines) for AF647-annexin A5 binding after 1- or 3-h stimulation with ABT737. Markers M1, M2, and M3 indicate fractions of platelets with no, moderate, or high annexin A5 binding, respectively. Gray curves show unstimulated platelets treated with vehicle. (B) Corresponding dot plots (forward scatter vs AF647 fluorescence) of platelets treated with ABT737 or vehicle. (C) Distribution of M1, M2, and M3 platelet populations upon ABT737 treatment (10 μM).
Proapoptotic agent ABT737 causes PS exposure in platelets from a patient with Scott syndrome. Washed platelets from healthy control subjects or a patient with Scott syndrome were treated with ABT737 (10 μM) in the presence of 1 mM CaCl2. (A) Representative histograms (black lines) for AF647-annexin A5 binding after 1- or 3-h stimulation with ABT737. Markers M1, M2, and M3 indicate fractions of platelets with no, moderate, or high annexin A5 binding, respectively. Gray curves show unstimulated platelets treated with vehicle. (B) Corresponding dot plots (forward scatter vs AF647 fluorescence) of platelets treated with ABT737 or vehicle. (C) Distribution of M1, M2, and M3 platelet populations upon ABT737 treatment (10 μM).
The ABT737-induced PS exposure in platelets was previously shown to be dependent on caspase proteases.14 This was confirmed by the measurement of caspase activity in platelets. Comparable caspase activity was detected in Scott syndrome platelets and platelets from controls (supplemental Figure 2A), ruling out the possibility that a difference in caspase activity attributed to the observed effects. Furthermore, the pan-caspase inhibitor Q-VD-Oph completely abolished ABT737-induced PS exposure in both Scott syndrome and control platelets (supplemental Figure 2B). These data suggest that the proapoptotic agent ABT737 activates 2 mechanisms leading to PS exposure, one of which causes a gradual increase in PS exposure that is independent of TMEM16F and one resulting in high PS exposure that requires the presence of a functional TMEM16F.
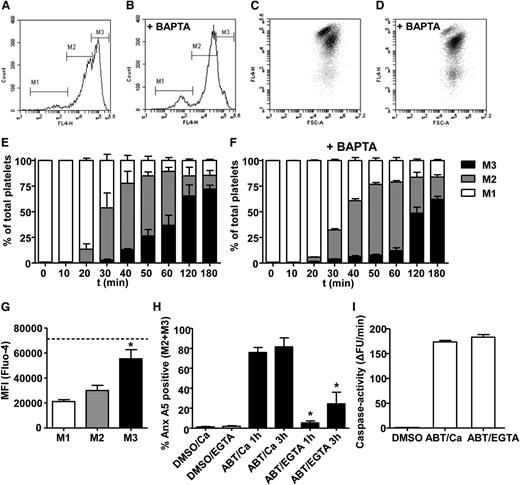
Because TMEM16F is considered to be essential for Ca2+-dependent phospholipid scrambling, we questioned to what extent the scrambling process observed after ABT737 treatment is regulated by intracellular Ca2+ levels. Indeed, chelation of intracellular Ca2+ levels in platelets loaded with dm-BAPTA resulted in strong suppression of the high annexin A5-binding population, with little effect on the moderate PS exposing fraction (Figure 2A-F). Furthermore, Fluo-4 measurements revealed that the high annexin A5-binding fraction had increased Ca2+ levels compared with the moderate PS-exposing fractions (Figure 2G). However, chelation of extracellular Ca2+ by 1 mM EGTA resulted in a significant suppression of ABT737-induced PS exposure in both the moderate and high annexin A5-binding populations (Figure 2H). The defective PS exposure in the presence of EGTA was not due to a reduction in caspase activity (Figure 2I). Further experiments with aspirin (100 μM), P2Y1 blocker MRS-2179 (50 μM), and P2Y12 blocker cangrelor (10 μM) indicated that the fractions of moderate and high annexin A5-binding platelets changed with <2% by blocking of the secondary mediator release.
