Key Points
Presence of more than 3 PET focal lesions after day 7 first cycle of induction chemotherapy can predict for inferior overall survival and progression free survival.
Abstract
Prognostic implications of 3 imaging tools, metastatic bone survey, magnetic resonance imaging, and positron emission tomography (PET), were evaluated in 2 consecutive Total Therapy 3 trials for newly diagnosed myeloma. Data including PET at baseline and on day 7 of induction as well as standard prognostic factors were available in 302 patients of whom 277 also had gene expression profiling (GEP)-derived risk information. According to multivariate analysis, more than 3 focal lesions on day 7 imparted inferior overall survival and progression-free survival, overall and in the subset with GEP-risk data. GEP high-risk designation retained independent significance for all 3 end points examined. Thus, the presence of > 3 focal lesions on day 7 PET follow-up may be exploited toward early therapy change, especially for the 15% of patients with GEP-defined high-risk disease with a median overall survival expectation of 2 years. This trial was registered at www.clinicaltrials.gov as #NCT00081939 and # NCT00572169.
Introduction
Magnetic resonance imaging (MRI) and fluoro-deoxy-glucose (FDG) positron emission tomography (PET) scanning are increasingly viewed as important state-of-the-art imaging tools in the initial workup of patients with multiple myeloma (MM).1,2 The prognostic implications of PET scanning performed at baseline and after induction and high-dose therapy interventions have recently been reported.3 Here we investigated the survival implications of the day 7 PET scanning of patients treated with Total Therapy 3A4 clinical trial and successor protocol Total Therapy 3B5 protocol.
Study design
Details of the TT3 protocols have been reported previously.4,5 The protocols and their modifications had been approved by the University of Arkansas Medical Sciences Institutional Review Board. Patients signed written informed consent in keeping with institutional, federal, and international guidelines (Helsinki Declaration). MM diagnostic and response criteria employed the European Bone Marrow Transplant criteria introduced by Blade et al.6
In addition to standard baseline variables, presence and type of metaphase cytogenetic abnormalities (CA, CA13) were considered.4,5 Imaging variables included radiograph-defined osteolytic lesions (OL), MRI-based focal lesions (FL), and diffuse hyperintense marrow involvement1 as well as PET-FL and maximum FL standard uptake value (SUV) (SUVmax).3 PET studies were repeated on day 7 of induction and before first transplant. Although MRI examinations were also performed before the second transplant, consolidation, and maintenance phases, this report considers only baseline and pretransplant data.
Survival distributions were estimated according to the Kaplan-Meier method.7 Cumulative incidence curves were estimated as described by Gooley.8 These estimates were compared using the log-rank test.9 As of March 9, 2012, median follow-up times are 6.8 years and 4.3 years for Total Therapy 3A and Total Therapy 3B, respectively. Univariate and multivariate regression analyses were performed using a stratified Cox regression model.10 Stepwise variable selection was used to select the multivariate models. Cut-points for imaging parameters were applied as previously reported, including multiple prognostic OL and FL number cutoffs (ie, >0 and >2 for metastatic bone survey (MBS)-OL, >0 and >7 for MRI-FL, and >0 and >3 for PET-FL).2 An accounting of the patients included in the tables and figures can be found in supplemental Table 1.
Clinical end points included overall survival (OS), progression-free survival (PFS), and complete response duration (CRD). OS events included death from any cause, whereas PFS events included disease relapse or progression. CRD was measured as the time from complete response onset to disease progression or death from any cause. Land-marking methods were employed in order to include post-induction PET variables in our analyses. In the absence of progressions, deaths, or censored follow-up before the day 14 landmark, baseline, and landmarked Cox models for OS and PFS are equivalent. Thus, only land-marked models for OS and PFS are included below. For CRD, additional land-marking is not required to include day-7 PET data since complete response onset was not observed for any patients within 14 days of initiation of therapy. Survival estimates for OS and PFS by pretransplant PET and MRI are landmarked at the first transplant date. CRD is not presented with pretransplant PET and MRI results, because this would exclude a number of posttransplant CRs.