High PS exposure induced by ABT737 depends on elevated intracellular Ca2+. (A-F) Washed platelets were stimulated by 10 μM ABT737 in the absence of CaCl2 or after loading with dimethyl-BAPTA (+ BAPTA) in the presence of 2 mM CaCl2. Representative flow cytometry histograms (A-B) and dot plots (C-D) of AF647-annexin (Anx) A5 binding after 1-h treatment with ABT737. Markers M1, M2, and M3 indicate fractions of platelets with no, moderate, or high Anx A5 binding, respectively. (E-F) Distribution of platelet populations over M1, M2, and M3 during treatment in time. (G) Platelets were loaded with the intracellular Ca2+ probe Fluo-4 and stimulated with ABT737 (10 μM, 1 h) in the presence of 1 mM CaCl2. Fluo-4 fluorescence was determined for the M1, M2, and M3 fractions. Dotted line represents maximal Fluo-4 fluorescence obtained with 10 μM ionomycin, as positive control. (H) Anx A5 binding of platelets stimulated by ABT737 in the presence of 1 mM CaCl2 or 1 mM EGTA after 1 to 3 h. (I) Caspase activity (arbitrary fluorescence units/min) of ABT737-stimulated platelets in the presence of 1 mM CaCl2 or 1 mM EGTA. Mean ± SEM (n = 3-6); *P < .05.
High PS exposure induced by ABT737 depends on elevated intracellular Ca2+. (A-F) Washed platelets were stimulated by 10 μM ABT737 in the absence of CaCl2 or after loading with dimethyl-BAPTA (+ BAPTA) in the presence of 2 mM CaCl2. Representative flow cytometry histograms (A-B) and dot plots (C-D) of AF647-annexin (Anx) A5 binding after 1-h treatment with ABT737. Markers M1, M2, and M3 indicate fractions of platelets with no, moderate, or high Anx A5 binding, respectively. (E-F) Distribution of platelet populations over M1, M2, and M3 during treatment in time. (G) Platelets were loaded with the intracellular Ca2+ probe Fluo-4 and stimulated with ABT737 (10 μM, 1 h) in the presence of 1 mM CaCl2. Fluo-4 fluorescence was determined for the M1, M2, and M3 fractions. Dotted line represents maximal Fluo-4 fluorescence obtained with 10 μM ionomycin, as positive control. (H) Anx A5 binding of platelets stimulated by ABT737 in the presence of 1 mM CaCl2 or 1 mM EGTA after 1 to 3 h. (I) Caspase activity (arbitrary fluorescence units/min) of ABT737-stimulated platelets in the presence of 1 mM CaCl2 or 1 mM EGTA. Mean ± SEM (n = 3-6); *P < .05.
Normal PS exposure in Scott syndrome platelets during prolonged storage
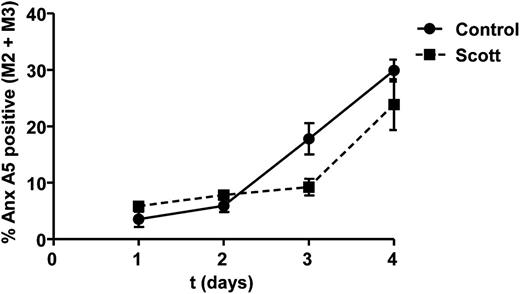
A gradual increase in surface-exposed PS can be observed in platelets that are stored for a prolonged time (C.S., unpublished data, 2012). To determine whether Scott syndrome platelets possess a similar mechanism to expose PS under aging conditions, Scott and control platelets were stored up to 4 d at room temperature. As shown in Figure 3, the patient’s platelets were equally able to expose PS upon storage as controls, indicating that in vitro aging-related PS exposure occurs independently of TMEM16F activity.
Prolonged storage causes PS exposure in Scott syndrome platelets. Percentage of annexin (Anx) A5-positive platelets (M2 and M3 fractions) from healthy controls and Scott patient during 1- to 4-h storage at room temperature in sterile buffer medium. Mean ± SEM (n = 3).
Prolonged storage causes PS exposure in Scott syndrome platelets. Percentage of annexin (Anx) A5-positive platelets (M2 and M3 fractions) from healthy controls and Scott patient during 1- to 4-h storage at room temperature in sterile buffer medium. Mean ± SEM (n = 3).
Convulxin-/thrombin-stimulated Scott platelets do not expose PS despite normal cyclophilin D-mediated mitochondrial depolarization and intracellular Ca2+ response
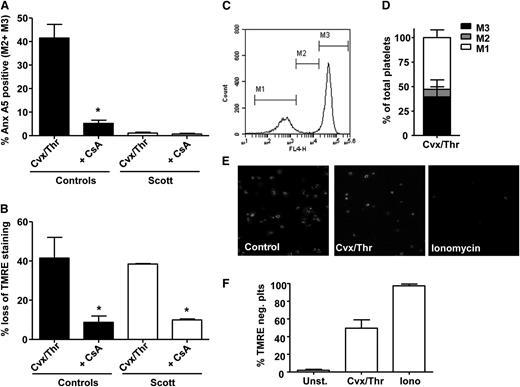
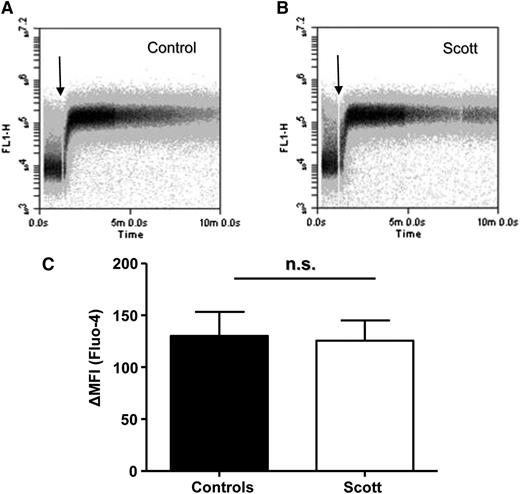
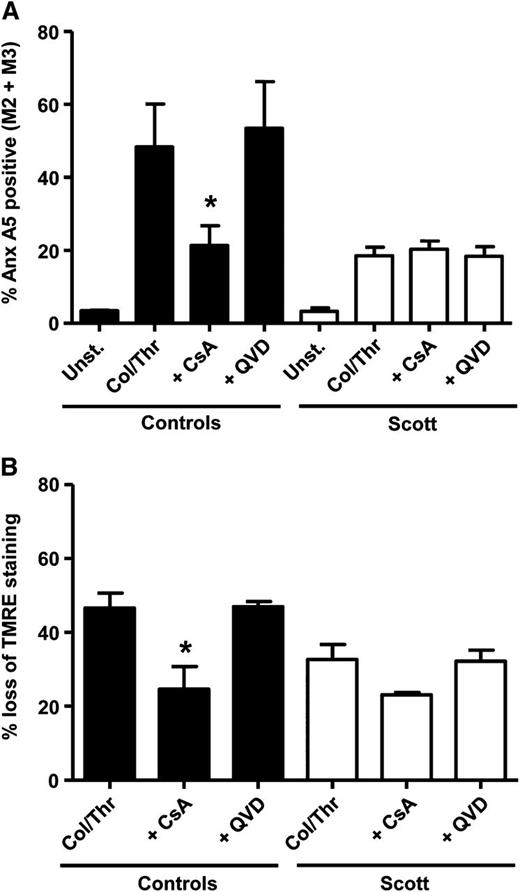
Mitochondrial depolarization through cyclophilin D-dependent formation of the MPTP has been shown to be crucial for convulxin-/thrombin-induced PS exposure in platelets.9 Involvement of cyclophilin D-mediated mitochondrial depolarization was assessed by measuring TMRE fluorescence and by investigating the effect of the MPTP inhibitor cyclosporin A. Stimulation of Scott syndrome platelets by convulxin/thrombin did not cause significant PS exposure (Figure 4A), whereas in healthy controls, 40% of the platelets became annexin A5 positive. This response was almost completely suppressed by cyclosporin A. The lack of PS exposure in convulxin-/thrombin-stimulated Scott platelets was not due to the absence of mitochondrial depolarization, as demonstrated by flow cytometry (Figure 4B). The loss of TMRE from mitochondria in Scott platelets stimulated with convulxin/thrombin was confirmed by confocal microscopy (Figure 4E-F). No differences in intracellular Ca2+ levels were found between Scott syndrome platelets and healthy controls after convulxin/thrombin stimulation (Figure 5A-C), which is in line with previous results showing normal store-operated Ca2+ entry with such agonists.11
Cvx-/Thr-induced PS exposure is absent in Scott syndrome platelets despite normal cyclophilin D-dependent mitochondrial depolarization. (A-D) Platelets from healthy controls or a Scott patient were loaded with TMRE and preincubated with vehicle (dimethylsulfoxide) or cyclosporin A (CsA) (4 μM) for 10 min prior to stimulation by Cvx/Thr (70 ng/mL, 4 nM, for 15 min) in the presence of 2 mM CaCl2. Graphs show annexin (Anx) A5 binding (A) and loss of TMRE (B) of washed control and Scott syndrome platelets. (C-D) Representative histogram and bar graph showing the distribution of Cvx-/Thr-stimulated control platelets (15 min) over the fractions M1, M2, and M3. (E-F) TMRE-loaded Scott platelets adhered to fibrinogen (10 min) were stimulated with Cvx/Thr (70 ng/mL, 4 nM) or ionomycin (10 μM) in the presence of 2 mM CaCl2. (E) Representative images after 15-min stimulation, (F) The percentage of TMRE-negative platelets. Mean ± SEM (n = 3-6); *P < .05.