Results and discussion
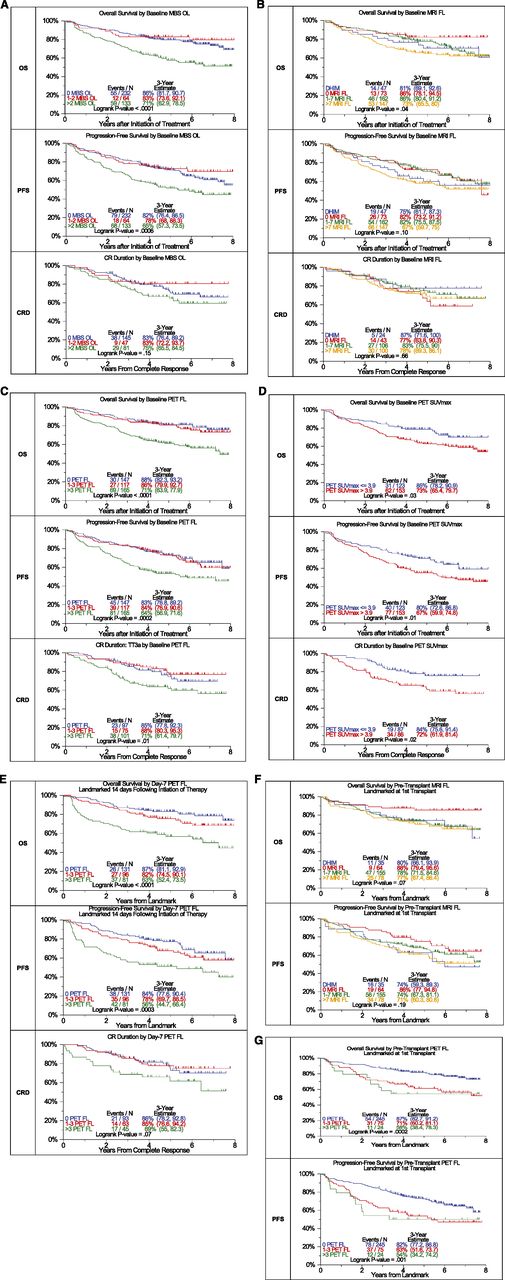
MBS-OL >2 adversely affected OS and PFS with a trend for CRD (OS: P < .0001, PFS: P = .0006, CRD: P = .15) (Figure 1A). OS differed among the 4 MRI baseline variables considered (P = .04) (Figure 1B). PFS tended to be superior in FL = 0 and FL 1-7 categories, whereas diffuse hyperintense marrow involvement and FL >7 baseline findings imparted borderline inferior outcomes (P = .1). PET-FL impacted all 3 outcome variables in a consistent manner (OS: P < .0001, PFS: P = .0002, CRD: P = .01) (Figure 1C). Patients with 0 or 1–3 FL had equally favorable OS, PFS, and CRD, whereas those presenting with FL >3 fared poorly. Similarly, PET-derived SUVmax of FL exceeding 3.9 conferred inferior OS, PFS, and CRD (OS: P = .03, PFS: P = .01, CRD: P = .02) (Figure 1D). A postinterventional PET scan follow-up examination on day 7 also provided prognostic value (Figure 1E). Presence of more than 3 FL was linked to inferior OS, PFS, and CRD with borderline significance (OS: P < .0001, PFS: P = .0003, CRD: P = .07). Day 7 SUVmax failed to affect outcomes significantly (data not shown). Consideration of pretransplant MRI FL suggested longer OS and PFS for cases without MRI FL. After 3 years, OS was >10% higher and PFS >12% higher for those without FL compared with all others (OS: P = .07, PFS: P = .19) (Figure 1F). PET-FL before transplant affected OS and PFS in a graded fashion (OS: P = .0002, PFS: P = .001) (Figure 1G).