Cvx-/Thr-induced PS exposure is absent in Scott syndrome platelets despite normal cyclophilin D-dependent mitochondrial depolarization. (A-D) Platelets from healthy controls or a Scott patient were loaded with TMRE and preincubated with vehicle (dimethylsulfoxide) or cyclosporin A (CsA) (4 μM) for 10 min prior to stimulation by Cvx/Thr (70 ng/mL, 4 nM, for 15 min) in the presence of 2 mM CaCl2. Graphs show annexin (Anx) A5 binding (A) and loss of TMRE (B) of washed control and Scott syndrome platelets. (C-D) Representative histogram and bar graph showing the distribution of Cvx-/Thr-stimulated control platelets (15 min) over the fractions M1, M2, and M3. (E-F) TMRE-loaded Scott platelets adhered to fibrinogen (10 min) were stimulated with Cvx/Thr (70 ng/mL, 4 nM) or ionomycin (10 μM) in the presence of 2 mM CaCl2. (E) Representative images after 15-min stimulation, (F) The percentage of TMRE-negative platelets. Mean ± SEM (n = 3-6); *P < .05.
Scott syndrome platelets display normal convulxin (Cvx)-/thrombin (Thr)-induced Ca2+ responses. (A-B) Time drive of fluorescence increase of washed control (A) and Scott syndrome (B) platelets loaded with Fluo-4 and activated by Cvx/Thr (70 ng/mL, 4 nM) in the presence of 2 mM CaCl2. (C) Average change in mean fluorescence intensity (MFI), representing cytosolic Ca2+, after 10 min stimulation. n.s., not significant.
Scott syndrome platelets display normal convulxin (Cvx)-/thrombin (Thr)-induced Ca2+ responses. (A-B) Time drive of fluorescence increase of washed control (A) and Scott syndrome (B) platelets loaded with Fluo-4 and activated by Cvx/Thr (70 ng/mL, 4 nM) in the presence of 2 mM CaCl2. (C) Average change in mean fluorescence intensity (MFI), representing cytosolic Ca2+, after 10 min stimulation. n.s., not significant.
Analysis of the flow cytometry histograms revealed a marked difference between ABT737 and convulxin/thrombin stimulation. Whereas ABT737 treatment resulted in 2 populations of platelets with moderate and high PS exposure, after convulxin/thrombin stimulation, even at early time points, only high PS-exposing platelets residing in marker region M3 were found (Figure 4C-D). Because this response was virtually absent in Scott platelets, we concluded that convulxin-/thrombin-induced PS exposure depends on a functional TMEM16F.
Collagen/thrombin stimulation induces an additional pathway to platelet PS exposure, which is independent of caspases, cyclophilin D, or TMEM16F
In previous studies, it was found that collagen-/thrombin-induced PS exposure is only partially impaired in platelets from patients with Scott syndrome.10,11 We questioned whether the residual PS exposure observed after collagen/thrombin stimulation in Scott platelets results from activation of the apoptotic pathway. Figure 6A shows that caspase inhibition by Q-VD-Oph did not affect the fraction of PS-exposing platelets induced by collagen/thrombin in either Scott platelets or healthy control platelets, indicating no appreciable contribution of the apoptotic pathway in collagen-/thrombin-induced PS exposure.