Effects of imaging variables on OS, PFS, and CRD in Total Therapy 3A and Total Therapy 3B combined. (A) Baseline number of MBS-defined osteolytic lesions (OL). Superior OS and PFS were linked to the presence of no more than 2 OL. A trend was noted in case of complete response duration. (B) Baseline number of MRI-defined FL and diffuse hyperintense marrow. Trends were observed for superior OS and PFS in case of MRI-FL not exceeding 7 FL. (C) Baseline PET-defined number of FL.The presence of more than 3 PET-defined FL affected all 3 survival end points adversely. (D) Baseline PET-defined SUVmax. Higher SUVmax of PET-defined FL conferred inferior OS, PFS, and complete response (CR) duration. (E) Day 7 PET-FL. OS and PFS were inferior when more than 3 FL persisted on day 7 after starting protocol therapy. In case of CR duration, a strong trend in the same direction was noted. (F) Pretransplant MRI-FL. Trends were observed for both OS and PFS with the best survival at 3 years observed in those with 0 MRI FL, followed by 1 to 7 MRI FL and >7 MRI FL. Similar to the baseline results, survival at 3 years for those with DHIM was more like patients with 1 to 7 or >7 MRI FL. (G) Pretransplant PET-FL. A graded negative impact was observed with increasing PET-defined FL remaining before first transplant.
Effects of imaging variables on OS, PFS, and CRD in Total Therapy 3A and Total Therapy 3B combined. (A) Baseline number of MBS-defined osteolytic lesions (OL). Superior OS and PFS were linked to the presence of no more than 2 OL. A trend was noted in case of complete response duration. (B) Baseline number of MRI-defined FL and diffuse hyperintense marrow. Trends were observed for superior OS and PFS in case of MRI-FL not exceeding 7 FL. (C) Baseline PET-defined number of FL.The presence of more than 3 PET-defined FL affected all 3 survival end points adversely. (D) Baseline PET-defined SUVmax. Higher SUVmax of PET-defined FL conferred inferior OS, PFS, and complete response (CR) duration. (E) Day 7 PET-FL. OS and PFS were inferior when more than 3 FL persisted on day 7 after starting protocol therapy. In case of CR duration, a strong trend in the same direction was noted. (F) Pretransplant MRI-FL. Trends were observed for both OS and PFS with the best survival at 3 years observed in those with 0 MRI FL, followed by 1 to 7 MRI FL and >7 MRI FL. Similar to the baseline results, survival at 3 years for those with DHIM was more like patients with 1 to 7 or >7 MRI FL. (G) Pretransplant PET-FL. A graded negative impact was observed with increasing PET-defined FL remaining before first transplant.
Next we examined by multivariate Cox regression analysis which baseline variables in the context of day 7 PET-FL affected clinical outcomes applying a 14-day landmark from the beginning of induction therapy (Table 1). Data are presented also for the subgroup of 277 of 302 patients in whom GEP-based risk designation was available. Univariate data are summarized in supplemental Table 2. In the absence of GEP data, OS was dominantly affected by the presence of metaphase cytogenetic abnormalities (CA), high beta-2-microglobulin (B2M) (>5.5 mg/L), day 7 persistence of more than 3 FL, baseline MBS-OL >2, and older age (≥65 years). With knowledge of GEP data, GEP-defined high-risk status (70-gene model)5 was the dominant adverse feature imparting a 4.1-fold higher risk of death (95% CI: 2.57-6.52), whereas baseline MBS-OL, day-7 PET-FL, and older age all posed additional risk with hazard ratio values of 1.7 to 1.8. In the case of PFS, day 7 PET-FL persistence of >3 FL was the dominant adverse variable, followed by high B2M, CA subtype CA13, high lactate dehydrogenase (≥190 U/L) and low albumin (<3.5 g/dL). With access to GEP information, high risk dominated with a 3.23-fold higher risk of relapse or death (95% CI: 2.09-4.99), whereas day 7 PET-FL and B2M were the other independently adverse features. CRD, finally, was inferior in case of high B2M and in the presence of CA13. GEP-defined high-risk replaced CA13 in the subset of patients with GEP data. Day 7 PET data failed to enter the CRD model.
Multivariate analysis of baseline and day 7 PET findings on clinical outcomes landmarked from day 14 of induction therapy: OS, PFS, and CRD
| . | Excluding GEP variables . | Including GEP variables . | ||||
|---|---|---|---|---|---|---|
| Variable . | n/N (%) . | HR (95% CI) . | P value . | n/N (%) . | HR (95% CI) . | P value . |
| OS | ||||||
| GEP70 high risk | 52/277 (19%) | 4.10 (2.57-6.52) | <.001 | |||
| Baseline MBS OL >2 | 96/302 (32%) | 1.82 (1.17-2.83) | .008 | 86/277 (31%) | 1.81 (1.16-2.85) | .010 |
| Day-7 PET FL >3 | 81/302 (27%) | 1.86 (1.19-2.91) | .006 | 75/277 (27%) | 1.67 (1.05-2.65) | .030 |
| Age ≥65 years | 78/302 (26%) | 1.79 (1.16-2.76) | .008 | 72/277 (26%) | 1.81 (1.16-2.83) | .009 |
| Cytogenetic abnormalities | 109/302 (36%) | 2.15 (1.40-3.30) | <.001 | |||
| B2M >5.5 mg/L | 73/302 (24%) | 1.95 (1.25-3.03) | .003 | |||
| PFS | ||||||
| GEP70 high risk | 52/277 (19%) | 3.23 (2.09-4.99) | <.001 | |||
| Day 7 PET FL >3 | 81/302 (27%) | 1.83 (1.23-2.71) | .003 | 75/277 (27%) | 1.81 (1.21-2.70) | .004 |
| B2M >5.5 mg/L | 73/302 (24%) | 1.82 (1.19-2.77) | .005 | 66/277 (24%) | 1.60 (1.05-2.45) | .030 |
| LDH ≥190 U/L | 75/302 (25%) | 1.69 (1.12-2.53) | .012 | |||
| CA13 | 57/302 (19%) | 1.75 (1.13-2.71) | .012 | |||
| Albumin <3.5 g/dL | 107/302 (35%) | 1.60 (1.07-2.37) | .021 | |||
| CRD | ||||||
| GEP-70 high risk | 28/180 (16%) | 3.61 (1.93-6.77) | <.001 | |||
| B2M >5.5 mg/L | 39/196 (20%) | 3.77 (2.11-6.75) | <.001 | 36/180 (20%) | 2.89 (1.56-5.37) | <.001 |
| CA13 | 40/196 (20%) | 1.98 (1.07-3.67) | .030 | |||
| . | Excluding GEP variables . | Including GEP variables . | ||||
|---|---|---|---|---|---|---|
| Variable . | n/N (%) . | HR (95% CI) . | P value . | n/N (%) . | HR (95% CI) . | P value . |
| OS | ||||||
| GEP70 high risk | 52/277 (19%) | 4.10 (2.57-6.52) | <.001 | |||
| Baseline MBS OL >2 | 96/302 (32%) | 1.82 (1.17-2.83) | .008 | 86/277 (31%) | 1.81 (1.16-2.85) | .010 |
| Day-7 PET FL >3 | 81/302 (27%) | 1.86 (1.19-2.91) | .006 | 75/277 (27%) | 1.67 (1.05-2.65) | .030 |
| Age ≥65 years | 78/302 (26%) | 1.79 (1.16-2.76) | .008 | 72/277 (26%) | 1.81 (1.16-2.83) | .009 |
| Cytogenetic abnormalities | 109/302 (36%) | 2.15 (1.40-3.30) | <.001 | |||
| B2M >5.5 mg/L | 73/302 (24%) | 1.95 (1.25-3.03) | .003 | |||
| PFS | ||||||
| GEP70 high risk | 52/277 (19%) | 3.23 (2.09-4.99) | <.001 | |||
| Day 7 PET FL >3 | 81/302 (27%) | 1.83 (1.23-2.71) | .003 | 75/277 (27%) | 1.81 (1.21-2.70) | .004 |
| B2M >5.5 mg/L | 73/302 (24%) | 1.82 (1.19-2.77) | .005 | 66/277 (24%) | 1.60 (1.05-2.45) | .030 |
| LDH ≥190 U/L | 75/302 (25%) | 1.69 (1.12-2.53) | .012 | |||
| CA13 | 57/302 (19%) | 1.75 (1.13-2.71) | .012 | |||
| Albumin <3.5 g/dL | 107/302 (35%) | 1.60 (1.07-2.37) | .021 | |||
| CRD | ||||||
| GEP-70 high risk | 28/180 (16%) | 3.61 (1.93-6.77) | <.001 | |||
| B2M >5.5 mg/L | 39/196 (20%) | 3.77 (2.11-6.75) | <.001 | 36/180 (20%) | 2.89 (1.56-5.37) | <.001 |
| CA13 | 40/196 (20%) | 1.98 (1.07-3.67) | .030 | |||