Residual collagen-/thrombin-induced PS exposure in Scott syndrome platelets is insensitive to Q-VD-Oph or cyclosporin A (CsA). Annexin (Anx) A5 binding (A) and TMRE loss (B) of washed control and Scott syndrome platelets stimulated by collagen (Col)/thrombin (Thr) (10 μg/mL, 4 nM) for 15 min in the presence of 2 mM CaCl2 preincubated with vehicle (dimethylsulfoxide), Q-VD-Oph (20 μM), or CsA (4 μM) for 10 min. Mean ± SEM (n = 3-6); *P < .05.
Residual collagen-/thrombin-induced PS exposure in Scott syndrome platelets is insensitive to Q-VD-Oph or cyclosporin A (CsA). Annexin (Anx) A5 binding (A) and TMRE loss (B) of washed control and Scott syndrome platelets stimulated by collagen (Col)/thrombin (Thr) (10 μg/mL, 4 nM) for 15 min in the presence of 2 mM CaCl2 preincubated with vehicle (dimethylsulfoxide), Q-VD-Oph (20 μM), or CsA (4 μM) for 10 min. Mean ± SEM (n = 3-6); *P < .05.
We then asked whether the residual PS-exposing activity is cyclophilin D-mediated. The collagen-/thrombin-induced PS exposure in Scott syndrome appeared insensitive to inhibition with cyclosporin A, whereas this compound effectively reduced mitochondrial depolarization (Figure 6A-B). Treatment of control platelets resulted in a reduction of collagen-/thrombin-induced PS exposure from 53% to 19% and a correspondingly reduced mitochondrial depolarization. These data suggest that collagen/thrombin activates 2 different pathways leading to PS exposure, one that requires MPTP formation and is dependent on TMEM16F and one that does not involve MPTP and functions independently of TMEM16F. Control studies showed that both collagen-/thrombin- and convulxin-/thrombin-induced PS exposure are entirely dependent on intracellular Ca2+, as platelet treatment with dm-BAPTA virtually abolished PS exposure (<4% PS-positive platelets).
Discussion
In this study, we show that Scott syndrome platelets have retained the capacity to expose PS upon ABT737-induced apoptosis. Remarkably, though, Scott syndrome platelets show only moderate levels of annexin A5 binding after 1 h of ABT737 treatment, whereas healthy controls have an additional platelet fraction with high annexin A5 binding despite the fact that the total fraction of annexin-positive platelets is virtually the same for the patient and healthy controls. This argues for a partial role of TMEM16F in apoptotic PS exposure. Because TMEM16F is reported to be crucial for Ca2+-induced phospholipid scrambling12 and the fraction of high annexin A5-binding platelets correlates with platelets high in intracellular Ca2+, we propose that next to a TMEM16F-independent mechanism, a TMEM16F-dependent mechanism is activated during platelet apoptosis, which accelerates the appearance of PS-exposing platelets. The high annexin A5-binding population was effectively suppressed by chelation of intracellular Ca2+, which is in concordance with TMEM16F being a Ca2+-dependent scramblase.12 Interestingly, the onset of the TMEM16F-dependent scrambling process (Figure 2E platelets in the region M3) appears to be delayed compared with the initiation of the TMEM16F-independent scrambling (region M2). Because the apoptotic process compromises Ca2+ homeostasis,23,24 it is not unlikely that a certain time span of increased cytosolic Ca2+ concentration is required to activate the TMEM16F-dependent scrambling mechanism. We do not know whether the actual rates of phospholipid scrambling differ between M3 and M2 platelets. It is tempting to speculate that the scrambling process in the M3 platelets is similar to that of ionomycin-induced scrambling, although it should be noted that apoptosis-induced PS exposure is caspase dependent, whereas ionomycin-induced PS exposure is not. Previously, induction of apoptosis in Scott syndrome lymphoid cells resulted in phospholipid scrambling similar to that in control lymphoid cells.25 In that study, the rate of lipid scrambling did not increase when apoptotic control B-lymphocytes were treated with Ca2+ ionophore, suggesting different pathways leading to lipid scrambling rather than the existence of more than one scramblase protein.
Although the role of high cytosolic Ca2+ in agonist-induced PS exposure is well recognized,1 it is unclear how Ca2+ affects phospholipid scrambling in apoptotic cells. In this paper, we show an inhibitory effect of chelating extracellular Ca2+ on ABT737-induced PS exposure. The lack of response upon removal of extracellular Ca2+ cannot be explained by decreased activity of the apoptotic machinery itself, as caspase activity is not affected by removal of extracellular Ca2+. Previously, Schoenwaelder et al14 have shown that ABT737-induced filamin and gelsolin cleavage was not affected by chelation of extracellular Ca2+. Our data indicate that, while the high PS-exposing platelet fraction M3 is absent upon chelation of intracellular Ca2+, the moderate PS-exposing fraction M2 also has a Ca2+-sensitive component. At present, we cannot distinguish whether this Ca2+ dependency is located at the level of signaling events downstream of caspase activity or at the level of the scramblase activity.
The most pronounced defect in Scott syndrome platelets was found upon activation with convulxin/thrombin; whereas these agonists induced ∼40% PS-positive platelets in healthy control platelets, PS exposure was virtually absent in the patient platelets. However, Ca2+ responses and mitochondrial depolarization were not affected in the patient platelets, indicating that the defect is downstream of Ca2+ signaling and MPTP formation. This finding is supported by a report showing that mitochondrial depolarization is normal in a canine model of impaired phospholipid scrambling.26 Inhibition of MPTP formation by cyclosporin A almost completely abrogated agonist-induced PS exposure in healthy control platelets (Figure 4A), in line with observations by Jobe et al9 Together, these data suggest that MPTP formation is required but not sufficient for agonist-induced platelet PS exposure via TMEM16F.
PS exposure induced by collagen/thrombin was only partially impaired in Scott syndrome platelets, confirming previous observations.11,27 The residual PS exposure typically was insensitive to inhibition by cyclosporin A (MPTP formation) or inhibition of caspases. In platelets from healthy controls, cyclosporin A was unable to completely suppress collagen-/thrombin-induced PS exposure. Earlier, Dasgupta and colleagues28 reported a partial impairment in collagen-/thrombin-induced PS exposure of cyclophilin D-deficient murine platelets. Taken together, these data suggest that 2 distinct pathways to PS exposure become activated by combined collagen/thrombin stimulation: a pathway sensitive to cyclosporin A inhibition and dependent on TMEM16F, and another pathway, which is cyclosporin A insensitive and operational in the absence of TMEM16F. This may explain the complete deficiency of PS exposure in Scott syndrome platelets activated by convulxin/thrombin, as the latter is more dependent on MPTP formation.9 The difference in PS exposure between stimulation with collagen/thrombin and convulxin/thrombin is interesting, because both collagen and convulxin are well-recognized GPVI agonists.21 We emphasize, however, that this difference has been repeatedly observed on different occasions. The distinct effects observed with collagen, compared with convulxin, may indicate the involvement of platelet collagen receptors other than GPVI (eg, integrin α2β1, GPIb via von Willebrand factor) in collagen-/thrombin-induced PS exposure.29
Acknowledging the inherent limitations of in vitro experimentation, this study demonstrates that TMEM16F-dependent and -independent phospholipid scrambling can be induced in platelets upon apoptosis, aging, and activation. However, it is still unclear whether TMEM16F by itself can act as a phospholipid scramblase or whether this protein has a regulatory function. If TMEM16F proves to be a scramblase itself, this will imply the existence of at least one additional and perhaps more proteins with scramblase activity in platelets. The identification of novel proteins with scramblase activity or the identification of novel regulators of scramblase activity will create new opportunities for interfering with phospholipid scrambling, with consequences for platelet procoagulant activity, life span, and storage.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by the Cardiovascular Centre, Maastricht, the Center for Translational Molecular Medicine INCOAG, and the Landsteiner Foundation for Blood Transfusion Research (1006).
Authorship
Contribution: R.v.K. designed the study, conceived experiments, analyzed data, and wrote the manuscript; N.J.A.M. and C.S. conceived experiments and analyzed data; M.A.H.F., J.L.N.W., and F.S. conceived experiments; P.W.C. contributed ideas and corrected the manuscript; J.W.M.H. designed the study and wrote the manuscript; and E.M.B. initiated and designed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: E.M. Bevers, Department of Biochemistry, Maastricht University, PO Box 616, 6200 MD Maastricht, The Netherlands; e-mail: em.bevers@maastrichtuniversity.nl.