Model selection and estimates based on the set of patients with complete data for the variables examined. P value from Wald χ-square test in Cox regression. Variables considered: age ≥65 years, albumin <3.5 g/dL, B2M ≥3.5 mg/L, B2M >5.5 mg/L, creatinine ≥2.0 mg/dL, C-reactive protein ≥8 mg/L, hemoglobin <10 g/dL, LDH ≥190 U/L, cytogenetic abnormalities, CA13, hypodiploid, CA 13/hypodiploid, GEP70 high risk, GEP Proliferation Index ≥10, GEP Centrosome Index ≥3, GEP Molecular Subgroup, baseline MBS OL (>0, >2), baseline MRI FL (>0, >7), baseline PET FL (>0, >3), baseline PET EMD, day 7 PET FL (>0, >3).
GEP, gene expression profiling; HR, hazard ratio; LDH, Lactate dehydrogenase
Collectively, our data confirm and extend observations by our group and others on the powerful prognostic implications of baseline and follow-up PET examinations.2,3 An important novel finding relates to the superiority of day 7 PET-defined FL >3 over baseline findings, retaining such significance for OS and PFS in the presence of GEP data, displacing CA as a variable. B2M survived the multivariate models for all 3 end points examined and is only displaced from the OS model with knowledge of GEP risk. Although MBS-OL is a later event than the development of FL detected on MRI or PET scans, the presence of more than 2 OL retained independent adverse implications for OS also in the presence of GEP data, confirming the pioneering work of Durie and Salmon.11 These findings strengthen the argument for validating such examinations in other trials as a basis for modifying therapy in the subset of patients with persisting FDG-avid FL >3. Such early corrective therapeutic measure should be of particular benefit in patients with GEP-defined high-risk MM.12,13
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by a grant from the National Cancer Institute and National Institutes of Health (CA 55813).
National Institutes of Health
Authorship
Contributions: S.Z.U. and B.B. designed the study and wrote the manuscript; S.Z.U., B.B., A.M., A.H., J.C., N.C., N.P., and E.A. analyzed the data; B.B., E.A., F.v.R., S.W., and S.Z.U. contributed patients; and S.Z.U., A.M, S.W., J.C., A.H., N.P, T. Brown, T. Bartel, E.A., F.v.R., and B.B. reviewed and approved the manuscript.
Conflict-of-interest disclosure: S.Z.U. is a consultant to Celgene, Millennium, and Onyx; has received research funding from Onyx and Celgene and speaking honoraria from Celgene. B.B. has received research funding from Celgene, Millennium, and Onyx; has served as consultant to Celgene, Millennium, Onyx, and Genzyme; is a co-inventor on patents and patent applications related to use of gene expression profiling in cancer medicine. He has no financial interest in SignalGenetics.
Correspondence: Saad Usmani, Myeloma Institute for Research and Therapy, University of Arkansas for Medical Sciences, 4301 West Markham, Little Rock, AR 72205; e-mail: susmani@uams.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal